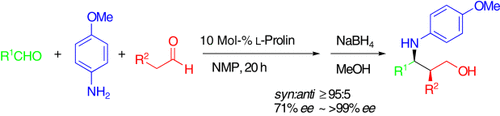
The Direct and Enantioselective, One-Pot, Three-Component, Cross-Mannich Reaction of Aldehydes†
Yujiro Hayashi Prof. Dr.
Department of Industrial Chemistry, Faculty of Engineering, Tokyo University of Science, Kagurazaka, Shinjuku-ku, Tokyo 162-8601, Japan, Fax: (+81) 3-5261-4631
Search for more papers by this authorWataru Tsuboi
Department of Industrial Chemistry, Faculty of Engineering, Tokyo University of Science, Kagurazaka, Shinjuku-ku, Tokyo 162-8601, Japan, Fax: (+81) 3-5261-4631
Search for more papers by this authorItaru Ashimine
Department of Industrial Chemistry, Faculty of Engineering, Tokyo University of Science, Kagurazaka, Shinjuku-ku, Tokyo 162-8601, Japan, Fax: (+81) 3-5261-4631
Search for more papers by this authorTatsuya Urushima
Department of Industrial Chemistry, Faculty of Engineering, Tokyo University of Science, Kagurazaka, Shinjuku-ku, Tokyo 162-8601, Japan, Fax: (+81) 3-5261-4631
Search for more papers by this authorMitsuru Shoji Dr.
Department of Industrial Chemistry, Faculty of Engineering, Tokyo University of Science, Kagurazaka, Shinjuku-ku, Tokyo 162-8601, Japan, Fax: (+81) 3-5261-4631
Search for more papers by this authorKen Sakai Prof. Dr.
Department of Applied Chemistry, Faculty of Science, Tokyo University of Science, Kagurazaka, Shinjuku-ku, Tokyo 162-8601, Japan
Search for more papers by this authorYujiro Hayashi Prof. Dr.
Department of Industrial Chemistry, Faculty of Engineering, Tokyo University of Science, Kagurazaka, Shinjuku-ku, Tokyo 162-8601, Japan, Fax: (+81) 3-5261-4631
Search for more papers by this authorWataru Tsuboi
Department of Industrial Chemistry, Faculty of Engineering, Tokyo University of Science, Kagurazaka, Shinjuku-ku, Tokyo 162-8601, Japan, Fax: (+81) 3-5261-4631
Search for more papers by this authorItaru Ashimine
Department of Industrial Chemistry, Faculty of Engineering, Tokyo University of Science, Kagurazaka, Shinjuku-ku, Tokyo 162-8601, Japan, Fax: (+81) 3-5261-4631
Search for more papers by this authorTatsuya Urushima
Department of Industrial Chemistry, Faculty of Engineering, Tokyo University of Science, Kagurazaka, Shinjuku-ku, Tokyo 162-8601, Japan, Fax: (+81) 3-5261-4631
Search for more papers by this authorMitsuru Shoji Dr.
Department of Industrial Chemistry, Faculty of Engineering, Tokyo University of Science, Kagurazaka, Shinjuku-ku, Tokyo 162-8601, Japan, Fax: (+81) 3-5261-4631
Search for more papers by this authorKen Sakai Prof. Dr.
Department of Applied Chemistry, Faculty of Science, Tokyo University of Science, Kagurazaka, Shinjuku-ku, Tokyo 162-8601, Japan
Search for more papers by this authorThis work was supported by a Grant-in-Aid for Scientific Research on Priority Areas (A; “Exploitation of Multi-Element Cyclic Molecules”) from the Ministry of Education, Culture, Sports, Science, and Technology, Japan.
Graphical Abstract
References
- 1Reviews,
- 1aB. List, Synlett 2001, 1675;
- 1bB. List, Tetrahedron 2002, 58, 5573;
- 1cR. O. Duthaler, Angew. Chem. 2003, 115, 1005;
10.1002/ange.200390228 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 975; for an excellent review of enantioselective organocatalysis, see
- 1dP. I. Dalko, L. Moisan, Angew. Chem. 2001, 113, 3840;
10.1002/1521-3757(20011015)113:20<3840::AID-ANGE3840>3.0.CO;2-M Google ScholarAngew. Chem. Int. Ed. 2001, 40, 3726.10.1002/1521-3773(20011015)40:20<3726::AID-ANIE3726>3.0.CO;2-D CAS PubMed Web of Science® Google Scholar
- 2
- 2aB. List, R. A. Lerner, C. F. Barbas III, J. Am. Chem. Soc. 2000, 122, 2395;
- 2bK. Sakthivel, W. Notz, T. Bui, C. F. Barbas III, J. Am. Chem. Soc. 2001, 123, 5260;
- 2cB. List, P. Pojarliev, C. Castello, Org. Lett. 2001, 3, 573;
- 2dW. Notz, B. List, J. Am. Chem. Soc. 2000, 122, 7386;
- 2eA. Cordova, W. Notz, C. F. Barbas III, J. Org. Chem. 2002, 67, 301;
- 2fL. Hoang, S. Bahmanyar, K. N. Houk, B. List, J. Am. Chem. Soc. 2003, 125, 16;
- 2gS. Bahmanyar, K. N. Houk, H. J. Martin, B. List, J. Am. Chem. Soc. 2003, 125, 2475; see also
- 2hZ. Tang, F. Jiang, L. Yu, X. Cui, L. Gong, A. Mi, Y. Jiang, Y. Wu, J. Am. Chem. Soc. 2003, 125, 5262;
- 2iA. Bøgevig, N. Kumaragurubaran, K. A. Jørgensen, Chem. Commun. 2002, 620;
- 2jA. B. Northrup, D. W. C. MacMillan, J. Am. Chem. Soc. 2002, 124, 6798.
- 3
- 3aZ. G. Hajos, D. R. Parrish, , German Patent DE 2102623, 1971 [ Chem. Abstr. 1972, 76, 59072x];
- 3bU. Eder, R. Wiechert, G. Sauer, , German Patent DE 2014757, 1971 [ Chem. Abstr. 1972, 76, 14180q];
- 3cU. Eder, G. Sauer, R. Wiechert, Angew. Chem. 1971, 83, 492;
10.1002/ange.19710831307 Google ScholarAngew. Chem. Int. Ed. Engl. 1971, 10, 496;
- 3dZ. G. Hajos, D. R. Parrish, J. Org. Chem. 1974, 39, 1612.
- 4
- 4aB. List, J. Am. Chem. Soc. 2000, 122, 9336;
- 4bB. List, P. Pojarliev, W. T. Biller, H. J. Martin, J. Am. Chem. Soc. 2002, 124, 827.
- 5
- 5aA. Cordova, W. Notz, G. Zhong, J. M. Betancort, C. F. Barbas III, J. Am. Chem. Soc. 2002, 124, 1842;
- 5bA. Cordova, S. Watanabe, F. Tanaka, W. Notz, C. F. Barbas III, J. Am. Chem. Soc. 2002, 124, 1866;
- 5cA. Cordova, C. F. Barbas III, Tetrahedron Lett. 2003, 44, 1923;
- 5dA. Cordova, C. F. Barbas III, Tetrahedron Lett. 2002, 43, 7749;
- 5eS. Watanabe, A. Cordova, F. Tanaka, C. F. Barbas III, Org. Lett. 2002, 4, 4519;
- 5fW. Notz, K. Sakthivel, T. Bui, G. Zhong, C. F. Barbas III, Tetrahedron Lett. 2001, 42, 199.
- 6A. G. Wenzel, E. N. Jacobsen, J. Am. Chem. Soc. 2002, 124, 12 964.
- 7
- 7aS. P. Brown, N. C. Goodwin, D. W. C. MacMillan, J. Am. Chem. Soc. 2003, 125, 1192;
- 7bD. Enders, A. Seki, Synlett 2002, 26;
- 7cJ. M. Betancort, K. Sakthivel, R. Thayumanavan, C. F. Barbas III, Tetrahedron Lett. 2001, 42, 4441;
- 7dJ. M. Betancort, C. F. Barbas III, Org. Lett. 2001, 3, 3737;
- 7eB. List, P. Pojarliev, H. J. Martin, Org. Lett. 2001, 3, 2423;
- 7fS. Hanessian, V. Pham, Org. Lett. 2000, 2, 2975;
- 7gA. Alexakis, O. Andrey, Org. Lett. 2002, 4, 3611.
- 8
- 8aB. List, J. Am. Chem. Soc. 2002, 124, 5656;
- 8bN. Kumaragurubaran, K. Juhl, W. Zhuang, A. Bøgevig, K. A. Jørgensen, J. Am. Chem. Soc. 2002, 124, 6254;
- 8cA. Bøgevig, K. Juhl, N. Kumaragurubaran, W. Zhuang, K. A. Jørgensen, Angew. Chem. 2002, 114, 1868;
10.1002/1521-3757(20020517)114:10<1868::AID-ANGE1868>3.0.CO;2-P Google ScholarAngew. Chem. Int. Ed. 2002, 41, 1790.10.1002/1521-3773(20020517)41:10<1790::AID-ANIE1790>3.0.CO;2-Y CAS PubMed Web of Science® Google Scholar
- 9For other notable examples of enantioselective organocatalytic reactions, see Diels–Alder reaction:
- 9aK. A. Ahrendt, C. J. Borths, D. W. C. MacMillan, J. Am. Chem. Soc. 2000, 122, 4243;
- 9bA. B. Northrup, D. W. C. MacMillan, J. Am. Chem. Soc. 2002, 124, 2458; hetero-Diels–Alder reaction:
- 9cK. Juhl, K. A. Jørgensen, Angew. Chem. 2003, 115, 1536;
10.1002/ange.200250652 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 1498; nitrone cycloaddition:
- 9dW. S. Jen, J. J. M. Wiener, D. W. C. MacMillan, J. Am. Chem. Soc. 2000, 122, 9874; pyrrole substitution:
- 9eN. A. Paras, D. W. C. MacMillan, J. Am. Chem. Soc. 2001, 123, 4370; indole substitution:
- 9fJ. F. Austin, D. W. C. MacMillan, J. Am. Chem. Soc. 2002, 124, 1172; aniline substitution:
- 9gN. A. Paras, D. W. C. MacMillan, J. Am. Chem. Soc. 2002, 124, 7894; Baylis–Hillman reaction:
- 9hY. Iwabuchi, M. Nakatani, N. Yokoyama, S. Hatakeyama, J. Am. Chem. Soc. 1999, 121, 10 219.
- 10
- 10aH. Fujieda, M. Kanai, T. Kambara, A. Iida, K. Tomioka, J. Am. Chem. Soc. 1997, 119, 2060;
- 10bH. Ishitani, M. Ueno, S. Kobayashi, J. Am. Chem. Soc. 1997, 119, 7153;
- 10cS. Kobayashi, H. Ishitani, M. Ueno, J. Am. Chem. Soc. 1998, 120, 431;
- 10dH. Ishitani, M. Ueno, S. Kobayashi, J. Am. Chem. Soc. 2000, 122, 8180;
- 10eS. Kobayashi, J. Kobayashi, H. Ishitani, M. Ueno, Chem. Eur. J. 2002, 8, 4185;
10.1002/1521-3765(20020916)8:18<4185::AID-CHEM4185>3.0.CO;2-6 CAS PubMed Web of Science® Google Scholar
- 10fE. Hagiwara, A. Fujii, M. Sodeoka, J. Am. Chem. Soc. 1998, 120, 2474;
- 10gA. Fujii, E. Hagiwara, M. Sodeoka, J. Am. Chem. Soc. 1999, 121, 5450;
- 10hD. Ferraris, B. Young, T. Dudding, T. Lectka, J. Am. Chem. Soc. 1998, 120, 4548;
- 10iD. Ferraris, B. Young, C. Cox, W. J. Drury III, T. Dudding, T. Lectka, J. Org. Chem. 1998, 63, 6090;
- 10jD. Ferraris, T. Dudding, B. Young, W. J. Drury III, T. Lectka, J. Org. Chem. 1999, 64, 2168;
- 10kD. Ferraris, B. Young, C. Cox, T. Dudding, W. J. Drury III, L. Ryzhkov, A. E. Taggi, T. Lectka, J. Am. Chem. Soc. 2002, 124, 67.
- 11
- 11aS. Matsunaga, N. Kumagai, S. Harada, M. Shibasaki, J. Am. Chem. Soc. 2003, 125, 4712;
- 11bS. Yamasaki, T. Iida, M. Shibasaki, Tetrahedron Lett. 1999, 40, 307;
- 11cS. Yamasaki, T. Iida, M. Shibasaki, Tetrahedron 1999, 55, 8857.
- 12K. Juhl, N. Gathergood, K. A. Jørgensen, Angew. Chem. 2001, 113, 3083;
10.1002/1521-3757(20010817)113:16<3083::AID-ANGE3083>3.0.CO;2-C Google ScholarAngew. Chem. Int. Ed. 2001, 40, 2995.10.1002/1521-3773(20010817)40:16<2995::AID-ANIE2995>3.0.CO;2-M CAS PubMed Web of Science® Google Scholar
- 13B. M. Trost, L. R. Terrell, J. Am. Chem. Soc. 2003, 125, 338.
- 14PMP=para-methoxyphenyl.
- 15
- 15aN. B. Ambhaikar, J. P. Snyder, D. C. Liotta, J. Am. Chem. Soc. 2003, 125, 3690;
- 15bF. Chemla, V. Hebbe, J. Normant, Synthesis 2000, 75;
- 15cM. Yamaguchi in Comprehensive Organic Synthesis, Vol. 1 (Ed ), Pergamon, Oxford, 1991, p. 325.
10.1016/B978-0-08-052349-1.00011-1 Google Scholar
- 16S. Kobayashi, S. Nagayama, J. Am. Chem. Soc. 1997, 119, 10 049.
- 17H. Nakamura, H. Iwama, Y. Yamamoto, J. Am. Chem. Soc. 1996, 118, 6641.
- 18T. Akiyama, J. Takaya, H. Kagoshima, Chem. Lett. 1997, 947.
- 19DMSO cannot be used at −20 °C because it solidifies.
- 20Crystal data: C17H20ClNO2, monoclinic, space group P21, a=10.206(4), b=6.187(2), c=12.962(5) Å, β=97.838(6)°, V=810.8(5) Å3, Z=2, R1=0.1427, wR2=0.4094 (refined on F2 using SHELXL97). CCDC-212084 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223-336-033; or [email protected]).
- 21S. Bahmanyar, K. N. Houk, Org. Lett. 2003, 5, 1249.
- 22HPLC conditions: Chiral AD-H column, iPrOH:hexane 1:30, 0.5 mL min−1, retention times: 74.6 min (minor) and 80.4 min (major).
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.