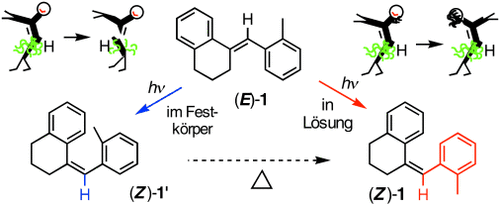
Photoisomerization by Hula Twist: 2,2′-Dimethylstilbene and a Ring-Fused Analogue†
Yasushi Imamoto Dr.
Graduate School of Materials Science, Nara Institute of Science and Technology, Ikoma, Nara 630-0192, Japan
Search for more papers by this authorTakayuki Kuroda
Graduate School of Materials Science, Nara Institute of Science and Technology, Ikoma, Nara 630-0192, Japan
Search for more papers by this authorMikio Kataoka Prof. Dr.
Graduate School of Materials Science, Nara Institute of Science and Technology, Ikoma, Nara 630-0192, Japan
Search for more papers by this authorSergey Shevyakov
Department of Chemistry, University of Hawaii, 2545 The Mall, Honolulu, HI 96822, USA, Fax: (+1) 808-956-5908
Search for more papers by this authorG. Krishnamoorthy
Department of Chemistry, University of Hawaii, 2545 The Mall, Honolulu, HI 96822, USA, Fax: (+1) 808-956-5908
Search for more papers by this authorRobert S. H. Liu Prof. Dr.
Department of Chemistry, University of Hawaii, 2545 The Mall, Honolulu, HI 96822, USA, Fax: (+1) 808-956-5908
Search for more papers by this authorYasushi Imamoto Dr.
Graduate School of Materials Science, Nara Institute of Science and Technology, Ikoma, Nara 630-0192, Japan
Search for more papers by this authorTakayuki Kuroda
Graduate School of Materials Science, Nara Institute of Science and Technology, Ikoma, Nara 630-0192, Japan
Search for more papers by this authorMikio Kataoka Prof. Dr.
Graduate School of Materials Science, Nara Institute of Science and Technology, Ikoma, Nara 630-0192, Japan
Search for more papers by this authorSergey Shevyakov
Department of Chemistry, University of Hawaii, 2545 The Mall, Honolulu, HI 96822, USA, Fax: (+1) 808-956-5908
Search for more papers by this authorG. Krishnamoorthy
Department of Chemistry, University of Hawaii, 2545 The Mall, Honolulu, HI 96822, USA, Fax: (+1) 808-956-5908
Search for more papers by this authorRobert S. H. Liu Prof. Dr.
Department of Chemistry, University of Hawaii, 2545 The Mall, Honolulu, HI 96822, USA, Fax: (+1) 808-956-5908
Search for more papers by this authorThe work was supported by a grant from the National Science Foundation (CHE-32250) and a grant from Sekisui Integrated Research Inc. We thank Dr. Alabugin for the calculated energies of the conformers of 2. X.-S. Wang, H.-R. Li, L. U. Colmenares, and D. Chang participated in the early part of the work described here.
Graphical Abstract
References
- 1
- 1aR. S. H. Liu, G. S. Hammond, Proc. Natl. Acad. Sci. USA 2000, 97, 11 153–11 158;
- 1bR. S. H. Liu, Acc. Chem. Res. 2001, 34, 555–562;
- 1cR. S. H. Liu, G. S. Hammond, Chem. Eur. J. 2001, 7, 4536–4544.
- 2See, for example,
- 2aJ. Saltiel, D. F. Sears, Jr.,D.-H. Ko, K.-M. Park in CRC Handbook of Organic Photochemistry and Photobiology (Ed ), CRC, Boca Raton, 1994, pp. 1–15;
- 2bW. J. Leigh in CRC Handbook of Organic Photochemistry and Photobiology (Ed ), CRC Press, Boca Raton, 1994, pp. 123–142;
- 2cH. J. C. Jacobs, W. H. Laarhoven in CRC Handbook of Organic Photochemistry and Photobiology (Ed ), CRC, Boca Raton, 1994, pp. 143–154.
- 3
- 3aR. S. H. Liu, A. E. Asato, Proc. Natl. Acad. Sci. USA 1985, 82, 259–263;
- 3bR. S. H. Liu, D. T. Browne Acc. Chem. Res. 1986, 19, 42–48.
- 4For selected papers on the photoisomerization pathways, torsional relaxation, and the Hula twist, see
- 4aR. S. H. Liu, Photochem. Photobiol. 2002, 76, 580–583;
- 4bM. Uda, T. Mizutani, J. Hayakawa, A. Momotake, M. Ikegami, R. Nagahata, T. Arai, Photochem. Photobiol. 2002, 76, 596–605;
- 4cS. Wilsey, K. N. Houk, Photochem. Photobiol. 2002, 76, 616–621;
- 4dM. Squillacote, T. Semple, J.-W. Chen, F. Liang, Photochem. Photobiol. 2002, 76, 634–639.
- 5A. M. Muller, S. Lochbrunner, W. E. Schmid, W. Fuss, Angew. Chem. 1998, 110, 520–522;
Angew. Chem. Int. Ed. 1998, 37, 505–507.
10.1002/(SICI)1521-3773(19980302)37:4<505::AID-ANIE505>3.0.CO;2-U CAS Web of Science® Google Scholar
- 6(E)-2: 1H NMR (300 MHz, CDCl3): δ=1.82 (dd, 2 H), 2.30 (s, 3 H), 2.58 (t, 2 H), 2.87 (t, 2 H), 7.01 (br s, 1 H), 7.1–7.3 (m, 7 H), 7.73 ppm (m, 1 H); UV/Vis (hexane): λmax 273 nm, ε=9500 mol−1 dm3 cm−1. (Z)-2: 1H NMR (300 MHz, CDCl3) : δ=2.02 (q, 2 H); 2.23 (s, 3 H), 2.58 (q, 2 H), 2.90 (dd, 2 H), 6.43 (m, 1 H), 6.76 (dd, 1 H), 6.88 (d, 1 H), 6.9–7.3 ppm (m, 6 H); UV/Vis (hexane): λmax 275 nm, ε=10 700 mol−1 dm3 cm−1. As expected, the Z isomer has a shorter retention time than the E isomer on silica gel.
- 7T. Yoshizawa, Y. Shichida, Methods Enzymol. 1982, 81, 333–354.
- 8U. Mazzucato, F. Momicchioli, Chem. Rev. 1991, 91, 1679–1719.
- 9Calculations were performed by Drs. Manoharan and Alabugin by using B3LYP6-31G* the density functional (DFT) method.
- 10The actinometer system (trans-stilbene, ϕ=0.49) was run at room temperature, see J. Saltiel, A. Marinari, D. W.-L. Chang, J. C. Mitchener, E. D. Megarity, J. Am. Chem. Soc. 1979, 101, 2982–2996.
- 11M. E. Squillacote, R. S. Sheridan, O. L. Chapman, F. A. L. Anet, J. Am. Chem. Soc. 1979, 101, 3657–3659.
- 12J. R. Ackerman, S. A. Forman, M. Hussain, B. Kohler, J. Chem. Phys. 1984, 80, 39–44.
- 13
- 13aM. V. Alfimov, V. F. Razumov, A. G. Rachinsky, V. N. Lisvan, Yu. B. Scheck, Chem. Phys. Lett. 1983, 101, 593–597;
- 13bN. Castel, E. Fischer, J. Mol. Struct. 1985, 127, 159–166.
- 14D. Sempedro Ruiz, A. Cembran, M. Garavelli, M. Olivucci, W. Fuss, Photochem. Photobiol. 2002, 76, 622–633.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.