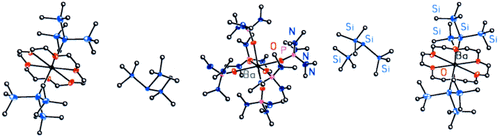
Syntheses and Structures of Barium Silanides: Contact and Separated Ions†
Weijie Teng
Department of Chemistry, Syracuse University, 1-014 Center for Science and Technology, Syracuse, NY 13244-4100, USA, Fax: (+1) 315-443-4070
Search for more papers by this authorUlrich Englich Dr.
Department of Chemistry, Syracuse University, 1-014 Center for Science and Technology, Syracuse, NY 13244-4100, USA, Fax: (+1) 315-443-4070
Search for more papers by this authorKarin Ruhlandt-Senge Prof. Dr.
Department of Chemistry, Syracuse University, 1-014 Center for Science and Technology, Syracuse, NY 13244-4100, USA, Fax: (+1) 315-443-4070
Search for more papers by this authorWeijie Teng
Department of Chemistry, Syracuse University, 1-014 Center for Science and Technology, Syracuse, NY 13244-4100, USA, Fax: (+1) 315-443-4070
Search for more papers by this authorUlrich Englich Dr.
Department of Chemistry, Syracuse University, 1-014 Center for Science and Technology, Syracuse, NY 13244-4100, USA, Fax: (+1) 315-443-4070
Search for more papers by this authorKarin Ruhlandt-Senge Prof. Dr.
Department of Chemistry, Syracuse University, 1-014 Center for Science and Technology, Syracuse, NY 13244-4100, USA, Fax: (+1) 315-443-4070
Search for more papers by this authorThis work was supported by the National Science Foundation (CHE-9702246 and CHE-0108098). Purchase of the X-ray diffractometer was made possible with grants from NSF (CHE-95-27898), the W. M. Keck Foundation and Syracuse University. We acknowledge D. Jenkins for technical assistance.
Graphical Abstract
Die Gegenwart bestimmter Donormoleküle entscheidet, ob Bariumhypersilanide als Koordinationsverbindungen (siehe Bild) oder als Ionenpaare vorliegen: In aromatischen Lösungsmitteln dissoziieren die Verbindungen unter Bildung diskreter Ionen. Die Silanide sind bedeutsam als effektive Initiatoren für Polymerisationen und wichtige Modellverbindungen in der metallorganischen Chemie der schweren Erdalkalimetalle.
References
- 1For recent reviews see:
- 1aT. P. Hanusa, Coord. Chem. Rev. 2000, 210, 329;
- 1bJ. Alexander, K. Ruhlandt-Senge, Eur. J. Inorg. Chem. 2002, 11, 2761, and references therein.
10.1002/1099-0682(200211)2002:11<2761::AID-EJIC2761>3.0.CO;2-2 Google Scholar
- 2
- 2aJ. D. Farwell, M. F. Lappert, C. Marschner, C. Strissel, T. D. Tilley, J. Organomet. Chem. 2000, 603, 185;
- 2bL. Rösch, Angew. Chem. 1977, 89, 257;
10.1002/ange.19770890413 Google ScholarAngew. Chem. Int. Ed. Engl. 1977, 16, 247;
- 2cL. Rösch, J. Pickardt, S. Imme, U. Börner, Z. Naturforsch. B 1986, 41, 1523;
- 2dA. R. Claggett, W. H. Ilsley, T. J. Anderson, M. D. Glick, J. P. Oliver, J. Am. Chem. Soc. 1977, 99, 1979;
- 2eL. Rösch, U. Starke, Z. Naturforsch. B 1983, 38, 1292.
- 3O. Shizuka (H. Kunio. Jpn. Kokai Tokkyo Koho), JP 0306806, 1991.
- 4D. Jenkins, K. Ruhlandt-Senge, unpublished results.
- 5U. Englich, K. Ruhlandt-Senge, F. Uhlig, J. Organomet. Chem. 2000, 613, 139.
- 6Crystal data for 1: C34H86BaO4Si8, Mr=921.09, orthorhombic, space group Pbca, a=19.157(3), b=17.219(3), c=31.602(5) Å, V=10 424(3) Å3, T=98(2) K, Z=8, μ=0.975 mm−1 (MoKα); yellow needles 0.70×0.30×0.20 mm3; 12 692 independent reflections, 3.34≤2θ≤56.60°; R1=0.0522, wR2=0.0891 for data I>2σ(I) and R1=0.0978, wR2=0.1011 for all data.
- 7
- 7aR. Shannon, C. T. Previtt, Acta Crystallogr. Sect. B 1969, 25, 925;
- 7bR. D. Shannon, Acta Crystallogr. Sect. A 1976, 32, 751.
- 8K. Ruhlandt-Senge, U. Englich, Chem. Eur. J. 2000, 6, 4063.
10.1002/1521-3765(20001117)6:22<4063::AID-CHEM4063>3.0.CO;2-Q CAS PubMed Web of Science® Google Scholar
- 9K. W. Klinkhammer, Chem. Eur. J. 1997, 3, 1418.
- 10D. M. Jenkins, W. Teng, U. Englich, D. Stone, K. Ruhlandt-Senge, Organometallics 2001, 20, 4600.
- 11Crystal data for 2: C54H162BaN18O6P6Si8, Mr=1707.90, triclinic, space group P1¯, a=19.192(2), b=19.636(2), c=14.347(12) Å, α=71.491(1), β=71.232(1), γ=81.048(2)°, V=4845.7(6) Å3, T=91(2) K, Z=2, μ=0.655 mm−1 (MoKα); yellow plate 0.60×0.48×0.10 mm3; 39 962 independent reflections, 3.26≤2θ≤50.30°; R1=0.0693, wR2=0.1740 for data I>2σ(I) and R1=0.1086, wR2=0.1991 for all data.
- 12J. Alexander, K. Ruhlandt-Senge, Angew. Chem. 2001, 113, 2732;
10.1002/1521-3757(20010716)113:14<2732::AID-ANGE2732>3.0.CO;2-X Google ScholarAngew. Chem. Int. Ed. 2001, 40, 2658.10.1002/1521-3773(20010716)40:14<2658::AID-ANIE2658>3.0.CO;2-Y CAS PubMed Web of Science® Google Scholar
- 13Crystal data for 3: C84H240Ba2N18O12P6Si16, Mr=2604.88, rhombohedral, space group R3¯c, a=17.4605(6), b=17.4605(6), c=84.697(5) Å, V=22 362(2) Å3, T=85(2) K, Z=6, μ=0.767 mm−1 (MoKα); yellow plate 0.26×0.18×0.10 mm3; 4313 independent reflections, 2.88≤2θ≤50.48°; R1=0.0541, wR2=0.1290 for data I>2σ(I) and R1=0.1062, wR2=0.1416 for all data. All crystals were mounted on the diffractometer as described elsewhere.[18] The data were collected using a Bruker SMART system, complete with three-circle goniometer and CCD detector as described earlier.[8] The crystal structure of 1 was solved using direct methods whereas Patterson methods were used to solve 2 and 3. All structures were refined by full-matrix least-squares refinement on F2.[19] An absorption correction was applied for all structures.[20] All non-hydrogen atoms in 1 and 3 were refined anisotropically. Significant disorder was detected for 2, resulting in the isotropic refinement of some of non-hydrogen atoms and the use of bond-distance restraints.[19] CCDC 206684–206686 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Centre, 12, Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223-336-033; or [email protected]).
- 14D. C. Allis, D. Green , W. Teng, U. Englich, W. Vargas, J. Alexander, B. S. Hudson, K. Ruhlandt-Senge, unpublished results.
- 15
- 15aG. B. Deacon, C. M. Forsyth, P. C. Junk, J. Organomet. Chem. 2000, 607, 112;
- 15bS. Chadwick, U. Englich, K. Ruhlandt-Senge, Organometallics 1997, 16, 5792.
- 16H. Bock, C. Näther, K. Ruppert, Z. Havlas, J. Am. Chem. Soc. 1992, 114, 6907.
- 17C. Marschner, Eur. J. Inorg. Chem. 1998, 221.
10.1002/(SICI)1099-0682(199802)1998:2<221::AID-EJIC221>3.0.CO;2-G CAS Web of Science® Google Scholar
- 18H. Hope, Prog. Inorg. Chem. 1994, 41, 1.
- 19G. M. Sheldrick, SHELXTL-Plus, Program for crystal structure solution and refinement, University of Göttingen, Germany, 1993.
- 20G. M. Sheldrick, SADABS, Program for Absorption Correction Using Area Detector Data, University of Göttingen, Göttingen, Germany, 1996.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.