Regional and Global Changes in Brain Structure 1-Year Post Pediatric “Mild” Traumatic Brain Injury
Abstract
Objective
Despite the high prevalence of pediatric “mild” traumatic brain injury (pmTBI), very little is known about the long-term effects of injury on brain structure and how injuries manifest in the context of dynamic and regionally specific neurodevelopmental changes.
Methods
Prospective study design characterizing long-term effects of pmTBI on both global (brain age) and regional (hippocampal volume, hippocampal subfields, and cortical thickness) brain structure at approximately 7 days, 4 months, and 1-year post-injury. A large sample of age- and sex-matched healthy controls was imaged at identical temporal intervals to account for typical neurodevelopmental changes, and to assess how trauma potentially affects developmental trajectories.
Results
A total of 269 pmTBI (age = 14.4 ± 2.9; 41.6% female) and 232 healthy controls (age = 14.1 ± 2.9; 44.8% female) were included in the final analyses (>70% 1-year retention). Results demonstrated that the presence of loss of consciousness and/or post-traumatic amnesia was associated with increased brain age up to 1-year post-injury, increased frontal cortical thickness at 4 months, as well as hippocampal atrophy across all time points relative to controls. “Mild” head trauma also interfered with hippocampal neurodevelopment in a dose-dependent fashion, including within the CA1 subfield. In contrast, post-concussive symptom burden was not associated with any structural abnormalities or alterations to neurodevelopment.
Interpretation
Current findings suggest a dose-dependent relationship between injury severity and alterations in both global and regional brain structure within the spectrum of pmTBI. Our results emphasize the importance of using objective biomarkers rather than subjective self-reported symptoms to better understand the long-term effects of injury. ANN NEUROL 2025;98:341–353
Pediatric “mild” traumatic brain injury (pmTBI) represents one of the most common neurological conditions in youth today.1, 2 There is increasing concern that alterations to the brain may persist for months to years post-injury.3, 4 However, high-quality, prospective studies on structural changes are sparse,5 have yielded mixed results, and have been limited to 6 months post-injury follow-ups.6-10 Previous mixed results may be partially attributable to heterogeneity in injury mechanisms and resultant injury foci, as well as heterogeneity in the clinical diagnosis/presentation of mTBI. For example, recent studies have suggested that imaging10, 11 and blood-based12-14 biomarkers are able to classify patients with more severe clinical signs (ie, presence of loss of consciousness and/or post-traumatic amnesia [LOC/PTA]) relative to reported symptoms within the spectrum of mTBI. Large prospective studies with parallel control cohorts are necessary to disambiguate injury-related atrophy or other trauma-related abnormalities from typical neurodevelopment, such as synaptic pruning, increases in cortical myelination, and hippocampal neurogenesis,15, 16 as well as how trauma interacts with neurodevelopment.
Post-traumatic alterations in brain structure have also been quantified through various metrics across studies. More global measures of structural change, including estimated brain age, are appealing because of clinical simplicity and excellent psychometric properties.17, 18 Global estimates may be more accurate at capturing diffuse changes in brain structure (edema or atrophy), but may be less sensitive to the significant spatial heterogeneity that exists in terms of injury foci.19 Global estimates may also be less sensitive for smaller brain structures where biomechanical forces are more likely to aggregate, such as deep gray/white matter regions or at tissue interfaces. For example, the hippocampi have been posited to be more vulnerable to injury because of their proximity to the fluid-filled temporal horns,20 and reduced hippocampal volumes have been reported in children with mild, moderate, and severe TBI several years post-injury in mostly cross-sectional studies.3, 10, 21 At an even more granular level, both clinical and preclinical studies suggest that the cornu ammonis subfield 1 (CA1) and dentate gyrus are highly sensitive to cell damage resulting from TBI.22-24 Importantly, contrary to thinning within the cortical mantle as a function of neurodevelopment, the hippocampi experience higher rates of neurogenesis during puberty.25, 26
The current study examined the long-term effects of pmTBI on global (ie, brain age) and regional (ie, hippocampi volume and prefrontal cortical thickness) brain structure up to 1-year post-injury. “Mild” TBI was classified using published criteria (see Methods) to maintain continuity with existing publications while simultaneously recognizing that the nomenclature of “mild” may not accurately reflect underlying injury conditions.27 It was hypothesized that regional, but not global changes in brain structure would persist to at least 4 months post-injury, with full recovery at 1-year. We further hypothesized that structural changes would be partially driven by the clinical signs of LOC and/or PTA (ie, greater loss/slower recovery in LOC/PTA+ patients) rather than post-concussive symptom burden.10-14 Secondary analyses examined whether hippocampal subfields, specifically CA (subfields 1 and 3) and the dentate gyrus would also be sensitive to injury.23, 24
Methods
Participants
Patients age 8 to 18 with pmTBI (n = 279) (Fig 1) were consecutively recruited from local emergency departments and urgent care settings from July 2016 to December 2023. Patients with a Glasgow Coma Score ≥13, LOC (if present) limited to 30 minutes, PTA (if present) limited to 24 hours, alteration in mental status at the time of injury, or the presence of at least 2 new post-concussive symptoms were enrolled. A semi-structured interview (the New Mexico Assessment of Pediatric Traumatic Brain Injury)28 was used to quantify LOC duration, PTA duration, alteration in mental status at the time of injury, or the presence of post-concussive symptoms. The inclusion criteria, therefore, combined elements from the American Congress of Rehabilitation Medicine (upper injury limit threshold) and the Zurich Concussion in Sport Group (minimal criteria threshold). Participants were evaluated within 11 days of injury (visit [V]1), approximately at 4 months (V2) and 1-year (V3) post-injury. Age- and sex-matched (n = 242) (see Fig 1) typically developing healthy controls (HC) were recruited from the local community using fliers and word of mouth and underwent identical assessments at comparable time intervals to account for potential neurodevelopmental confounds and/or effects associated with repeat assessment. A subset of the data from the V1 and V2 (76.7%) has already been published.10, 29
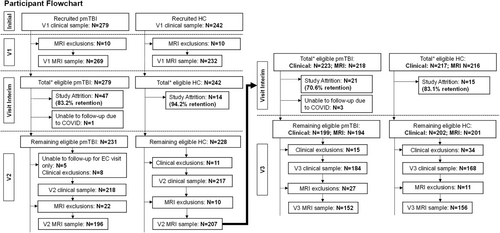
Participants from both groups were excluded for history of: (1) prior TBI with LOC exceeding 30 minutes, (2) any neurological diagnosis, (3) any psychiatric diagnosis other than adjustment disorder, (4) autism spectrum disorder or intellectual disability, (5) history of substance abuse/dependence, (6) contraindications for magnetic resonance imaging (MRI) including pregnancy and braces, or (7) non-English speaking. Participants with pmTBI were further excluded if injury affected the dominant hand or if general anesthesia was administered immediately post-injury. HC were further excluded for diagnoses of attention-deficit/hyperactivity disorder (ADHD) or a learning disability (LD). A positive urine drug screen (see Supplemental Materials) also led to exclusion with the exception of recreational marijuana use. All procedures received approval from the University of New Mexico Health Sciences Human Research Review Committee (HRRC), and participants and their parents provided informed consent or assent based on institutional guidelines.
Clinical Assessments
A battery of Common Data Elements tests was administered across all study visits, including retrospective ratings for multiple measures (Supplemental Materials and Table S1 for primary vs secondary outcomes). Primary clinical outcome measures included a modified version of the Post-Concussion Symptom Inventory (PCSI), the Conflict and Behavioral Questionnaire, and the Pediatric Quality of Life Inventory. A normative rather than simple change approach was used to binarily define individuals with high post-concussive symptoms burden (ie, symptomatic vs asymptomatic) and reduce false positive rates.30 A semi-structured interview was used to gather information on previous and current TBI history.28 Secondary measures included scales from the Patient-Reported Outcomes Measurement Information System (sleep, anxiety, and depression), self-reported pain and headache ratings, Glasgow Outcome Scale Extended (GOS-E), and parent-reported Strengths and Difficulties Questionnaire. Parental distress was assessed using the Brief Symptom Inventory-18. Additionally, a comprehensive battery of neuropsychological tests (see Supplemental Materials) was administered to evaluate potential cognitive deficits in attention, processing speed, working memory, executive functioning, and long-term memory recall, as well as to estimate reading ability and effort.
Image Acquisition
Imaging data were acquired using a 3 Tesla TrioTim or a PRISMA fit MRI scanner (Siemens, Erlangen, Germany) with a 32-channel head coil (see Supplemental Materials). High resolution T1-weighted (voxel size = 1.0 mm3), T2-weighted (voxel size varied across the TrioTim [1.1 × 1.1 × 1.5 mm, 2D T2 TSE] and PRISMA fit [1mm3, 3D T2-SPACE] platforms), susceptibility-weighted (SWI) sequence (voxel size = 1.0 × 1.0 × 1.5 mm), and fluid attenuated inversion recovery (voxel size = 0.8 × 0.8 × 3 mm). The scanner version remained consistent for each participant. A board-certified neuroradiologist, blinded to participant groups, conducted a review of all MRI images to identify any abnormal findings using previously published rating scales.31 A subset of pmTBI participants (n = 146) underwent computed tomography (CT) scans as part of routine care.
Brain age was estimated based on the T1-weighted image using a deep learning network (DeepBrainNet) architecture32 implemented in Python (v3.10) with AntsPy (v0.3.4). The algorithm was initially trained using a diverse cohort of 11,729 participants age 3 to 95 years old from multiple imaging sites and, therefore, is applicable to the current pediatric cohort. Preprocessing steps for T1-weighted images included N4 bias field correction, brain extraction, and affine registration to the Montreal Neurological Institute (MNI) anatomical template. From the registered volume, 80 axial slices (range, 45–125) were extracted and converted to 3-channel RGB format.29 Brain age was predicted on a slice-by-slice basis using the trained model with TensorFlow and Keras (2.10). The predicted age difference (PAD) was calculated by subtracting actual subject age from the median value of all slice-wise predictions (Fig 2A).

The FreeSurfer (version 7.1.1) package was used to produce individual maps of cortical thickness surfaces and hippocampi regions of interest (ROI) using standard segmentation labels33 based on the T1-weighted images. Individual cortical thickness maps were transformed to the FreeSurfer average template (fsaverage), spatially smoothed using a 10 mm full width at half maximum Gaussian kernel and converted to GIFTI format. Secondary analyses examined the following a priori hippocampal subfields: CA subfields 1 and 3, as well as the granule cell layer of the dentate gyrus.23
Statistical Analysis
All clinical data were analyzed using 2 (group [HC vs pmTBI]) × 3 (visit [V1 vs V2 vs V3]) generalized estimating equations (GEE) full factorial models (see Supplemental Materials). Statistical analyses of the cortical thickness surface data were conducted using linear mixed effects (Analysis of Functional NeuroImages [AFNI] 3dLME). Freesurfer data were converted to GIFTI format for use in AFNI. Imaging data were analyzed using separate LME models to test our a priori hypotheses regarding the different metrics of injury severity. A series of 3 × 3 models (group [LOC/PTA+ vs LOC/PTA− vs HC]) × (visit [V1 vs V2 vs V3]) investigated effects associated with LOC/PTA. Individuals reporting LOC or PTA were grouped together, because these medical concepts are often conflated in injury reports from a lay perspective.34, 35 All models included age-at-injury (unit = months) and the imaging models also included scanner version (Siemens TrioTim vs PRISMA fit) as covariates, with intracranial volume included as an additional covariate for hippocampi analyses. All pmTBI were evaluated within 11 days of injury, such that age-at-injury typically corresponded to chronological age for this sub-acute study design. Similarly, chronological age corresponded to age at the first visit for HC, such that age-at-injury is used interchangeably with age for the remainder of the manuscript. The second set of LME models used a modified 2 × 3 (group [pmTBI vs HC]) × (visit [V1 vs V2 vs V3]) factor structure including total post-concussive symptom burden as an additional covariate. Simplified 2 × 3 (group [pmTBI vs HC]) × (visit [V1 vs V2 vs V3]) LMEs are presented in Supplemental Materials for thoroughness and to facilitate comparisons with previous studies.
Precomputed Freesurfer Monte-Carlo simulations were used for family-wise error correction for thickness data.36 To ensure attrition did not affect results, sensitivity analyses comparing V1 data for returners and non-returns were conducted for all clinical and imaging data. All clinical outcomes were categorized into the following patterns: complete recovery (group × visit interaction with no significant group effects at V3), partial recovery (group × visit interaction with reduced but statistically significant group differences at V3), no recovery (main effect of group), or no deficits (absence of main effect of group or group × visit interaction).
Results
Demographics
Final MRI analyses (see Fig 1; Supplemental Materials) included 269 pmTBI (112 females; age 14.4 ± 2.9; 7.3 ± 2.2 days post-injury) and 232 HC (104 females; age 14.1 ± 2.9) at V1. A total of 196 pmTBI (83 females; 131.9 ± 15.0 days post-injury; 124.5 ± 14.8 days between V1 and V2) and 207 HC (88 females; 127.6 ± 18.8 days between V1 and V2) completed V2, and 152 pmTBI (62 females; 367.1 ± 31.9 days post-injury; 363.6 ± 29.1 days between V2 and V3) and 156 HC (71 females; 367.0 ± 28.7 days between V2 and V3) completed V3. Patients who did not complete all visits were included in all statistical models using available data. A higher number of participants (pmTBI = 279 and HC = 242 at V1) were included in clinical analyses because of a variety of image quality assurance reasons (braces, poor data quality, etc.) (see Supplemental Materials).
The pmTBI and HC groups did not differ in terms of biological sex, age, handedness, post-pubertal stage, or self-reported Tanner stage of development (all p ≥ 0.05) (see Table 1). The groups differed for self-reported history of previous head injuries (χ2 = 19.60, p < 0.001; pmTBI = 17.2%; HC = 5.8%), parental self-reported psychopathology (Wald-χ2 = 28.43, p < 0.001; pmTBI > HC), premorbid reading ability (Wald-χ2 = 54.01, p < 0.001; pmTBI < HC), and effort (Wald-χ2 = 25.80, p < 0.001; pmTBI < HC). We, therefore, used both reading ability and effort as covariates in neuropsychological analyses. Thirteen pmTBI participants had a positive CT scan, and a partially overlapping sample (n = 9) had TBI-related abnormalities (see Table 1) on standard structural MRI (eg, skull fracture, subdural hematoma, subarachnoid hemorrhage, and contusions)31 based on radiological read.
| V1 pmTBI (n = 279) | V1 HC (n = 242) | |
|---|---|---|
| Age | 14.8 (12.4–16.8) | 14.0 (12.0–16.3) |
| Sex (% female) | 41.9% | 45.0% |
| Tanner stage of development | 4 (3–4) | 4 (2–4) |
| % Post-pubertal | 12% | 14.5% |
| Parent BSI-18a | 3 (1–7) | 1 (0–4.5) |
| pmTBI Hxa | 17.2% | 5.8% |
| Injury characteristics | ||
| Loss of consciousness | 49.3% | – |
| Post-traumatic amnesia | 38.8% | – |
| CT collected/positive | 53.1%/8.9% | – |
| MRI collected/positive | 100%/3.2% | – |
| Mechanism of injury | ||
| Struck by object | 14.4% | – |
| Struck by person | 27.4% | – |
| Fall | 19.9% | – |
| MVC | 29.2% | – |
| Assault | 4.7% | – |
| Bicycle-related | 3.6% | – |
| Other | 0.7% | – |
| Sport or recreation related | 56.0% | – |
- Data are formatted at mean ± standard deviation or median (interquartile range) based on distribution properties.
- a Group main effect.
- BSI = Brief Symptom Inventory-18; CT = computed tomography; HC = healthy control; Hx = history; MRI = magnetic resonance imaging; MVC = motor vehicle crash; pmTBI = pediatric “mild” traumatic brain injury; V= visit.
Clinical Outcomes
Measures of central tendency are presented in Table 2 for clinical and neuropsychological results. Primary clinical measures revealed a significant group × visit interaction for self-reported post-concussive symptoms severity. Follow-up tests indicated partial recovery (see Table 3 for associated statistics), with symptom magnitude greater (pmTBI > HC) at V1 (37.9% symptomatic) relative to V2 (18.4% symptomatic) and V3 (20.4% symptomatic). A main effect of group was observed for a primary measure of self-reported behavioral disturbances (pmTBI > HC), suggesting limited recovery in this domain. In contrast, there was no evidence of differences in quality of life at V2 or V3 (p > 0.0167 following Bonferroni correction), suggestive of full recovery. Secondary clinical measures demonstrated complete recovery in the domains of pain, headache, and global TBI outcomes (GOS-E) following the first visit. Sleep disturbance showed partial recovery, with increased disturbances at V1 (pmTBI > HC) relative to milder sleep disturbances at V2 and V3. Anxiety showed a mixed pattern of recovery, with non-significant findings at V2 following Bonferroni correction, but a re-emergence of symptoms at V3 (pmTBI > HC). Finally, no evidence of recovery was observed for a secondary measure of behavioral/emotional difficulties and depression across all visits (pmTBI > HC).
| Metric | V1 pmTBIa (n = 279) | V1 HCa (n = 242) | V2 pmTBIa (n = 218) | V2 HCa (n = 217) | V3 pmTBIa (n = 184) | V3 HCa (n = 168) |
|---|---|---|---|---|---|---|
| Symptom measures | ||||||
| PCSI (% max)b, c (P) | 16.7 (5.6–37.3) | 3.2 (0–8.8) | 5.6 (0.8–18.3) | 4 (0.8–8.8) | 4 (0.4–16.3) | 2.4 (0–7.5) |
| PROMIS sleepb (S) | 19.3 ± 7.1 | 14.2 ± 4.8 | 18.2 ± 7.0 | 15.0 ± 4.8 | 18.4 ± 6.7 | 14.8 ± 4.9 |
| PROMIS anxietyb (S) | 3 (0–8) | 1 (0–4) | 2 (0–7) | 1 (0–5) | 2 (0–5.5) | 0.5 (0–3) |
| PROMIS depressiond (S) | 2 (0–10) | 1 (0–4) | 1 (0–7) | 1 (0–4) | 1 (0–6) | 0 (0–4) |
| Pain scaleb, c (S) | 3 (1–5) | 0 (0–1) | 0 (0–3) | 0 (0–1) | 0 (0–2) | 0 (0–1) |
| HIT-6b (S) | 52 (44–60) | 40 (36–46) | 46 (40–55) | 42 (38–48) | 46 (40–53) | 42 (38–48) |
| Behavioral and outcome measures | ||||||
| CBQd, e (P) | 1 (0–3) | 1 (0–2) | 1 (0–3) | 1 (0–2) | 1 (0–3) | 0 (0–2) |
| PedsQL (P) | NA | NA | 83.0 ± 13.6 | 87.1 ± 10.0 | 84.8 ± 12.9 | 88.8 ± 9.6 |
| SDQd (S) | NA | NA | 7 (4–10.5) | 4 (2–7.5) | 7 (3–9) | 4 (2–6) |
| GOS-Eb (S) | 1 (1–4) | 1 (1–1) | 1 (1–2) | 1 (1–1) | 1 (1–1) | 1 (1–1) |
| Cognitive measures | ||||||
| TOMMe10d (S) | 10 (9–10) | 10 (10–10) | 10 (10–10) | 10 (10–10) | 10 (9–10) | 10 (10–10) |
| WRAT4d (S) | 49.4 ± 10.0 | 56.2 ± 11.0 | 51.0 ± 11.1 | 58.2 ± 11.6 | 51.4 ± 11.0 | 57.7 ± 11.5 |
| PSb (P) | 46.4 ± 7.7 | 50.2 ± 8.2 | 50.4 ± 8.3 | 52.7 ± 8.9 | 51.9 ± 8.4 | 55.4 ± 9.4 |
| ATb (P) | 46.8 ± 8.7 | 51.2 ± 7.1 | 49.3 ± 7.9 | 51.8 ± 7.3 | 50.1 ± 7.4 | 52.8 ± 7.2 |
| WMd (S) | 46.7 ± 8.0 | 50.8 ± 10.2 | 47.2 ± 9.3 | 51.6 ± 11.0 | 47.8 ± 8.8 | 52.0 ± 10.2 |
| EFa (S) | 46.9 ± 7.2 | 50.5 ± 6.7 | 50.3 ± 6.8 | 53.2 ± 6.2 | 52.2 ± 6.2 | 54.7 ± 6.7 |
| HVLT delaya, c (S) | 7.5 ± 2.6 | 8.8 ± 2.1 | 7.3 ± 2.3 | 8.6 ± 2.3 | 8.2 ± 2.4 | 9.6 ± 1.9 |
- Data are either formatted at mean ± standard deviation or median (interquartile range).
- a Clinical data includes a larger sample because of quality assurance exclusions for the imaging data. NA denotes that data not available since data were not collected at V1 because of instrument instructions. Outcomes are classified as primary (P) or secondary (S).
- b Group × visit interaction.
- c Group × age interaction.
- d Group main effect.
- e Group × age × visit interaction.
- AT = attention; CBQ = Conflict Behavior Questionnaire; EF = executive function; GOS-E = Glasgow Outcome Scale Extended; HC = healthy control; HIT-6 = Headache Impact Test; HVLT Delay = Delayed recall on Hopkins Verbal Learning Task (measure of long-term memory); max = maximum; PCSI = Post-Concussion Symptom Inventory (presented as percent of maximum score to account for age-related scale differences); PedsQL = Pediatric Quality of Life Inventory; pmTBI = pediatric “mild” traumatic brain injury; PROMIS = Patient Reported Outcomes Measurement Information System; PS = processing speed; SDQ = Strengths and Difficulties Questionnaire; TOMMe10 = Test of Memory Malingering – 10-item short version; V1 = visit 1 (~7 days post-injury); V2 = visit 2 (~4 months post-injury); V3 = visit 3 (~1 year post-injury); WM = working memory; WRAT-IV = Wide Range Achievement Test 4.
| Pattern | Metric | Measure | Group × visit model (2 × 3) | Sensitivity analyses V2 (p-value) | Sensitivity analyses V3 (p-value) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Effect (Wald-χ2; p-value) | V1 (p-value) | V2 (p-value) | V3 (p-value) | pmTBI | HC | pmTBI | HC | |||
| Complete | Clinical | Pain scale (S) | G × V (χ2 = 36.90; p < 0.001) | <0.001 | 0.007 | 0.096 | 0.877 | 0.470 | 0.882 | 0.554 |
| Headache (S) | G × V (χ2 = 57.60; p < 0.001) | <0.001 | 0.057 | 0.052 | 0.220 | 0.621 | 0.766 | 0.679 | ||
| Global outcome (S) | G × V (χ2 = 11.72; p = 0.003) | <0.001 | 0.001 | 0.027a | 0.625 | 0.713 | 0.304 | 0.812 | ||
| Cognitive | Attention (P) | G × V (χ2 = 11.25; p = 0.004) | 0.001 | 0.190 | 0.178 | 0.476 | 0.002 | 0.175 | 0.003 | |
| PS (P) | G × V (χ2 = 11.54; p = 0.003) | 0.001 | 0.206 | 0.040a | 0.575 | 0.969 | 0.505 | 0.143 | ||
| Partial | Clinical | PCS (P) | G × V (χ2 = 36.13; p < 0.001) | <0.001 | <0.001 | <0.001 | 0.439 | 0.756 | 0.629 | 0.479 |
| Sleep (S) | G × V (χ2 = 13.12; p = 0.001) | <0.001 | <0.001 | <0.001 | 0.287 | 0.557 | 0.904 | 0.452 | ||
| Anxiety (S) | G × V (χ2 = 9.92; p = 0.007) | <0.001 | 0.042a | 0.007 | 0.662 | 0.188 | 0.353 | 0.959 | ||
| No recovery | Clinical | Behavioral and emotional difficulties (S) | G (χ2 = 16.22; p < 0.001) | NA | NA | NA | NA | |||
| Behavioral disturbance (P) | G (χ2 = 6.06; p = 0.014) | 0.075 | 0.931 | 0.068 | 0.435 | |||||
| Depression (S) | G (χ2 = 11.90; p < 0.001) | 0.982 | 0.660 | 0.413 | 0.879 | |||||
| Cognitive | EF (S) | G (χ2 = 13.94; p < 0.001) | 0.664 | 0.229 | 0.493 | 0.416 | ||||
| WM (S) | G (χ2 = 8.12; p = 0.004) | 0.526 | 0.623 | 0.418 | 0.274 | |||||
| Long-term memory (S) | G (χ2 = 35.66; p < 0.001) | 0.652 | 0.923 | 0.389 | 0.718 | |||||
| No deficits | Clinical | PedsQL (P) | NA | NA | NA | NA | ||||
- All significant main effects of group exhibited opposite patterns for clinical (pmTBI > HC) relative to neuropsychological (pmTBI < HC) findings. Sensitivity analyses compared data from V1 for those participants who remained in the study versus attrition. NA denotes that sensitivity analyses not available since data were not collected at V1 because of instrument instructions.
- a Denotes p-value not significant following Bonferroni correction, which were performed separately for primary and secondary clinical and cognitive domains.
- EF = executive function; G = group; HC = healthy controls; V = visit; P = primary domain; PCS = post-concussive symptoms; PedsQL = Pediatric Quality of Life; pmTBI = pediatric “mild” traumatic brain injury; PS = processing speed; S = secondary domain; V = visit; WM = working memory.
Results for primary neuropsychological domains indicated complete recovery for attention and processing speed following V1. Secondary cognitive measures indicated continued deficits in executive function, working memory, and long-term memory for pmTBI relative to HC across all other follow-up visits.
Uncorrected sensitivity analyses comparing returning and non-returning participants were negative for all clinical and cognitive measures (42 total tests) (see Table 3) with the exception of attention in HC at both V2 and V3. See Supplemental Materials for effects associated with age and other factors.
Effects of LOC/PTA on Global and Regional Brain Structure
Results (Fig 2B) from a 3 × 3 (group [LOC/PTA+, LOC/PTA−, and HC] × visit [V1, V2, and V3]) LME examining the effects of LOC/PTA on predicted brain age difference indicated a significant group × visit interaction (F = 9.03, p < 0.001) and main effect of group (F = 4.53, p = 0.011). Simple effects tests revealed a significant group effect at V1 (F = 8.23, p < 0.001) in which predicted brain age difference was greater for pmTBI with LOC/PTA+ relative to both HC (p < 0.001; Cohen's d = 0.36) and pmTBI with LOC/PTA− (p = 0.004; Cohen's d = 0.35), with no difference between HC and LOC/PTA− (p > 0.05). Although there were no significant differences at V2 (F = 0.05, p > 0.05), at V3 (F = 3.27, p = 0.040) predicted brain age difference was again significantly increased for pmTBI with LOC/PTA+ relative to HC (p = 0.013; Cohen's d = 0.32) only.
Results (Fig 3) from the 3 × 3 3dLME cortical thickness analysis revealed a significant group × visit interaction in the right superior frontal gyrus and a significant group × visit × age interaction in the left postcentral gyrus. Follow-up analysis in the right superior frontal gyrus indicated a significant group effect only at V2 (F = 3.06, p = 0.048), with increased thickness for LOC/PTA + relative to HC (p = 0.014; Cohen's d = 0.27), and no difference between the other groups (all p's > 0.05). Follow-up analysis for the left postcentral gyrus indicated that the group × age interaction was not significant at any of the visits (all p > 0.05). In addition to group effects, decreased cortical thickness as a function of increased age was observed across the entire cortical mantle with the exception of bilateral primary sensory and motor cortices (Fig S1). Similarly, decreased cortical thickness was also observed in several bilateral regions as a function of visit (Fig S2).

Results (Fig 4) from a 3 × 3 LME examining the effects of LOC/PTA on hippocampal volumes indicated a significant effect of group (F = 6.95, p = 0.001), group × visit (F = 2.82, p = 0.025) and group × age (F = 5.34, p = 0.005) interactions. Hippocampal volumes were decreased for the LOC/PTA+ group relative to HC at all 3 visits (all p < 0.014; Cohen's d range = [−0.37 to −0.24]), and significantly decreased for the LOC/PTA− group relative to HC at V1 (p = 0.002; Cohen's d = −0.34) and V3 (p = 0.009; Cohen's d = −0.38). There was no significant difference between the LOC/PTA+ and LOC/PTA− groups (p > 0.05). Additional follow-up tests indicated that the main effect of visit was significant only for the pmTBI LOC/PTA+ group (F = 5.82, p = 0.004), with evidence of hippocampal atrophy (ie, a decreasing volume as a function of time) at both V2 (p = 0.042) and V3 (p = 0.001) relative to V1. Follow-up analyses for the group × age interaction further indicated significant positive relationships between age and hippocampal volume for HC (F = 5.25, β = 14.68, p = 0.023), non-significant positive associations for LOC/PTA− (β = 13.88, p > 0.05), and significant negative relationships for LOC/PTA+ (F = 5.46, β = −18.26, p = 0.021).

There were no significant differences (p > 0.05) for any of the structural measures at V1 between returning and non-returning participants. Supplementary analyses eliminated pmTBI with diagnoses of ADHD or LD from primary imaging models, but results remained statistically similar. Similarly, additional supplemental analyses separately removed pmTBI with positive imaging findings on CT or MRI, but results again remained statistically similar.
Secondary Analyses of Hippocampal Subfields
Results from secondary 3 × 3 LMEs examining the effects of LOC/PTA on hippocampal subfields (see Table 4 for statistics) indicated a significant group × age interaction in CA1 only. Age was significantly and positively correlated with subfield volume for HC, significantly and negatively correlated for LOC/PTA+, and non-significantly correlated for the LOC/PTA− cohort (ie, similar patterns as primary results from the entire hippocampi) within CA1. The main effect of group was significant for CA1 and dentate gyrus subfields, with HC demonstrating larger subfield volumes relative to both LOC/PTA+ (Cohen's d = 0.19) and LOC/PTA− (Cohen's d = 0.20), with no significant differences between the 2 patient groups (all p's > 0.05). None of the subfields exhibited a significant group × visit interaction (all p > 0.05). There were no significant main effects or interactions for CA3.
| Subfield area | LOC/PTA | |
|---|---|---|
| Effect (F, p) | Simple effect | |
| CA1 | Group (F = 6.84, p = 0.001) | HC > LOC/PTA+ (p = 0.001) |
| HC > LOC/PTA− (p = 0.005) | ||
| LOC/PTA+ ~LOC/PTA− (p = 0.901) | ||
| Group × age (F = 4.52, p = 0.011) | HC: age (F = 5.54; p = 0.019; β = 1.48) | |
| LOC/PTA+: age (F = 3.95; p = 0.049; β = −1.50) | ||
| LOC/PTA−: age (F = 0.00; p = 0.992; β = −0.01) | ||
| CA3 | Group (F = 3.696, p = 0.025)a | |
| DG | Group (F = 5.10, p = 0.006) | HC > LOC/PTA+ (p = 0.013) |
| HC > LOC/PTA− (p = 0.007) | ||
| LOC/PTA+ ~LOC/PTA− (p = 0.582) | ||
- β value (β) indicates the direction of the relationship between age and volume.
- a Denotes p-value not significant following Bonferroni correction.
- CA = cornu ammonis; DG = dentate gyrus; HC = healthy controls; LOC/PTA: presence (denoted by + sign) or absence (denoted by − sign) of post-traumatic amnesia and/or loss of consciousness.
Effects of Post-Concussive Symptom Burden on Global and Regional Brain Structure
There were no significant main effects or interactions associated with post-concussive symptoms for predicted brain age difference when it was entered into a 2 × 3 (group [pmTBI vs HC] × visit [V1, V2 and V3]) LME. However, the main effect of group and the group × visit interaction remained significant (both p's < 0.05) in this analysis with or without the presence of post-concussive symptom load as a covariate in the model (see Supplemental Results for simplified model). These results are not discussed further to reduce redundancy with simplified models.
The main effect of group and the group × visit interaction were not significant for cortical thickness when the presence of post-concussive symptom load was entered into a 2 × 3 vertex-wise 3dLME with overall diagnostic status as a group factor (pmTBI vs HC). A significant group × PCSI interaction existed in right middle occipital gyrus/lunate sulcus during cortical thickness analyses. However, follow-up analyses indicated a significantly negative relationship between PCSI and cortical thickness for HC only (F = 7.64, β = −0.002, p = 0.006) with non-significant associations for pmTBI (β < 0.001, p > 0.05).
Hippocampi volume results indicated a significant main effect of PCSI (F = 5.88, β = 0.044, p = 0.016) and a significant PCSI × visit (F = 3.50, p = 0.031) interaction, as well as expected main effects of group, group × visit and group × age interactions (see Supplemental Results for simplified model). However, follow-up analyses of the PCSI × visit interaction were negative for the main effect of PCSI at any of the visits (all p > 0.05).
Discussion
Middle childhood and adolescence are associated with a myriad of regionally specific changes in brain structure including synaptic pruning and increased myelination that have been associated with higher order cognitive processes and emotional maturation.15 The current study prospectively examined the impact of pmTBI on both global (ie, brain age) and regional (ie, hippocampi volume and prefrontal cortical thickness) changes in brain structure up to 1-year post-injury relative to a large sample of sex- and age-matched HC. Current results suggest persistent effects of injury across emotional, behavioral, and cognitive domains. Within the spectrum of pmTBI, there were multiple structural changes that occurred in a dose-dependent fashion based on traditional clinical signs of injury severity. Specifically, the presence of LOC/PTA was associated with increased brain age up to 1-year post-injury, increased frontal cortical thickness at 4 months post-injury, and hippocampal atrophy (ie, volume decreases as a function of time) across visits. In contrast, there was no association between structural abnormalities and post-concussive symptom burden within the pmTBI group. Finally, there was indirect evidence that trauma also interacted with neurodevelopment through associations between age and brain structure.
In line with previous studies,37 results from a comprehensive clinical battery revealed deficits that persisted 1-year post-injury for several behavioral, emotional, and cognitive domains. Specifically, executive functioning, working memory, and long-term memory remained significantly below HC performance across all visits, even when controlling for premorbid reading abilities and effort. In contrast, attention and processing speed showed evidence of complete recovery by 4 months post-injury. Similarly, the somatic symptoms of pain and headache demonstrated complete resolution by 4 months post-injury, whereas sleep disturbances and post-concussive symptom load remained elevated up to 1-year post-injury. Although the magnitude of differences on all clinical measures were not clinically meaningful (ie, small effect sizes), current findings suggest the need for comprehensive clinical assessments at multiple timepoints post-injury for patients with persistent symptoms. Future studies are also needed to determine optimal testing measures at different timepoints post-injury to reduce patient burden. Addressing the clinical effects of pmTBI soon after injury may reduce negative consequences on overall quality of health, academic progress, and likelihood of future neuropsychiatric conditions that have been reported to occur for a subset of patients.38
Previous studies suggest that mTBI may exist on a spectrum both in terms of clinical findings (ie, signs and symptoms) and biomarkers.12, 13 In the current study, pmTBI with LOC/PTA+ exhibited increased brain age compared to both HC and LOC/PTA− at V1, with no difference at V2, but increased brain age relative to the control group at 1-year post-injury. The specificity of injury severity for changes in global brain age was further confirmed by supplementary analyses in which the collapsing of groups across the presence/absence of LOC/PTA suggested potentially misleading evidence of full recovery for all pmTBI participants. These findings align with our previous study in which there were no group differences across the entire pmTBI cohort at 4 months, and where PTA duration was associated with brain age.29 Similarly, other studies in chronic moderate–severe TBI cohorts have also reported that injury severity can lead to progressive increases in brain age over time.39 Collectively, there is a growing recognition that TBI may initiate a lifelong process contributing to neurodegenerative changes in an age dependent fashion.40
Consistent with our previous study,10 both LOC/PTA+ and LOC/PTA− groups exhibited volume reductions compared to HC at V1 and V3, with the LOC/PTA+ group also showing significant decreases relative to HC at V2. In contrast to brain age findings, Supplementary Results from the combined pmTBI cohort also indicated reduced hippocampal volumes at each visit, supporting a growing body of evidence that suggests that medial temporal lobe structures may be particularly sensitive to head trauma across multiple severity levels.3, 10, 21 However, only patients with LOC/PTA+ demonstrated evidence of reduced hippocampal volumes between visits (ie, atrophy), which persisted in this group up to 1-year post-injury. Previous research has demonstrated that there is significant neurogenesis in the hippocampi during middle childhood and adolescence, particularly within the dentate gyrus,26 as well as other age-related volume increases secondary to increased myelination and/or dendritic arborization.15, 41 Findings of significant, age-dependent increases in hippocampal volumes were replicated for the HC group in the current study, whereas either null (LOC/PTA−) or negative (LOC/PTA+) associations existed between age and hippocampal volumes/CA1 following pmTBI. Collectively, these findings are suggestive of disruption to critical neurodevelopmental functions within the medial temporal lobes23, 24 following pmTBI.
Changes in cortical thickness were also observed in patients with LOC/PTA+, but findings were contrary to a priori hypotheses. Specifically, increased cortical thickness was observed within the right superior frontal gyrus at 4 months post-injury for patients with LOC/PTA+ relative to HC. Increased cortical thickness was also evident at V1 and V2 when results were collapsed across the entire pmTBI cohort. The relatively fewer studies that have reported increased cortical thickness following trauma typically attribute this finding to inflammation and a relative increase in astrocytes or glial cells,42, 43 with other evidence suggesting an increase in growth-promoting gene expression shortly post-injury in the perilesion area.44 Alternatively, dual findings of reduced hippocampal volume and increased prefrontal cortical thickness may be indicative of compensatory response such as increased plasticity to support functional recovery.45 However, previous studies have typically shown reduced cortical thickness in pediatric moderate–severe TBI cases up to 3-years post-injury relative to typically developing children.46 Decreased cortical thickness has also previously been reported at approximately 3 to 4 months post-pmTBI relative to HC,7, 8 including our recent study with a subset of participants.10 Therefore, additional studies are required to determine patterns of cortical thinning in pediatric TBI and how they interact with neurodevelopment.
Strengths of the study include a prospective design, which minimizes between subject variability and increases power to detect subtle volumetric and cortical changes that might be missed in cross-sectional studies.7, 47 Additionally, current findings underscore the importance of a large control group, which is essential for accurately modeling complex and regionally specific structural changes because of neurodevelopment. Limitations of the study include the exclusive use of T1-weighted sequence. Although T1-weighted sequences demonstrate strong test–retest reliability, it is heavily dependent on prior atlas information and may provide limited representation of the complex structure of hippocampi.48 Second, current findings on hippocampal subfields should be approached with caution given the acquisition of 1 mm3 isotropic voxels.49 However, multiple studies have demonstrated that hippocampal subfield estimates at this resolution are both reliable and spatially reproducible.50 Third, although the current study used semi-structured interviews to determine history of remote TBI and injury severity characteristics, a higher percentage of pmTBI endorsed LOC relative to PTA. This is likely a result of the frequent conflation of these terms in lay personnel34, 35 and represents a primary motivation for collapsing data across these 2 groups. Last, although all participants were seen within 48 hours of injury at a medical center to qualify for study enrolment, we did not track the recruitment site (emergency department vs urgent care center), or if the initial presentation for medical attention was sought on the day of injury or delayed by a day.
In summary, our prospective study provides novel insights into the interplay and persistent vulnerability of frontal and temporal lobes to trauma-related changes that can last up to a year following pmTBI. Current results suggest a dose-dependent relationship between injury severity, as indexed by the clinical signs of presence/absence of LOC/PTA, and changes in both global (brain age) and regional (hippocampal) brain structure within the spectrum of “mild” TBI. In contrast, there were minimal significant relationships between structural abnormalities and post-concussive symptom load. Collectively, these results highlight the importance of using objective biomarkers (eg, blood and imaging) to better understand the long-term effects of pmTBI, as well as recent international efforts to diagnose “mild” TBI based on both symptoms and signs.27 Our results highlight the need for further longitudinal research to better understand the long-term impacts of pmTBI, especially as youth are in a dynamic phase of neurodevelopment, where processes like neurogenesis, synaptic pruning and cortical maturation are actively co-occurring in a regionally dependent fashion.
Acknowledgments
This research was supported by grants from the National Institutes of Health (NIH) (https://www.nih.gov; grant numbers NIH 01 R01 NS098494-01A1, R01 NS098494-03S1A1, and P30 GM122734, A.R.M). The NIH had no role in study review, data collection and analysis, decision to publish, or preparation of the manuscript. Research reported in this publication was also supported by the Office of The Director, NIH of the NIH under award number S10OD025313. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Contributions
U.N. and A.R.M. contributed to the conception and design of the article; U.N., T.W., J.L.L., J.R.M., D.S.K., S.D.M., J.W., V.D., and A.R.M. contributed to the acquisition and/or analysis of data; U.N., T.W., T.B.M., H.j.v.d.H., J.P.P., R.A.C., R.E.S., R.K., A.A.V., and A.R.M. contributed to drafting the text or preparing the figures.
Potential Conflicts of Interest
Nothing to report.
Open Research
Data Availability
The data that support the findings of this study will be openly available in FITBIR at fitbir.nih.gov on the conclusion of the study (reference number FITBIR-STUDY0000339).




