Priorities and Recommendations to Make ALS a Livable Disease Emanating from the 2024 National Academies of Sciences, Engineering, and Medicine Report Living with ALS
Abstract
Amyotrophic lateral sclerosis (ALS) is a relentless, fatal neurodegenerative disease. The progressive loss of voluntary muscle function, diagnostic delays, lack of effective treatments, and challenges accessing multidisciplinary care and resources have tremendous impact on quality of life. The congressionally directed ALS committee of the National Academies of Science, Engineering, and Medicine, in their 2024 report “Living with ALS,” recommends critical actions for specific United States stakeholders to make ALS a livable disease over the next decade. This review summarizes the context and recommendations of the report. Advocacy efforts are critical to make these recommendations a reality for the ALS community. ANN NEUROL 2024;96:1035–1039
Amyotrophic lateral sclerosis (ALS) is a fatal multi-system neurodegenerative disease hallmarked by progressive motor neuron degeneration in the brain, brainstem, and spinal cord.1 The resulting weakness and inevitable loss of voluntary muscle function, respiratory failure, and impairments in speech and swallowing, along with the potential for cognitive impairment and secondary symptoms such as fatigue, pain, anxiety, and depression, requires people living with ALS, as well as their families and caregivers, to have access to a constantly evolving and expanding range of medical and support services and resources. Evidence supports the critical importance of multidisciplinary care to improve outcomes in ALS,2 but receiving the services and resources needed to best navigate the changing disease course can be fraught with challenges. These hurdles are further compounded by the diagnostic delays common in ALS that preclude early initiation of supportive care and eligibility for clinical trials because of advancement of symptoms by the time a diagnosis is made. Factors contributing to the delays include the heterogeneous presentation of early disease phases, lack of recognition by general practitioners, potential for misdiagnosis, and need for definitive biomarkers or tests for ALS.3 As approximately 90% of ALS is sporadic without a known genetic mutation, more research is needed to define the mechanisms underlying ALS, including the complex role of environmental risk factors and gene–environment interactions, to support earlier diagnosis, therapeutic development, and prevention efforts.4, 5 Ultimately, there is no cure for ALS and effective treatments are needed to temper the relentless disease course and prolong survival.
To enhance the quality of life for those living with ALS and address the need for treatment advances, the National Academies of Science, Engineering, and Medicine (NASEM) was tasked by Congress to establish a plan to make ALS a livable disease within a decade. The Committee on Amyotrophic Lateral Sclerosis: Accelerating Treatments and Improving Quality of Life was formed, comprising 18 individuals with expertise in specialty and primary care, health care delivery and financing, research and therapeutic development, and law/ethics/policy, as well as individuals with an ALS lived experience. The committee's goal was to establish the recommendations, priorities, and actions needed for implementation in the public, private, and nonprofit sectors to make ALS livable. Considering the priorities and lived experience of individuals with ALS and their families, emphasis focused on equitable access to affordable multidisciplinary care, services, and caregiver support and on facilitating advances in basic science, clinical care, and population health research to improve survival and enhance quality of life for those living with or at-risk for ALS. The resulting report presents key recommendations (numbering below reflects that used in the NASEM report, which is based on the chapter and order of mention)6 that fall under four major domains of focus. These domains include short-term actions to improve access to ALS care and services and longer-term actions to establish systems for ALS care and research, to improve research, therapeutic development, and best care practices, and to advance ALS prevention (Fig 1).
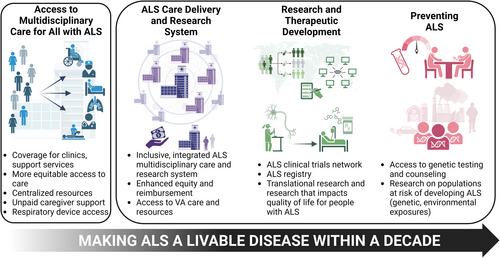
Improving Access to Care Resources and Services
In the near-term, addressing gaps in current clinical practice and support for caregivers is critical to ensure individuals living with ALS have access to supportive care known to improve both quantity and quality of life. The rapidly progressive nature of ALS and average life expectancy of 2 to 5 years from onset does not fit well into the traditional reimbursement model for equipment and services, which are often predicated on a slower timeline with a reactive model and based on a potential for functional improvement. Delays in approval and delivery of equipment in ALS can result in catastrophic safety risks rather than a simple inconvenience. Recommendation 3–1 expands on recently published rules under the Centers for Medicare and Medicaid Services (CMS).7 These rules mandate 72-hour prior authorization responses for urgent requests, and the recommendation posits that all requests for equipment and services for ALS be considered urgent and handled expeditiously, not only by CMS-administered programs, but likewise by private insurers. Additionally, this recommendation urges authorization of home health services despite progression of the primary disease resulting in “failure to improve.”
Multidisciplinary clinics are considered standard of care for ALS2 and are associated with decreased health care utilization,8 prolonged survival,9 and higher quality of life.10 Despite this, access to this care is out of reach for many because of insurance typically covering less than half of the costs for services rendered in ALS centers.11 Although expedited Medicare enrollment for persons with ALS benefits some, many individuals remain ineligible for Medicare despite receiving an ALS diagnosis. Recommendation 3–2 urges Congress to expand Medicare eligibility for all persons with ALS regardless of age, employment history, or other factors, and to require adequate reimbursement of multidisciplinary ALS care. It is safe to assume that more ALS clinics may open once this care model is at least cost-neutral to the hosting facility. Additionally, Recommendations 3–3 and 3–4 focus on urgent needs of unpaid caregivers, including tax relief and financial support, and centralized resources compiled by non-profits and patient-centered organizations.
Most persons with ALS will succumb to respiratory infections at the end of life, and use of respiratory assist devices (RAD) offers greater quantity and quality of life.12, 13 Medicare qualification for RAD reimbursement for ALS is outdated and misaligned with current American College of Chest Physicians and international best practices, requiring a forced vital capacity (FVC) below 50%.13-17 Recommendation 3–5 urges CMS and private insurers to align coverage for RAD and services for ALS to the current best practices and provide in-home respiratory care and services.
Creating a Sustainable and Accessible ALS Clinical Care and Research System
Building on the fact that not all individuals impacted by ALS have access to evidence-based, high-quality ALS care and research, the committee also detailed four critical recommendations addressing the inherent structural challenges impacting long-term ALS care and research. As the foundation of this domain, Recommendation 4–1 proposes a reimagined, inclusive, and integrated multidisciplinary care and research system. The proposed hub-and-spoke system of ALS care and research expands the existing network of care via three distinct, but interconnected care settings: community-based-, regional-, and comprehensive-ALS centers. To be most effective, this system will be National Institute of Neurological Disorders and Stroke (NINDS)-designated, analogous to the National Cancer Institute (NCI) tiered designations for cancer centers. The community-based ALS multidisciplinary care centers will expand access to currently underserved areas. The new value-based payments and NINDS designation would incentivize existing multidisciplinary clinics and foster new partnerships among specialists (neurologists, physiatrists, pulmonologists, etc.), local allied health professionals, and community-based organizations to deliver multidisciplinary care closer to patients' homes. Importantly, through this network, all ALS families cared for in this system will have access to complex specialized support and research opportunities throughout their illness. Although the NINDS financing model would support ALS research and workforce training for all centers, Recommendation 4–3 asks insurers to align clinical payments with indicators of high-quality care at all levels in the hub-and-spoke care delivery system.
In parallel, efforts to improve the structural and financial barriers to ALS care that impede timely diagnosis and access to inclusive multidisciplinary care and research are required. Recommendation 4–2 seeks to improve racial and ethnic equity in ALS care and research. To address the finding that Black Americans face significantly worse health outcomes living with ALS, ALS multidisciplinary care centers should partner with community-serving organizations and pursue targeted approaches to understand and improve care and outcomes for underrepresented communities. This would build a bridge to the broader community to bring people into the care network who might otherwise go unnoticed.
Finally, Recommendation 4–4 recommends that Congress allocate funding to create a network for ALS clinical care, education, research, and innovation within the Department of Veterans Affairs (VA). These resources will enhance equitable access to high-quality ALS care for veterans and will align the VA with the broader hub-and-spoke system. Investment in the VA will benefit the broader community by addressing the workforce shortage through the VA's extensive health professional training programs and increase opportunities for ALS research within a large national system of care. Notably, research understanding the relationship between military service and increased disease susceptibility has recently stalled, but the potential for critical discoveries on ALS causality could be within reach if innovative, well-designed studies, including those considered outside-the-box, are conducted and not siloed. These recommendations are essential to meeting health equity challenges, providing funding and reimbursement support for hub-and-spoke centers and better integrating VA networks.
Advancing ALS Research, Accelerating Therapeutic Development, and Preventing ALS
There has been rising momentum in drug development for ALS over the past decade and multiple companies are currently developing drugs for ALS. Despite this, there are notable gaps slowing the pace of ALS therapeutic development. Critical factors include, but are not limited to, complex disease biology, diagnostic delays, demand–supply mismatch for access to clinical trials because of fewer large-scale trials and trial sites, and unaffordable research and development costs for drug developers.
Recommendation 5–1 calls on the National Institutes of Health (NIH) and NINDS to fund an end-to-end supported ALS clinical trials network that is distributed across diverse geographic regions and integrated within the ALS hub-and-spoke system. This dedicated ALS clinical trials network would leverage and build on existing infrastructure (Northeast ALS Consortium of 150+ sites and central institutional review board, NeuroNEXT, ALL ALS), support both exploratory and confirmatory ALS clinical trials, and mandate centralized data sharing platforms, thereby driving all-round clinical and translational research development. An ALS clinical trial network integrating new rural sites also offers opportunities for thoughtful implementation of video-conferencing technology across state-lines. This technology enables enrollment into clinical trials and natural history studies, as well as opportunities for remote monitoring and local home-based outcome assessment measures. Such decentralized clinical research methodologies are currently being implemented in the NIH-led ALL ALS consortium. In the reimagined hub-and-spoke system, optimal utilization of expanded telehealth technology and services would dramatically increase outreach to underserved or underrepresented communities for both care and research. Again, NCI provides a robust model for this with the National Clinical Trials Network ($170 M budget, 2,200+ trial sites, ~20,000 cancer participants enrolled annually).18
For advancing translational, epidemiological, and key population health outcomes research, Recommendation 5–3 urges the Centers for Disease Control and Prevention and NINDS to create a comprehensive ALS registry with interoperable data platforms to integrate new and current data sources and collect data on all symptomatic and at-risk ALS individuals. To make this registry more useful, ALS should be added to the National Notifiable Diseases Surveillance System, mandating states report all cases of ALS. The registry should be embedded into routine care whereby every ALS patient receiving care at any NINDS-designated ALS center is automatically enrolled, with a portion of supplemental funding based on registry participation. Recommendation 5–2 calls on NINDS to create an indefinite, ongoing natural history study across diverse patient populations that could serve as external and concurrent controls for some clinical trials. This would help reduce placebo exposures in ALS trials, especially for early phase trials and/or longer treatment duration randomized controlled trials. Moreover, such robust natural history study datasets will enable research promoting deeper understanding of ALS biology and protective factors among different subgroups, within individuals at various disease stages, and in at-risk individuals. This includes, but is not limited to, how ALS varies in clinical phenotypes, disease plateaus, and rates of progression. Such knowledge is essential for identifying new therapeutic targets based on improved understanding of sporadic ALS pathophysiology.
The drug discovery and biomarker development fields are rapidly evolving and there are continuous gene and novel therapeutic target discoveries in ALS. Therefore, the committee opined on promoting scientific creativity and allowing adjustable research priorities based on emerging science over the next decade instead of focusing on today's knowledge alone. Recommendations 5–2 and 5–4 call on multiple stakeholders, including NINDS, ACT for ALS-led public-private partnerships, Agency for Healthcare Research and Quality, and ALS funders, to consider additional research priorities, such as clinical prognostication, application of artificial intelligence, patient-friendly drug delivery approaches, improving expanded access pathways, health services, and social and behavioral research.
Finally, Recommendations 6–1 and 6–2 focus on preventing ALS and unique unmet needs of at-risk genetic carriers. There is a need to develop specific research programs for individuals at-risk for ALS, including large-scale natural history studies, and to tackle the issue of genetic discrimination for carriers. To better support clinical care and prevention efforts, insurance coverage of genetic testing and counseling services is needed for all living with ALS and their families.
Conclusion
Overall, the critical gaps in ALS diagnosis, care, and treatment can be addressed by implementing the near- and longer-term recommendations emanating from the NASEM committee discussions. These actions will help individuals living with ALS, their families and caregivers, and those at-risk receive the multidisciplinary support and resources needed to navigate the complex disease course, while in parallel establish the infrastructure for equitable access to care and research and support diagnostic and prognostic biomarker discovery, therapeutic development, and prevention strategies. To see these substantial improvements, advocates in the ALS community must now unite in advocacy with the responsible parties identified in each recommendation. With successful implementation, these recommendations have potential to make ALS a livable disease within a decade.
Acknowledgments
Funding support during the preparation of this manuscript was provided by the NIH (R01ES030049, R01NS127188, OT2NS136939, to E.L.F.; UF1NS131791-01, 1U01NS136020-01, 1U01NS136021-01, 1OT2NS136938-1 to S.B.; 1U01NS077179-01 to M.E.C.), the Centers for Disease Control and Prevention/Agency for Toxic Substances and Disease Registry (R01TS000327, R01TS000344 to E.L.F.); the American Academy of Neurology, ALS Association, and Muscular Dystrophy Association to S.B.; ALS Finding a Cure and ALS One to M.E.C., the Neurological Clinical Research Institute, Sean M. Healey & AMG Center for ALS, and the Charles H. Abdalian, Jr. ALS Research Fund to S.B. and M.E.C.; and Scott L. Pranger and the NeuroNetwork for Emerging Therapies to E.L.F.
Author Contributions
All authors contributed to the conception and design of the manuscript, acquisition of data, and writing of the manuscript. S.A.S. created the figure. All authors contributed to reviewing/editing and final approval of the manuscript.
Potential Conflicts of Interest
S.B. reports research funding from Biogen, Novartis, Ionis Pharmaceuticals, OrphAI Therapeutics, Denali Therapeutics, and uniQure. M.E.C. reports consulting fees from Denali Therapeutics, ITB-MED, and Calico and personal compensation from Transposon Therapeutics, Ono Pharmaceutical, InFlectis BioScience, QurAlis, Novartis, NeuroSense Therapeutics, Biogen, Locust Walk, Pasitnea, Roche, Denali Therapeutics, Immunity Pharma, RRD, Takeda Pharmaceuticals, Mitsubishi Tanabe Pharma Corporation, Arrowhead Pharmaceuticals, Servier Pharmaceuticals, Eledon Pharmaceuticals, Cytokinetics, and Regeneron Pharmaceuticals. I.M.H., S.B., C.C., J.E.K., and E.L.F. served on the National Academies of Science, Engineering, and Medicine Amyotrophic Lateral Sclerosis: Accelerating Treatments and Improving Quality of Life committee. S.A.S has no relevant conflicts to disclose.