Clinical, Radiological and Pathological Features of a Large American Cohort of Spinocerebellar Ataxia (SCA27B)
Abstract
Objectives
Spinocerebellar ataxia 27B due to GAA repeat expansions in the fibroblast growth factor 14 (FGF14) gene has recently been recognized as a common cause of late-onset hereditary cerebellar ataxia. Here we present the first report of this disease in the US population, characterizing its clinical manifestations, disease progression, pathological abnormalities, and response to 4-aminopyridine in a cohort of 102 patients bearing GAA repeat expansions.
Methods
We compiled a series of patients with SCA27B, recruited from 5 academic centers across the United States. Clinical manifestations and patient demographics were collected retrospectively from clinical records in an unblinded approach using a standardized form. Post-mortem analysis was done on 4 brains of patients with genetically confirmed SCA27B.
Results
In our cohort of 102 patients with SCA27B, we found that SCA27B was a late-onset (57 ± 12.5 years) slowly progressive ataxia with an episodic component in 51% of patients. Balance and gait impairment were almost always present at disease onset. The principal finding on post-mortem examination of 4 brain specimens was loss of Purkinje neurons that was most severe in the vermis most particularly in the anterior vermis. Similar to European populations, a high percent of patients 21/28 (75%) reported a positive treatment response with 4-aminopyridine.
Interpretation
Our study further estimates prevalence and further expands the clinical, imaging and pathological features of SCA27B, while looking at treatment response, disease progression, and survival in patients with this disease. Testing for SCA27B should be considered in all undiagnosed ataxia patients, especially those with episodic onset. ANN NEUROL 2024;96:1092–1103
Autosomal dominant spinocerebellar ataxias (SCAs) are a genetically and clinically diverse group of neurodegenerative diseases characterized by progressive ataxia due to degeneration of the cerebellum and varying involvement of other brain regions. SCAs have been attributed to pathogenic variants in more than 50 genes, including at least 14 that are disrupted by pathological repeat expansions.1 Recently, a deep intronic GAA repeat expansion in the gene FGF14 was identified as a novel cause of late-onset hereditary cerebellar ataxia (LOCA) and has now been identified among diverse populations of patients with LOCA.2 Its frequency has been reported to be as high as 61% in various cohorts of patients with genetically undefined late-onset cerebellar ataxia,2 rendering it one of the most common cerebellar ataxias. To date, more than 400 individuals with GAA-FGF14 (SCA27B) have been identified. The disease was first described in 2023 by Pellerin et al.2 in French Canadian, German, French, Australian, and Indian cohorts, and by Rafehi et al.3 in German and Australian cohorts, but reports on the prevalence and clinical characteristics of SCA27B have appeared from populations in Spain,4 Brazil,5 France,6, 7 Greece,8 China,9 and Japan,10 highlighting the global nature of this disease.11 GAA repeat expansions of 300 and larger are highly penetrant and fully pathogenic while those with of 250–299 repeats may exhibit reduced penetrance. Here, we present the first study on this disease in the US population, characterizing its clinical manifestations, imaging findings, pathological abnormalities, and response to 4-aminopyridine (4-AP) in a cohort of patients bearing GAA repeat expansions in the incomplete penetrant and fully expanded range. To assess prevalence, we screened 732 patients with undiagnosed ataxia, identifying 55 patients (7.5%) with expansions in FGF14 equal to or greater than 250 tandem repeats.
Materials and Methods
Cohort
We compiled a consecutive series of 102 patients with GAA-FGF14 (SCA27B), recruited from a consortium of 5 academic centers across the United States (University of Chicago, University of California Los Angeles, University of Texas Southwestern, University of Michigan, and University of Minnesota) after genetic testing had excluded other repeat expansions. For prevalence screening, we examined a cohort of 732 patients evaluated at a tertiary referral ataxia center, clinically unselected by age or phenotype aside from having undiagnosed familial or sporadic cerebellar ataxia following testing for common genetic causes of cerebellar ataxia,12 including RFC1-Ataxia (tested in 580/732, 79.2%). This study adheres to the Strobe checklist.
Genetic Analysis
Repeat expansion analysis of the FGF14 repeat locus was performed using the method described by Bonnet et al.13 with some minor modifications. The intronic FGF14 repeat region was amplified by long-range polymerase chain reaction (PCR) using primers flanking the repeat region.2, 3 Fluorescent long-range PCR amplification products were sized by capillary electrophoresis (ABI 3730xl, Foster city, CA, USA) to determine the number of repeats. Least squares method was used to correct for sizing as trinucleotide repeat-containing fragments are known to affect capillary electrophoresis migration.7 Amplification products containing ≥400 repeats that could not be sized by capillary electrophoresis were sized on TapeStation (Agilent Inc., Santa Clara, CA, USA) or by agarose gel electrophoresis. For samples with more than 200 repeats, repeat-primed PCR was performed to confirm the presence of the GAA repeat motif. Repeat-primed PCR was performed in both the 5′ and 3′ directions using published primers2, 3 and products were analyzed by capillary electrophoresis (ABI 3730×l, Foster city, CA, USA). GAA repeat expansions ≥250 repeat units were considered pathogenic. Genetic testing for the repeat expansion in the FGF14 gene is available in some diagnostic laboratories, in the United States and in Europe, and can be performed for clinical testing purposes. In the United States, only The University of Chicago and Variantyx currently offer SCA27B testing. The University of Chicago Labs offer the only direct CLIA-approved test. Variantyx testing is indirect via bioinformatic methods.
Data Collection/Phenotyping
Clinical manifestations and patient demographics were collected retrospectively from clinical records in an unblinded approach using a standardized form, including symptoms, neurological examination, genetics, imaging findings, and disease severity. A standardized patient questionnaire was completed when possible upon re-evaluation in the clinic. Patients with missing information were considered lost to follow-up. Disease severity and neurological features were assessed by the Scale for the Assessment and Rating of Ataxia (SARA) and the Inventory of Non-Ataxia Signs (INAS) when available. Functional impairment was defined as the use of a walking aid (cane, walker, or wheelchair dependence). Video head impulse testing (vHIT), complemented by bedside testing, was performed on seven patients to document vestibular abnormalities.
MRI Imaging
Thirty-seven MRI scans were reviewed for the presence of cerebellar atrophy, midbrain atrophy, and other abnormalities, in either the axial or sagittal planes or both, and assessed by 2 independent raters (midbrain atrophy by T.X. and J.C.; cerebellar atrophy by C.G. and J.C.). Agreement between the 2 raters was reached in 79% of cases for midbrain atrophy and 88% of cases for cerebellar atrophy. In the few cases where there was no agreement, the presence of atrophy was settled by a third rater (W.A.). Since the MRI images were acquired for clinical utility and each contained different sequences, they could not be analyzed quantitatively. An additional 48 MRI reports were reviewed when images were not available.
Neuropathology
Post-mortem analysis was done on 4 brains of patients with genetically confirmed SCA27B. Histological analysis of formalin-fixed paraffin embedded tissue included hematoxylin and eosin (H&E) staining, Bielschowsky and ubiquitin antibody (P4D1).
Treatment Response to 4-AP
Treatment response to 4-AP or dalfampridine ER (ranging 5–10 mg twice daily) was recorded in a non-standardized manner, either retrospectively or prospectively depending on the institution, in 28 subjects. Response was defined as a subjective improvement as reported by the patients in any of the following symptoms: unsteadiness, dizziness, diplopia, dysarthria, appendicular ataxia, and episodes. Response was considered positive when sustained for over 1 week.
Statistical Analysis
Summary statistics were described as mean with standard deviation or median with the interquartile range (IQR) for continuous variables, based on data normality, and frequency with percentages for categorical variables. We used median regression models to assess the associations between demographic or clinical variables and continuous outcomes, including SARA at first visit, progression in SARA score between first and last visit, and repeat size (expanded allele), without any assumptions on the distribution of the observations or the residuals of the regression model. Overall survival (OS) was assessed using Kaplan–Meier methods. Univariate Cox proportional hazards models were used to evaluate the association between OS and potential variables, including race, age at onset, gender, and number of repeats. Fine-Gray subdistribution hazard model was applied to identify factors related to the use of a walking aid with patients' deaths as competing events. Potential risk variables with a p-value ≤0.15 on univariate analysis were included in multivariate analyses to conduct the final models. Median regression coefficient and subdistribution hazard ratio (SHR) were presented with 95% confidence interval (CI). All statistical tests were two-sided with a p-value <0.05 as statistically significant. Analyses were performed using STATA/SE version 18.0 (StataCorp LP, Texas, USA).
Standard Protocol Approvals and Patient Consents
All study methods were approved by the Institutional Review Board from the respective University centers. Written informed consent was obtained from all patients participating in the study.
Results
Prevalence Screening for SCA27B in an Unselected Clinical Ataxia Cohort
A cohort of 732 predominantly adult-onset patients with undiagnosed familial or sporadic cerebellar ataxia was evaluated at a tertiary referral ataxia center, excluded for other common genetic causes of cerebellar ataxia, and then screened for the SCA27B repeat expansion. To allow for the observation of phenotypes outside those previously reported, no further restrictions were made with regard to age or clinical presentation. Average age for the cohort was 56.4 ± 16.8 years. The cohort was 52.5% female and the ancestral makeup was 71.7% White, 9.6% Asian, 2.7% Black, 1.0% Native American, with 8.1% of Hispanic/Latino ethnicity. Fifty-five patients (7.5%) were found to have tandem repeats greater than or equal to 250. Of these 55 subjects, 31 (56%, 4.2% of cohort) were deemed fully pathogenic, with uninterrupted expansions greater than 300 GAA tandem repeats, and 24 (44%, 3.3% of cohort) were designated reduced penetrance, with uninterrupted expansions between 250 and 300 GAA tandem repeats. An additional 11 (1.5% of cohort) subjects had expansions greater than 250 GAA tandem repeats but containing interruptions with non-GAA motifs and therefore unclear significance for pathogenicity.14 Clinically, of the 55 patients with diagnostic findings, 45 (81.8%) experienced unsteadiness, 45 (81.8%) demonstrated gait ataxia, 33 (60%) had episodic onset of symptoms, 27 (49.1%) experienced dizziness, 26 (47.2%) showed saccadic pursuit of eye movements, and 24 (43.6%) showed cerebellar atrophy on MRI. The average age of onset for patients with SCA27B was 58.6 ± 13.8 years, which was significantly older than the average age of onset for the entire cohort (47.7 ± 19.6 years, p < 0.0001).
Establishment of a Multi-Center Cross-Sectional Study Cohort of Patients with SCA27B: Demographics and Genetics
Along with the above patients, an additional 57 patients with genetically-confirmed SCA27B from 4 other US centers were enrolled in this study. A total of 102 patients from 101 families with FGF14 GAA repeat expansions (greater than or equal to 250 repeats) were included in our analysis (Table 1). Fifty-eight patients (57%) were male and 44 patients were female (43%). Ninety-two of the patients (90%) identified as White, while 10 patients (10%) were classified as other (Latino-Hispanic, Japanese, or undisclosed). Median repeat size of the expanded allele was 337 (maximal repeat size 521). 59 (60%) patients had a positive family history of ataxic symptoms while 39 (40%) patients did not. Median duration of follow-up was 2.8 (0.3–6) years.
| Parameter | Value |
|---|---|
| Male/female (ratio) | 58/44 (1.32) |
| Race | |
| White | 92/102 (90.2%) |
| Othersa | 10/102 (9.8%) |
| Deceased | 17/98 (17%) |
| Mean age at first visit | 67 ± 10.5 |
| Family history | 59/98 (60%) |
| Median duration of follow-up (in years) | 2.8 (0.3–6)b |
| Median repeat size 1 | 18 (9.5–51)b |
| Median repeat size 2 | 337 (297–392)b |
- a Latino-Hispanic, Japanese, or undisclosed.
- b Interquartile range (IQR).
Overall Survival of the SCA27B Multi-Center Study Cohort
Of 98 patients, 17 (17%) were deceased at the time of analysis. Mean age at death was 78 ± 11 years. Median overall survival from time of disease onset till death was estimated at 39 years (Fig S1 in supplementary material) using Kaplan–Meier analysis. Of 102 patients, 14 (14%) had a history of cancer (mainly prostate, thyroid, and skin cancer).
Symptom Onset in the SCA27B Multi-Center Study Cohort
Symptoms at onset were defined as the presence of any: unsteadiness, dysarthria, diplopia, vertigo, or hand incoordination. Mean age of onset in our cohort was 57 years (± 12.5) (Table 2), with unsteadiness as initial symptom in 88% of patients. Fifty-one patients (51%) had episodic symptoms at onset. The average age at first visit was 67. Larger repeat size was associated with a younger age at disease onset (Table A in supplementary material).
| Parameter | Age (Years) |
|---|---|
| Disease onset (mean) | 57 ± 12.5 |
| Unsteadiness (mean) | 57.8 ± 12.3 |
| Episodes (mean) | 55 ± 13 |
| Diplopia (median) | 63 (53–72) |
| Hand coordination (mean) | 66 ± 11 |
| Dysarthria (mean) | 62 ± 12 |
| Dysphagia (mean) | 67 ± 11 |
| Use of walking stick (median) | 68.5 (62–75)a |
| Use of walker (median) | 73 (68–78)a |
| Use of wheelchair (median) | 74 (68–80)a |
- a Interquartile range (IQR).
Phenotypic Profile of the SCA27B Multi-Center Study Cohort
The phenotypic profile of SCA27B ataxia in our cohort consisted of a cerebellar syndrome with mainly balance and gait impairment (98%) (Fig 1). Ocular motor signs were present in 89% of patients (nystagmus, impaired smooth pursuits, diplopia). Downbeat nystagmus (DBN) was present in 70% of patients. Regarding non-cerebellar findings, 51% of patients had sensory changes in the lower extremities (numbness, decreased vibration, or proprioception), and 39% had a positive Romberg sign. Deep tendon reflexes were normal in 45 patients (50%), decreased in 13 (15%), and increased in 31 (35%). Of 46 patients, 33 (72%) had impaired downgaze. Dysautonomia: urinary dysfunction (33%), postural hypotension (22%), and constipation (25%), was less common, although erectile dysfunction was relatively high (50%). Cognitive impairment (assessed subjectively) was infrequent in 20/92 (22%).
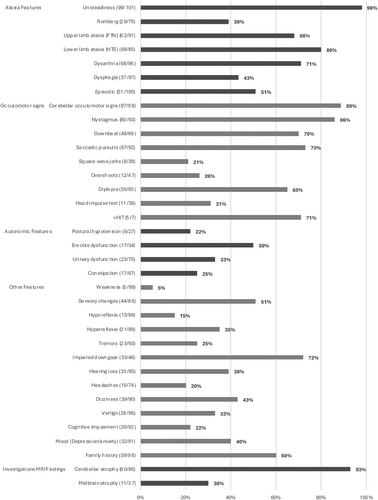
Episodic Characteristics of the SCA27B Multi-Center Study Cohort
Episodic features were reported in 51% of patients, defined as an abrupt and transient exacerbation in symptoms. The mean age of onset of episodes was 55 ± 13 years. Only 13/44 (30%) of patients reported a return to interictal baseline, while the majority had residual symptoms between episodes. Symptoms reported during the episodes included gait ataxia, appendicular ataxia, dysarthria, diplopia, or vertigo. The average duration of the episodes ranged from minutes to days, and frequency varied greatly between individuals from a few episodes a year to a few episodes a week (Table C in supplementary material). Common episode triggers were physical exertion (58%), heat (32%), caffeine (32%), alcohol (32%), or stress (29%). The episodes, when present, preceded the onset of progressive symptoms usually by 2 years, but all patients eventually developed progressive ataxia.
Imaging Findings of the SCA27B Multi-Center Study Cohort
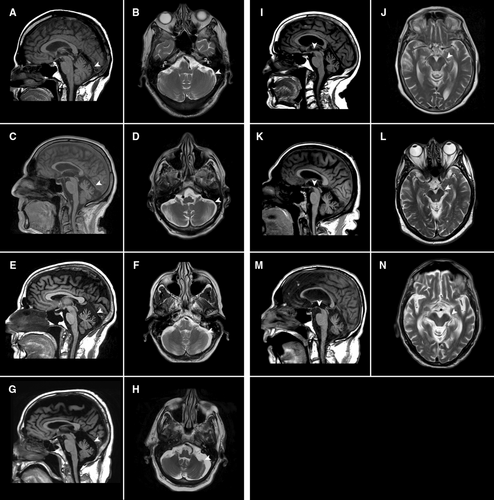
Of 86 patients, 80 (93%) had cerebellar atrophy on routine MRI, mainly vermian (Fig 2A–H). Given the relatively high frequency of impaired downgaze, midbrain atrophy was also assessed and identified in 11/37 (30%) of scans (Fig 2I–N).
Phenotypic Evolution and Functional Impairment of the SCA27B Multi-Center Study Cohort
Balance and gait impairment were almost always present at disease onset, with 42/102 (41%) of patients progressing to a cane by 11 years [median age 68.5 years (IQR 62–75)], 47/102 (46%) to a walker by 16 years [median age 73 (68–78)], and 18/102 (18%) to a wheelchair by 17 years [median age 74 (68–80)]. Use of a walking stick was significantly associated with older age at disease onset and the presence of increased tone (Table B in supplementary material). Use of a walker was significantly associated with older age at onset, presence of muscle weakness, and prior use of a cane. The use of a wheelchair was significantly associated with older age at disease onset.
Dysarthria was present in 68/96 (72%) and dysphagia in 37/87 (43%) of patients, but both remained mild and presented later in the disease course (mean age of onset 62 ± 12 and 67 ± 11 years of age, respectively). Similarly, upper limb ataxia and impairment in hand coordination were present later, at 66 ± 11 years of age. Cognitive impairment remained less frequent, even in later stages.
Disease Severity within the SCA27B Multi-Center Study Cohort
Disease severity was defined by SARA and progression to a walking aid. The mean SARA score at the first visit was 8 (±5.5), which progressed to a total of 12 (± 6.6) over a median of 2.8 years. The abnormal components of the SARA score consisted mainly of gait ataxia and lower limb symptoms before progressing to mild upper limb and speech disturbances. Factors associated with a greater progression of SARA from first to last visit were the use of a walking stick and a longer disease duration. Repeat size, gender and age at disease onset were not significantly associated with SARA score.
Pathological Findings within the SCA27B Multi-Center Study Cohort
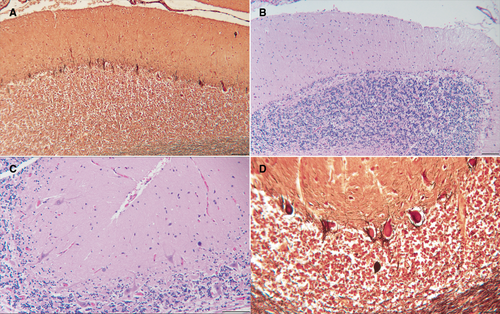
Based on 4 post-mortem brain examinations from patients with genetically confirmed SCA27B, the neuropathological changes were largely confined to the cerebellar cortex (Fig 3). The severity of changes differed between the four patients. The principal finding was loss of Purkinje neurons, which was most severe in the anterior vermis. In the patient with more severe changes, there also was partial loss of Purkinje cells in the cerebellar hemispheres, with the superior and posterior portions more affected than the inferior. “Empty baskets” were frequent in less chronically affected areas and axonal torpedoes were occasionally present. An infrequent finding was the heterotopic displacement of Purkinje cells into the molecular layer. The inferior olivary nuclei had mild neuronal loss and gliosis and there was mild gliosis in the vestibular nuclei. The cerebellar nuclei, basal pons, and all cerebellar peduncles were intact, as were the red nuclei and striatum. The pars compacta of the nigra and the midbrain tegmentum were intact with no evidence of neuronal tangles or “tufted” or “thorned” astrocytes. No ubiquitin-positive inclusions were detected. Spinal cords were not available for examination.
4-Aminopyridine Treatment Response within the SCA27B Multi-Center Study Cohort
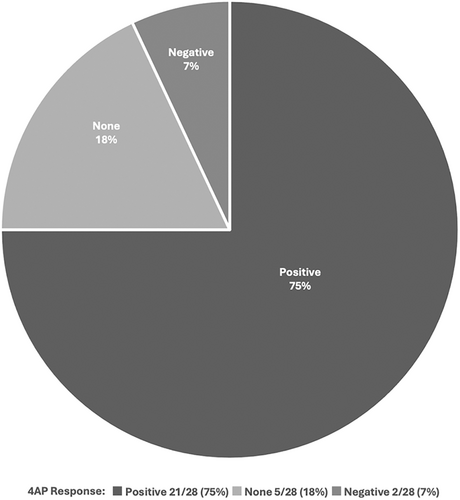
Based on previously reported positive results with 4-AP in small cohorts of patients,2, 15-17 we looked at the response to 4-AP or dalfampridine ER in a subset of our patients. Twenty-eight patients in our cohort received 4-AP or dalfampridine ER (ranging 5–10 mg BID). Treatment response was recorded based on subjective reports by the patients. Twenty one (75%) of patients reported positive results, mainly in terms of improved balance, speech, diplopia, limb coordination, and frequency and intensity of episodes (Fig 4). Five (18%) reported no effect after treatment for at least 2 months. Two (7%) reported a worsening of symptoms, mainly increased imbalance and vertigo and had to discontinue the drug. Aside from 1 report of heartburn, most patients tolerated the medication well with no side-effects. Of the patients who reported a positive response, 17/21 (81%) had DBN on exam, while 3/5 (60%) of those with no response had DBN, and the remaining 2 patients with a negative response both had DBN. Of 20 patients, 12 had a beneficial response to dalfampridine ER compared to 8/8 patients with 4-AP.
Discussion
Our study is the first and largest cohort to date to report on patients with SCA27B in a US population. With recognition of FGF14 GAA repeat expansions as one of the most common causes of late-onset cerebellar ataxia, our study aimed at describing the clinical, pathological, and radiological features of this disease among US patients, as well as drawing longitudinal assessment of disease severity and functional impairment. We also performed a prevalence screen in 732 patients with undiagnosed ataxia, identifiying SCA27B in 7.5%. Last, we report on treatment response to 4-AP in a subset of patients to inspire formal clinical trials.
Prevalence estimates for SCA27B world wide have ranged from 1.2% (n = 940) in Japan10 to a high of 61% (n = 66) in French Canada.2 The 7.5% diagnostic rate in our cohort is in the lower range compared with other studies.2-5, 7, 8 This difference may be influenced by variations in patient selection in some of these studies based on age and/or phenotype,2, 4, 5, 8 compared with our relatively unbiased clinical ataxia population. Overall, our findings suggest that older age, episodic onset, DBN, and gait ataxia are the most useful clinical clues for SCA27B diagnosis in a US clinical population and use of these criteria yields higher diagnostic rates. Excluding subjects with age of onset less than 40 years and phenotypes overlapping with multiple system atrophy2, 4, 5, 8 increases our diagnostic rate to 11.7% (50/428). Additional factors to consider include the racial/ethnic composition of our subjects (9.6% Asian, 8.1% Hispanic, 2.7% Black, and 1% Native American) versus the predominantly European ancestry in many prior studies. Profiling of the FGF14 locus has indicated a smaller repeat size in non-European populations.2, 3
Our findings are similar to previous reports describing SCA27B as a late-onset slowly progressive cerebellar syndrome, beginning with impaired gait and balance, with nearly half the patients presenting with episodic symptoms at clinical onset. Compared to previous studies (Table 3), our cohort had a similar mean age of onset (57 years). There does not seem to be a gender predominance among the different studies. Family history is positive in over 50% of patients. Gait ataxia is a prominent feature in over 90% of patients, and episodic symptoms were noted in 51% of our patients, compared with 11 to 48% in other cohorts. Prevalence of appendicular ataxia, visual disturbances, vertigo, and dysarthria were mostly similar in other cohorts. Cerebellar atrophy in most studies ranged from 64% to 97%, aside from 38% in one study (3). DBN was a prominent feature in this cohort. DBN, which most likely reflects involvement of the flocculus of the cerebellum, is not unique to SCA27B and is quite common in other cerebellar disorders, particularly SCA6.18
| Parameter | Our Cohort, 2024 | Pellerin et al.2 | Méreaux et al.7 | Rafehi et al.3 | Wilke et al.15 | Wirth et al.6 | Novis et al.5 |
|---|---|---|---|---|---|---|---|
| Region | US | Canada, Germany, Australia, India | France | Australia | Germany | France | Brazil |
| Total cohort (N) | 102 | 122 | 127 | 13 | 50 | 15 | 9 |
| Male sex (%) | 57 | 51 | 48 | 62 | 50 | 73 | 56 |
| Positive family history (%) | 59 | 69 | 74 | 0 | 51 | 0 | 56 |
| Average age at onset | 57 | 55 | 55 | 61 | 60 | 67.5 | 53 |
| Episodic onset (%) | 51 | 46 | 48 | NA | 12 | NA | 11 |
| Gait ataxia (%) | 98 | 96 | 95 | 96 | 95 | NA | 100 |
| Nystagmus (%) | 87 | NA | 83 | NA | NA | 66.7 | 78 |
| Downbeat nystagmus (%) | 72 | 42 | 67 | NA | NA | NA | 0 |
| Visual disturbancesa (%) | 64 | 48 | 59 | NA | 48 | 40 | 56 |
| Dysarthria (%) | 72 | 53 | 49.5 | 48 | 60 | 43 | 0 |
| Appendicular ataxia (%) | 80 | 80 | 73–81 | 74 | 60 | NA | 100 |
| Dizziness or vertigo (%) | 33 | 29 | NA | NA | 21 | 67 | 44.40 |
| Tremor (%) | 24 | 16 | 14–27 | NA | 52 | 64 | 11 |
| Cerebellar atrophy (%) | 93 | 74 | 88 | 38 | 97 | 64 | 87.5 |
| Findings on brain pathology | Loss of Purkinje neurons in the anterior vermis, partial loss of Purkinje cells in the cerebellar hemispheres, “empty baskets”, axonal torpedoes, heterotopic displacement of Purkinje cells into the molecular layer | Widespread depletion of Purkinje cells, gliosis in the molecular layer, and overall mild cell loss in the granule-cell layer | NA | NA | Pathological changes characteristic of PSP with typical tau-pathology; and additional cerebellar atrophy with severe loss of Purkinje cells in the absence of tau aggregation | NA | NA |
- a Oscillopsia, diplopia, blurring.
Large cohorts of European patients (n >120) have been described elsewhere (Pellerin et al, NEJM, 2023; Méreaux et al, eBioMedicine, 2024). The phenotypic spectrum is highly consistent across populations, and has already been well characterized. However, the phenotype of our cohort was highly consistent in this very large cohort drawn from different centers. This is important as it confirms the core phenotype of this condition and makes it highly recognizable clinically. There have been several smaller reports not reporting DBN, or episodic symptoms, or other key symptoms, but our large study essentially confirms the core key phenotype of SCA27B.
Noncerebellar features reported subjectively in this study included cognitive impairment (22%) similar to another study (16%)15 and mood disorder (36%), although whether this preceded or followed the onset of the disease was unclear. Dysautonomia was not frequent in our cohort, except for erectile dysfunction (50%), although this may relate to an increased incidence in this age group.19 INAS scores ranged only between 2 and 5 in our cohort, with abnormalities found predominantly in the brainstem oculomotor section. Sensory changes reported in 51% of these patients, consisted of lower extremity numbness, decreased vibration or proprioception, which was also not unusual in this age group.
vHIT testing was performed on a subset of 7 patients, in which RFC1 expansions were excluded. Five had evidence that is clear, or suggestive, of bilateral vestibular weakness, similar to findings by previous studies that also showed evidence of vestibular hypofunction.3, 17, 20 These findings suggest that vestibular impairment is not unique to patients with CANVAS.21 In addition, 33/46 (72%) of SCA27B patients in this cohort had impaired downgaze, along with 30% of scans demonstrating midbrain atrophy, although there were no findings suggestive of progressive supranuclear palsy (PSP) in our pathological study. These studies, as has been noted by others,22 suggest that several of the clinical findings in these patients, including DBN, vestibular areflexia, and impaired vertical gaze are not unique to individual disorders, but must be supplemented by molecular genetic studies.
The inheritance pattern in our study is consistent with an autosomal dominant transmission. However, similar to other studies, 41% had no known affected family member. The lack of family history in some cases likely results from the early death of the parent, or incomplete penetrance of smaller alleles or repeat expansion instability, particularly in light of the previously reported observation that the size of the GAA repeat is more likely to expand with maternal transmission and to contract with paternal transmission.23
Our study is one of the first to look at the overall survival in patients with SCA27B and the association of repeat size with the severity and progression of the disease. We found that median survival was 39 years from disease onset, suggesting that SCA27B does not significantly affect an individual's lifespan, similar to previous findings.6 With our report, we are able to more convincingly state that survival does not appear to be affected by the disease. Wirth et al. have looked at this but in a much smaller cohort. Furthermore, there is conflicting literature suggesting that FGF14 may, in the periphery, act as a tumor suppressor or oncogene (lung, colon, etc.).24, 25 Of our patients, 14% had a history of cancer, compared to a 5.5% prevalence in the general US population, a finding likely consistent with the older age of our cohort, and the fact that 20% of people older than 70 are living with cancer in the United States.26 The cancer frequency in our cohort was not higher than the general population, which has not been examined previously. Given the relatively high frequency of SCA27B, this observation is clinically relevant.
We also found that a higher repeat size of the expanded allele was associated with a younger age at disease onset. Similar to previous studies,15 there was no statistically significant association between repeat size and gender, age at onset of episodes, SARA score, or use of a walking aid. However, larger studies are needed to confirm these results.
Few studies2, 15-17, 27 have been undertaken thus far to assess response to different medications and, to our knowledge, no large clinical trials have been done. An open-label treatment study14 with 4-AP in 7 patients reported an 86% treatment response, as defined by improvement in daily living. Another study27 also reported on the response to acetazolamide in a subset of 34 patients with SCA27B. Unlike with 4-AP, the results were less promising with 56% of patients reporting no improvement. We found that 75% of a subset of 28 patients reported subjective improvement in their ataxia symptoms when treated with 4-AP or dalfampridine. The beneficial response to dalfampridine (12/20 subjects) may be have been less robust than 4-AP (8/8 subjects) because of the slower CNS penetration. Our results, however, complement those of others,15, 17 and support the implementation of a formal clinical trial to study the objective response to 4-AP in patients with SCA27B.
As distinct from patients with the previously identified genetic form SCA27A, SCA27B appears to be a late-onset cerebellar ataxia in most, but not all,28 patients described. As with other forms of SCA, symptoms begin initially with imbalance and gait ataxia, which later progresses to involve the upper limbs, speech, and swallowing function. Episodic symptoms in this study closely resemble those of episodic ataxia type 2 (EA2), with similar semiology and triggers. However, unlike EA2, in which episodes may appear in childhood and may never progress to interictal ataxia, episodes in SCA27B appear in the late and immediate years prior to the onset of progressive disease (within 2–5 years). The late onset and slow progression of a pure cerebellar syndrome can be often mistaken with SCA6, which itself may present with episodic symptoms.18
Our study is the third to report on pathological findings with patients with SCA27B and the first in the United States. Similar to the other reported autopsy cases,2, 15 our post-mortem analysis showed loss of Purkinje cells in the cerebellum. However, none of the present cases had any changes suggestive of PSP, which was noted in a previous study15 and may have represented an incidental disease association. The pars compacta of the substantia nigra and the midbrain tegmentum were intact with no evidence of neuronal neurofibrillary tangles or the classical “tufted” or “thorned” astrocytes. The neuropathological changes seen in patients in this report who had autopsies were primarily confined to the cerebellar cortex, specifically Purkinje cells, with the degree and distribution of their loss greater with prolonged duration of the disease. The cerebellar nuclei and basal pons were spared, but there was mild loss of inferior olivary neurons, likely secondary to retrograde trans-synaptic degeneration, and gliosis with equivocal loss of neurons in the vestibular nuclei in one patient with a protracted course. The changes seen in the present study are similar to those reported previously and to what is seen in other hereditary ataxias that primarily affect the cerebellar cortex.18, 29, 30
This study has several limitations. As a retrospective study, it introduces the risk of information and recall bias. It also includes a homogeneous population with most patients studied being White, although others have reported on patients of non-European ancestry.2, 5 The sample size for the 4-AP subset is small with open-label subjective assessment. Larger trials with objective measures are needed to confirm the findings. In addition, response to 4-AP was subjective and based on patients' reports rather than standardized testing.
Acknowledgements
W. Abou Chaar acknowledges Dr. Mei Polley for her mentorship. B. Fogel acknowledges support through donations to the University of California. V. Shakkottai ackowledges support through the O'Donnell Brain Institute, UT Southwestern Medical Center for genetic testing of patients seen through the Ataxia Clinic. D. Pellerin has received a fellowship award from the Canadian Institutes of Health Research (CIHR). M. Burmeister is supported by NIH R01 NS078560, the Raynor Cerebellum project, and National Ataxia Foundation. C. Gomez is supported by NINDS R35 NS116868.
Author Contributions
W.A.C., C.M.G., and B.L.F. contributed to the conception and design of the study. W.A.C., C.M.G., B.L.F., C.L., H.B.C., S.D., D.P., and B.C.B. contributed to drafting a significant portion of the manuscript. W.A.C., C.M.G., B.L.F., C.L., H.B.C., S.D., T.X., A.N.E., H.A.S., S.L.W., D.Y.W., V.S.A., M.D., A.J.G., S.T., M.T., M.C., P.J.L., M.B., M.J.C., M.D., S.Z., S.P., M.B., H.P., S.S., L.S., M.B., K.B., V.S., and J.C. contributed to the acquisition and analysis of data.
Potential Conflicts of Interest
None to report.
Open Research
Data Availability
The anonymized data that support the findings of this study are available from the corresponding author upon reasonable request.