Metagenomics of the Gut Microbiome in Parkinson's Disease: Prodromal Changes
Alberto Ascherio, Curtis Huttenhower these authors are co-senior authors.
Abstract
Objective
Prior studies on the gut microbiome in Parkinson's disease (PD) have yielded conflicting results, and few studies have focused on prodromal (premotor) PD or used shotgun metagenomic profiling to assess microbial functional potential. We conducted a nested case–control study within 2 large epidemiological cohorts to examine the role of the gut microbiome in PD.
Methods
We profiled the fecal metagenomes of 420 participants in the Nurses' Health Study and the Health Professionals Follow-up Study with recent onset PD (N = 75), with features of prodromal PD (N = 101), controls with constipation (N = 113), and healthy controls (N = 131) to identify microbial taxonomic and functional features associated with PD and features suggestive of prodromal PD. Omnibus and feature-wise analyses identified bacterial species and pathways associated with prodromal and recently onset PD.
Results
We observed depletion of several strict anaerobes associated with reduced inflammation among participants with PD or features of prodromal PD. A microbiome-based classifier had moderate accuracy (area under the curve [AUC] = 0.76 for species and 0.74 for pathways) to discriminate between recently onset PD cases and controls. These taxonomic shifts corresponded with functional shifts indicative of carbohydrate source preference. Similar, but less marked, changes were observed in participants with features of prodromal PD, in both microbial features and functions.
Interpretation
PD and features of prodromal PD were associated with similar changes in the gut microbiome. These findings suggest that changes in the microbiome could represent novel biomarkers for the earliest phases of PD. ANN NEUROL 2023;94:486–501
Introduction
Parkinson's disease (PD) is the second most common neurodegenerative disease globally, impacting approximately 1% of the population 65 years and older.1 Compelling evidence suggests that PD may start outside the central nervous system: in animal models, aggregated αSyn propagated from the intestinal wall along the vagal nerve to the dorsal motor nucleus of the vagus in the brainstem,2-4 demonstrating a retrograde transport from the gut to the brain. The αSyn aggregation has been identified in intestinal biopsies from patients with PD, and truncal vagotomy has been associated with a reduced risk of PD5, 6 suggesting a possible gastrointestinal initiation of PD.7 Over 80% of patients with PD suffer from constipation, with onset of gastrointestinal symptoms up to 20 years before the first motor symptoms.1, 8
The role of the gut microbiome in PD has been explored a growing number of studies,9-29 which have yielded heterogeneous results, likely due to limitations in study design and microbiome sequencing technology. Abundance of some bacterial genera have been somewhat consistently reported to be increased in PD, including Akkermansia9, 14, 26, 27, 30-32 and Bifidobacterium,10, 13, 15, 18, 22, 26, 31-33 whereas others were decreased, including Prevotella,9, 10, 19, 22, 33 Faecalibacterium,15, 19, 26, 34 Blautia,10, 15, 26, 30 and Roseburia.10, 18, 26, 30, 32 Increased intestinal permeability and inflammation has also been noted in PD.35, 36 A recent study in monozygotic twins noted only minor differences in abundance of Bifidobacterium species and bile acid biosynthesis pathways, but was of limited sample size.13 Earlier studies used 16S rRNA gene amplicon profiling (abbreviated 16S), which in many cases cannot differentiate closely related taxa (including species). Recently, several metagenomic studies have begun to identify species-level taxonomic as well as bacterial pathways in relation to PD,9, 12, 13, 21, 23, 26 with some noting disruption in microbial carbohydrate metabolism.9, 12, 23, 26 Most of these prior studies used a traditional case–control design prone to selection bias,9 and although several have examined the microbiome in prodromal PD,14 none have explicitly considered the trend across phenotype groups that include constipated controls and participants with prodromal PD.
Biomarkers that can detect patients during their prodromal phase are highly sought after, because this period can last a decade or more, during which patients experience a range of subtle non-motor symptoms.37-40 Our group has, for the past 10 years, rigorously assessed specific prodromal symptoms, including constipation, hyposmia, and probable RBD (pRBD) in 2 large cohort studies, showing that a combination of these symptoms is likely to be predictive of PD.41, 42 By using these symptoms, we have identified a unique cohort of individuals with a constellation of features that are common in prodromal PD, from here on denoted as prodromal Parkinson's syndrome (PPS). Here, we sought to newly determine whether gut microbial changes might also accompany these early PPS symptoms, preceding clinical onset of PD. This would both provide additional information for biomarkers of early PD detection, as well as potentially better explaining the causal or responsive role of the gut microbiome during progression toward disease. To thus explore the role of the gut microbiome in PD and PPS, we conducted a large, nested case–control study within these 2 large cohorts. We included both healthy and constipated controls to account for microbial differences related to constipation and used metagenomic sequencing to profile the microbiome.
Methods
The study was approved by the institutional review boards at the Brigham and Women's Hospital and the Harvard T.H. Chan School of Public Health.
Enrollment and Study Participants
The study population was selected among participants in 2 prospective cohort studies: the Nurses' Health Study (NHS) and the Health Professionals Follow-up Study (HPFS). The NHS enrolled 121,700 female nurses aged 30 to 55 at baseline in 1976. The HPFS similarly enrolled 51,529 male health professionals aged 40 to 75 at baseline in 1986.
Premotor Parkinson's Syndrome
The procedures for PPS assessment in the NHS and HPFS have been described previously.41 Briefly, on the 2012 questionnaire, all NHS and HPFS participants were asked about bowel movement frequency, and those with a frequency of every other day or less or who reported use of laxatives at least twice a week were classified as having constipation. In addition, on the 2012 questionnaire in both cohorts, we assessed pRBD using a screening question from the Mayo Sleep Questionnaire (“Has your spouse [or sleep partner] told you that you appear to ‘act out your dreams’ while sleeping [punched or flailed arms in the air, shouted or screamed], which has occurred at least 3 times?”).43 In a prior study, the above question was shown to have 100% sensitivity and 95% specificity for the diagnosis of polysomnography-confirmed RBD.44 In 2014 (HPFS) and 2015 (NHS), participants were also administered the Brief Smell Identification Test (BSIT),45 based on which we calculated an olfactory score as the sum of correctly identified odors. Based on prior literature, an olfactory BSIT score ≤ 7 was considered as substantial loss of smell. In this study, we classified participants as having PPS if they had all 3 of (1) constipation, (2) pRBD, and (3) substantial loss of smell.
Recent Onset Parkinson's Disease
On biennial study questionnaires, all NHS and HPFS participants were asked to self-report diagnosis of PD, among other disorders. All NHS and HPFS participants self-reporting PD were asked to release medical records, which then underwent review by a study neurologist specializing in movement disorders who rated the certainty of PD diagnosis as “clinically confirmed” or “possible,” we considered only confirmed PD cases in our analyses. Our analyses included PD cases with a date of diagnosis of 2006 or later.
Healthy Controls and Controls with Constipation
NHS and HPFS participants without diagnosed PD, under 85 years of age, who responded negatively to the 2012 constipation and pRBD questions, and who scored above the BSIT cutoff of 7 on the 2014 (HPFS) and 2015 (NHS) hyposmia were eligible for the study as healthy controls. Similarly, participants meeting the above criteria (no diagnosed PD, no pRBD, and no hyposmia), but with a positive response to the 2012 constipation question were eligible for selection as constipated controls. Constipated controls and healthy controls were frequency matched to the PD and PPS participants on age within sex/cohort.
Stool Sample Collection and Storage
A total of 837 NHS and 880 HPFS participants were selected and invited into the study. The stool collection protocol in our study followed that of the Micro-N study, designed and implemented by the Harvard Chan Microbiome Collection Core (HCMCC), as previously described.46 Briefly, an invitation letter was mailed to eligible NHS and HPFS participants identified as PPS, PD, constipated controls, and healthy controls. Participants were asked to review and return a consent form for the stool collection and study. Consenting participants were mailed, via US Postal Service, a package containing a toilet accessory and up to 4 different specimen collection kits including (1) an OMNIgeneGUT tube (OMR-200, DNA Genotek), (2) a tube with 95% ethanol preservative, (3) a tube with Anaerobe Systems liquid dental transport medium, and (4) an OMNIgeneORAL tube (OMR-120, DNA Genotek). Participants were asked to collect a small amount of stool, with the help of the toilet accessory, into the OMNIgeneGUT kit according to provided instructions. Only the material in the OMNIgeneGUT kit was used for the work described in this paper. Participants returned their kits through standard pre-paid mail. Upon receipt at the HCMCC, the specimen provided in the OMNIgeneGUT kit was aliquoted into 5 approximately 500 ul subsamples, which were then stored in a Hamilton BiOS robotic freezer at −80°C until sequencing.
The collection occurred in 2 stages: a total of 269 participants provided stool samples during the first phase of the collection (2019–2020), and 151 provided stool samples during the second phase of the collection (2020–2021). Shipping and receipt times for all kits were recorded and batch effects were examined, as well as adjusted for in the statistical analyses.
Shotgun Metagenomic Sequencing and Profiling
Shotgun metagenomic sequencing was conducted in 2 batches, with 269 samples sequenced in the first batch and 151 in the second. We examined differences in overall alpha and beta diversity, as well as in taxonomic and functional composition, according to batch, and the batch was included as a covariate in all analyses.
DNA extraction and sequencing from stool aliquots was performed at the Alkek Center for Metagenomics and Microbiome Research (CMMR) at the Baylor College of Medicine. Libraries were constructed and sequenced on the Illumina NovaSeq S4 platform, targeting a minimum of 3 Gnt/sample with paired 150 bp length reads. Artificial communities of microbes (MSA-100347 and MSA-200247) as positive controls and extraction reagent as negative controls were included to quality-control the resulting data and maximize rigor and reproducibility.
Taxonomic and functional profiling was performed on the Harvard Faculty of Arts and Sciences Cannon Research Computing cluster, using the bioBakery, a read-based microbiome bioinformatics analysis suite.48, 49 The KneadData version 0.3 pipeline (http://huttenhower.sph.harvard.edu/kneaddata)50 was used with default parameters for quality control and removal of host “contaminant” sequences, which incorporates Trimmomatic51 (filtering) and BMTagger52 (decontamination). Subsequently, pan-microbial (bacterial, archaeal, viral, and eukaryotic) taxonomic profiles were be determined using MetaPhlAn version 3.0 profiler (http://huttenhower.sph.harvard.edu/metaphlan),53, 54 which identifies taxa to the species level and quantifies their relative abundances. We excluded microbial species with a relative abundance < 10−5 (0.01%) in over 10% of all observations from most subsequent analyses, although to ascertain whether rare species may be associated with PD, we additionally conducted sensitivity analyses without filtering.
HUMAnN v3.0.0.alpha.4 (http://huttenhower. sph.harvard.edu/humann)55, 56 was used to quantify the relative abundances of microbial pathways from metagenomic data (also using default parameters). HUMAnN inputs short microbial DNA reads and outputs abundance of microbial pathways, providing taxon-specific profiles of UniRef orthologous gene families,57 MetaCyc,58 UniPathway,58 and KEGG pathways59 to survey microbial community metabolic potential in each metagenome. We again filtered out pathways with a relative abundance < 10−5 (0.01%) in over 10% of all observations prior to subsequent analyses.
Assessment of Covariates
Biennial Cohort Questionnaires
All NHS and HPFS participants were administered follow-up questionnaires every 2 years, on which they reported information on lifestyle exposures, medical histories, and anthropometric variables. Covariates in this study were drawn from the biennial NHS and HPFS questionnaires: smoking status, pack-years smoking, and body mass index (BMI; weight in kilograms divided by height in meters squared). Dietary information was drawn from a food frequency questionnaire (FFQ) administered biennially in NHS and HPFS, and included data on alcohol and caffeine intake; the Mediterranean Diet Score based on the FFQ was used as an aggregate measure of diet quality. The 2016 and 2014 HPFS and NHS biennial questionnaires were used for this covariate information, as those were the most recently available questionnaires prior to the collection. For patients with recently onset PD, dates of diagnosis and first symptoms were assessed based on review of medical records by a study neurologist (author M.S.).
Supplemental Stool Questionnaire
At the time of stool collection, participants completed a 2-page supplemental stool questionnaire that included questions regarding recent diet, major lifestyle factors, medication (including laxative and stool softener use), and medical history, as well as stool consistency and bowel movement pattern. The supplemental questionnaire used in this study was developed for the Micro-N study and has been described in detail and published previously.46
Statistical Analysis
Our dataset contained 519 microbial species after quality control from MetaPhlAn2, and of these 157 were retained after filtering (as detailed above) and used in our statistical analyses. Likewise, of 453 pathways profiled by HUMAnN2, there were 341 that were available for statistical analyses after filtering.
Overall Community Patterns of Microbial Variation (Omnibus Tests)
Alpha Diversity
We examined overall intra-sample (alpha) microbiome diversity, as a simple summary statistic of microbiome structure, using Shannon and Simpson alpha diversity indexes.
Beta Diversity
We used the Bray–Curtis dissimilarity metric for all beta-diversity analyses, both of taxonomic composition and functional potential. This included omnibus testing with permutational multivariate analysis of variance (PERMANOVA) of Bray-Curtis dissimilarities to quantify the percent variance explained by phenotype group (PD/PPS/constipated control/healthy control) and study covariates (age, sex, pack years of smoking, BMI, Mediterranean diet adherence, and batch). Beta-diversity relationships were visualized using ordination via Principal Coordinates Analyses (PCoA).
Feature-Wise Testing
Data Pre-Processing Prior to Feature-Wise and Classifier Analyses
In preparation for regression (MaAsLin 2) and classification (Random Forest [RF]) models, we removed outlying features (taxa and pathways) by applying Tukey's fences with k = 3 prior to modeling. Additionally, in MaAsLin 2 and RF models, we only considered microbial pathways that were not strongly correlated to any individual species (r < 0.7). The Benjamini-Hochberg false discovery rate (FDR) was used to control type I errors, considering FDR p values < 0.20 as statistically significant. Results with the highest level of statistical significance and biological/mechanistic relevance are generally presented in the main manuscript figures.
To further examine whether microbiome alterations in PPS resembled those of PD, we fit a series of fully adjusted MaAsLin 2 models comparing (1) participants with PD to healthy controls, and (2) participants with PPS to healthy controls, and assessed the β coefficients from these models. We performed an analogous analysis for functional features.
Microbiome-Based Random Forest Classifier
We used an RF classifier, a microbiome-appropriate discriminative prediction model, as implemented in the scikit-learn package in Python, to generate a microbiome-based biomarker for PD, as well as PPS, versus controls. The model was fit with cross validation based on 100 random splits (ShuffleSplit in Python) and an 80 of 20 random split of training and testing folds. The performance of the classifier was quantified by calculating the mean areas under the receiver operating characteristic curve (ROC AUC) over 100 RF iterations. We identified the top 20 microbial features contributing to the classification, via the mean Gini importance (decrease impurity) from the RF model.
A Priori Species of Interest
Because of the postulated protective role of strict anaerobes associated with reduced inflammation in chronic disease, including PD,9, 30 we calculated the total (sum/summary) relative abundance of several anti-inflammatory species: Eubacterium rectale, Roseburia inulinivorans, Roseburia intestinalis, Eubacterium hallii, Anaerostipes hadrus, and Faecalibacterium prausnitzii,61 in our dataset and compared the overall levels in PD and PPS versus the other groups using the Wilcoxon rank-sum test. We tested for linear trend across phenotype groups by fitting a logistic regression model with the ordinal phenotype groups (healthy, constipation, PPS, and PD) in relation to anti-inflammatory species abundance adjusting for sequencing batch, age, pack-years of smoking, BMI, and MED diet adherence.
Sensitivity Analyses
We conducted sensitivity analyses additionally adjusting for region of the US (Northeast/Midwest/South/West) and whether the sample was collected prior to or after January 1, 2020. We also conducted sensitivity analyses with RF models restricted to participants with Bristol Scores between 1 and 3, to examine whether our prediction was robust among participants with harder stool consistency.
The dataset is publicly available at dbGap (phs002193.v1.p1) and the complete results of our analyses are available at https://doi.org/10.7910/DVN/ZOAWNF.
Results
Study Design, Cohort, and Metagenomic Profiling
Stool samples were returned by 82 participants with PD (48% response rate), 114 of participants with PPS (59% response rate), 116 constipated controls (63% response rate), and 133 healthy controls (66% response rate). Collection progress is summarized in Figure 1. We collected and performed metagenomic profiling on stool samples from 420 NHS (N = 194) and HPFS (N = 226) participants. Samples from 3 participants were sequenced repeatedly during each batch for quality control; the resulting taxonomic profiles were highly correlated (Spearman r > 0.90), and replicates were subsequently analyzed as averages.
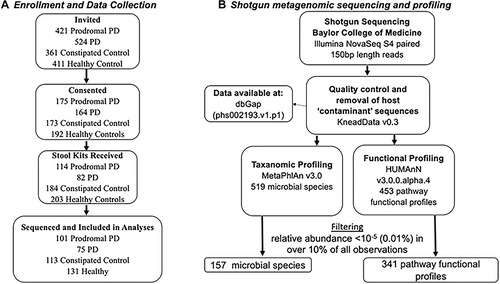
Table 1 outlines the characteristics of the study participants. Because participants were frequency-matched on age, the mean age of participants did not differ substantially across groups. Participants with PD were more likely to be women (due to a higher proportion of kits returned by female NHS participants than male HPFS participants with recent onset PD) and had lower alcohol and caffeine intake. They also had lower pack-years of smoking, which is consistent with observations in previous studies.64 As expected, PD, and to a lesser extent PPS, participants reported harder stool on the Bristol scale compared to healthy controls, and long-term use of fiber supplements, laxatives, and prebiotics/probiotics was elevated in participants who were recently diagnosed with PD relative to healthy controls. Participants with PD also had slightly lower BMI than healthy or constipated controls.
| Healthy controls (N = 131) | Constipation controls (N = 113) | PPS (N = 101) | Recently diagnosed PD (N = 75) | |
|---|---|---|---|---|
| Age, years | 80.7 (5.5) | 81.1 (5.8) | 80.5 95.5) | 79.5 (5.0) |
| Sex, female (%) | 43.5% | 46.0% | 41.6% | 57.3% |
| Body mass index, kg/m2 | 26.3 (4.9) | 25.5 (4.3) | 26.0 (4.0) | 25.0 (6.1) |
| Med Diet Score | 4.6 (2.1) | 4.4 (1.9) | 4.0 (2.0) | 4.6 (2.1) |
| Alcohol intake, g/day | 10.6 (11.8) | 9.7 (11.3) | 11.3 (15.1) | 7.5 (9.0) |
| Caffeine intake, g/day | 154.1 (115.0) | 157.0 (121.3) | 151.8 (122.2) | 132.0 (94.7) |
| Pack years smoking | 7.7 (13.8) | 8.8 (13.3) | 9.3 (15.6) | 4.6 (10.1) |
| Year first PD symptoms (median) | 2010 | |||
| Year PD diagnosis (median) | 2012 | |||
| Antibiotic use in last 1–6 mo, % | 12.7 | 22.5 | 18.3 | 17.4 |
| Bristol score | ||||
| 1–2, hard stool | 10.2% | 16.7% | 23.7% | 34.8% |
| 3–5 normal stool | 76.3% | 69.6% | 62.4% | 56.5% |
| 6–7 loose stool | 13.6% | 13.7% | 12.9% | 5.8% |
| Fiber supplement use | ||||
| Percentage more than 1/day in last wk | 8.5% | 23.5% | 21.5% | 8.7% |
| Laxative use | ||||
| Percentage more than 1/day in last wk | 0.0% | 6.9% | 12.9% | 8.7% |
| Stool softener use | ||||
| Percentage more than 1/day in last wk | 1.7% | 13.7% | 12.9% | 14.5% |
| Prebiotic use | ||||
| Long term: % more than 1/day | 3.4% | 1.0% | 1.1% | 0.0% |
| Probiotic use | ||||
| Percentage more than 1/day in last wk | 12.7% | 11.8% | 9.7%% | 10.1% |
- Note: Values are presented as means (SD) for continuous variables, proportion (%) for categorical variables, and median for dates (PD diagnosis and first symptoms).
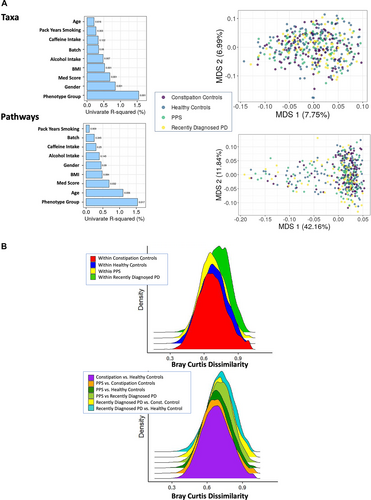
Collection/batch (first vs second) explained 0.34% (p = 0.08) and 0.24% (p = 0.35) in overall taxonomic and functional variation in the gut microbiome (based on Bray Curtis dissimilarity, Fig 2A). Therefore, all subsequent analyses are presented combining the 2 collections with a total sample size of 420 samples.
Overall Patterns of Microbial Community Variation in PD and PPS
Shannon and Simpson alpha diversity indexes were similar across disease groups (data not presented). Applying univariate PERMANOVA of Bray-Curtis dissimilarities, we observed that age, smoking, MED diet, sex, caffeine intake, BMI, and alcohol intake all explained relatively small amounts of variation in gut microbiome taxonomic composition (see Fig 2A, Taxa) and functional potential (see Fig 2A, Pathways). Outcome group (PD/PPS/constipated control/healthy control) also explained a small amount of variation in both taxonomic (R2 = 1.55%; p = 0.001) and functional composition (R2 = 1.54%; p = 0.017), but this was both statistically significant and larger than variance from other covariates. In complementary analyses, we examined the within—and between-group differences in taxonomic Bray–Curtis dissimilarity comparing healthy controls, constipated controls, PPS, and PD. Overall, we observed (1) more variability in the PD population and (2) a small gradient of dysbiosis from health through PPS to PD (Fig 2B). Results were similar when considering Bray–Curtis dissimilarity based on functional profiles (data not presented).
Individual Species and Functions Are Similarly Associated with Recently Onset PD and PPS
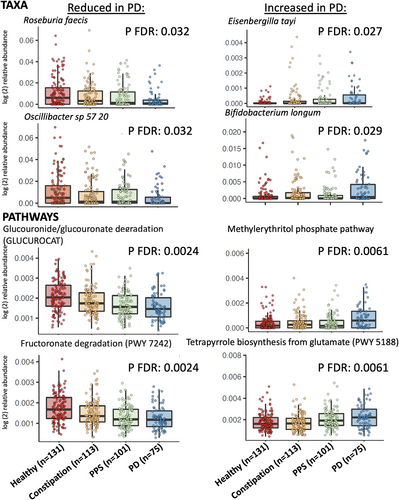
Subsequently, we identified individual microbial species, associated with group (PD/PPS/constipated control/healthy control) membership in our study using feature-wise microbiome-specific multivariate linear modeling in MaAsLin 2.65 In feature-wise models adjusted for sequencing batch, age, pack-years of smoking, BMI, and MED diet adherence, we observed significantly reduced abundance of several bacterial species, including Roseburia faecis, Eubacterium rectale, and E. ramulus (known for flavonoid degradation),66 as well as Oscillibacter_sp_57_20, Bacteroides xylanisolvens, and E. siraeum in PD samples compared to healthy controls (Fig 3A). Furthermore, in the majority of analyses, the abundance of these species followed a trend, progressing from healthy controls to constipation controls, to PPS, to PD (see Fig 3A). Similarly, we observed increased abundance of Bifidobacterium dentium and B. longum, which is consistent with prior reports of increased Bifidobacteria in PD,10, 13, 15, 18, 22, 26, 31-33 Our report of higher abundance E. tayi, C. leptum and R. lactatiformans in our recently onset PD samples compared to healthy controls is also consistent with published metagenomics work on PD.26 Abundance of these species also increased gradually from healthy controls to constipated control, PPS, and recently onset PD (see Fig 3A).
Microbial Pathways in PD and PPS
MaAsLin 2 identified 36 microbial pathways significantly depleted in PD, including several involved in microbial carbohydrate utilization (glucose, hexose, and other sugar and polysaccharide metabolism; Fig 3B). Akin to the taxonomic analyses described above, this depletion followed a trend with, primarily, decreasing abundance across our study groups, from healthy controls to constipation controls, PPS, and PD. Starch and tetrapyrrole biosynthesis pathways were elevated in PD compared to controls (see Fig 3B), also with a gradient across study groups.
The PPS Microbiome Lies on a Taxonomic and Functional Continuum between Healthy Controls and PD
When comparing key microbiome alterations observed in PPS, we observed that species over—or under—represented in PD relative to healthy controls were similarly related to PPS (Fig 4A). When plotted against each other, βPD and βPPS exhibited a linear relationship, indicating that taxa are similarly related to both PD and PPS. An even stronger linear relationship between βPD and βPPS was observed for metagenomic pathways (Fig 4B). Spearman correlations between βPD βPPS were 0.44 (p = 1.2 × 10−8) and 0.65 (p = 2.2 × 10−16) for species and pathways, respectively.

Discriminative Classification of PPS/PD Using Microbiome Profiles
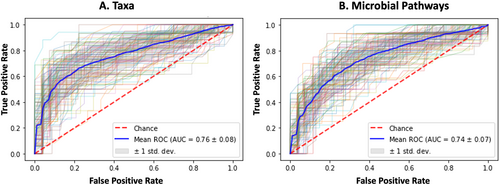
A RF classifier based on bacterial taxa discriminated between PD and healthy control participants with a mean ROC of 0.76 (+/−0.08) over 100 folds. An analogous RF classifier based on bacterial pathways had a comparable ROC of 0.74 (+/−0.07). When an RF was used to discriminate PD from constipated instead of healthy controls (Fig 5), discriminatory power was somewhat attenuated, with a resulting AUC of 0.69 (+/−0.07) for taxa and 0.68 (+/−0.09) for microbial pathways. Finally, the discriminatory power of the RF classifier was also attenuated but was still moderate when comparing PPS to healthy controls AUC of 0.67 (+/−0.07) for taxa and 0.68 (+/−0.08) for pathways. These results are concordant with a gradient of gut microbial change occurring between health, constipation, PPS, and PD. In sensitivity analyses restricted to participants with Bristol scores of 3 or below, RF classification restricted to participants with Bristol Scores between 1 and 3, remained robust for PD AUC of 0.70 (+/−0.12) for taxa and 0.71 (+/−0.11) for microbial pathways (Harvard Dataverse: https://doi.org/10.7910/DVN/ZOAWNF).
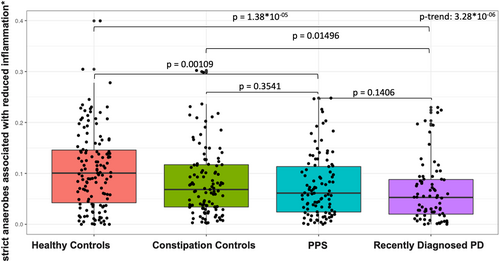
Anti-Inflammatory Species and PD
The total abundance of a priori defined anti-inflammatory species was lower in PD compared to healthy controls (p = 1.38 × 10−05) and to constipated controls (p = 0.015) and decreased gradually across study groups, with the highest levels in healthy controls and the lowest in PD (p trend = 3.28 × 10−06; Fig 6). In a multivariate model adjusting for sequencing batch, age, pack-years of smoking, BMI, and MED diet adherence, total abundance of these taxa was associated with decreased odds of PD (OR top vs bottom tertile = 0.20, 95% CI = 0.09–0.46, p trend 0.000152), and to a lesser extent decreased odds of PPS (OR top vs bottom tertile = 0.36, 95% CI = 0.18–0.71, p trend = 0.003), versus healthy controls.
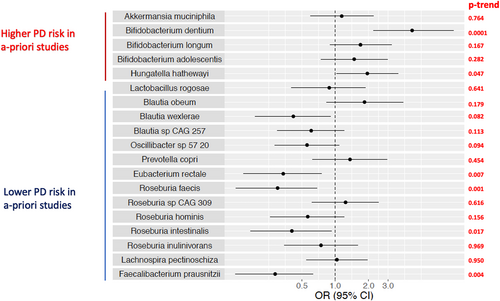
Supplemental Analyses, Species Previously Associated with PD
In logistic regression models focused on taxa previously associated with PD, we confirmed previously reported associations for abundance of Bifidobacterium dentium (p trend = 0.0001), and Hungatella hathewyi (p trend = 0.047) with higher PD risk, as well as between abundance of Eubacterium rectale (p trend = 0.007), Roseburia faecis (p trend = 0.001), Roseburia intestinalis (p = 0.0017) and Faecalibacterium prausnitzii (p = 0.004) and lower PD risk (Fig 7).
Sensitivity Analyses
Results of sensitivity analyses adjusting for region of the US (Northeast/Midwest/South/West) and timing of sample collection relative to the start of the coronavirus disease (COVID) pandemic were similar to our primary results. Taxa and pathways associated with PD were analogous, and the same gradient across phenotypes was observed, with, as without adjustment and the corresponding β coefficients were similar. The Spearman correlation between the MaAsLin2 β coefficients from these models and our primary results (Spearman r = 0.996 p = 2.2 × 10−16 for taxa), suggesting that adjustment for these additional covariates did not play a major role in the microbiome-phenotype association in this study.
Discussion
In this rigorously designed case–control study nested within 2 large prospective epidemiological cohorts, the NHS and the HPFS, we observed that the microbiome of participants with prodromal PD lies on a continuum between healthy and PD microbiomes. We identified several common bacterial species and microbial pathways that were differentially abundant in both prodromal and recently onset PD relative to healthy controls. Overall, our results suggest that the microbial shifts in PD precede PD diagnosis, potentially paving the way for prodromal PD biomarkers that include microbiome features.
Some, although not all, microbial alterations observed in our study are consistent with a state of elevated intestinal inflammation in PD.35, 36 Gastrointestinal disturbance has been recognized as a prodromal feature of PD and is present in up to 80% of patients.67 Inflammatory gastrointestinal conditions, such as the inflammatory bowel diseases (IBDs), have been associated with an increased risk of PD in several large cohort studies,68-70 and the use of antitumor necrosis factor (anti-TNF) therapy has been associated with reduced PD risk.70 IBD and PD have been shown to share genetic risk factors, most notably in LRRK2, where the N2081 Crohn's disease risk allele lies on the same kinase domain as a G2019S, a mutation shown to be the major genetic cause of familiar PD and a contributor to sporadic PD.71
Further, intestinal inflammation has been shown to lead to onset or worsening of PD-related changes in animal models.72 In human studies, levels of pro-inflammatory cytokines, including TNF-α, interferon-y, IL-1, IL-1β, and CRP have been shown to be elevated in PD compared to controls,73, 74 suggestive of intestinal immune dysregulation and inflammation in PD. Patients with PD also have higher levels of intestinal permeability compared to controls.75 Unlike prior studies that have also reported an inflammatory microbiome state in patients with PD compared to controls, our work goes further, demonstrating that dysbiosis reflective of immune activation is present at the earliest stages of PD, and may thus play an important role in PD pathogenesis. This provides additional evidence in favor of a causal role of gastrointestinal inflammatory changes in PD pathogenesis.
Our RF species and pathway-based classifiers were able to discriminate between recently onset PD and healthy controls, suggesting promise for a microbiome-inclusive biomarker for PD. As expected, this discriminatory ability of the classifier was attenuated, but not lost, when comparing PPS (instead of PD) to healthy controls, and the resulting PPS classifier relied on largely the same sets of species and pathways. This provides further evidence that the shared microbial changes observed both in PD and PPS may be part of, or at least parallel to, the pathological process in PD and are not the result of post-diagnosis factors, such as medication and lifestyle changes. The RF classifier was also able to discriminate PD from constipated controls, again largely on the basis of the same set of features.
This study's depletion of pathways and species involved in microbial carbohydrate utilization in PD is highly consistent with prior published metagenomic studies.9, 12, 23, 26 This seems to be particularly the case for near-terminal monosaccharide products of polysaccharide degradation. The pathway most depleted in PD in our cohorts was beta glucuronide and glucuronate degradation (GLUCUROCAT-PWY), which was also significantly depleted in participants with PPS compared to controls. This result is consistent with 3 of the 4 published PD metagenomic studies that also reported depletion of the same pathways.9, 12, 26 Several other polysaccharide degradation pathways that were depleted in our PD population, including fructuronate degradation (PWY-7242), mannan degradation (PWY 7456), galacturonate degradation (GALACTUROCAT-PWY), and stachyose degradation (PWY-6527) have also been reported.26 Conversely, we observed enrichment of glycogen degradation (GLYCOCAT-PWY); this is consistent with the same prior study, which interpreted it as a potential microbiota preference for non-plant-based polysaccharides during PD.
Other changes in PD microbiota functional profiles are more difficult to interpret, sometimes due to the lack of precise pathway cataloging for typical gut microbes. Amino acid pathways, for example, are often altered in the PD metagenome (eg, lysine and isoleucine biosynthesis enrichment in our study). However, these pathways are highly central to both microbial housekeeping and response to dietary products.26 Bedarf reported an increase in tryptophan degradation,9 and Wallen reported reduction in tryptophan biosynthesis in PD in tandem with general enrichment of a number of proteolytic pathways.26 In our study, tryptophan pathways were not associated with either PD or PPS. Similarly, pathways related to neuroactive metabolites are also sometimes metagenomically perturbed, but the interpretation of these changes in the human gut can be difficult.76-78 We observed a reduction in glutamate biosynthesis (PWY-5505, βPD = −0.0002, FDR = 0.10) and increase in glutamate degradation (PWY-5188, βPD = 0.0006, FDR = 0.006). Wallen observed analogous changes,26 and our observed reduction in reduction in the GABA shunt (GLUDEG-I-PWY) was also consistent with their report of dysregulation in GABA metabolism.
Finally, we observed alterations in several B-vitamin metabolism pathways. Flavin biosynthesis (PWY-6168, bPD = −0.0006, FDR = 0.002), was reduced in PD, consistent with observations from Boktor.12 We also observed reduction in thiamin (vitamin B1) pathways including thiamin formation (PWY-7357, βPD = −0.0006, FDR = 0.02) and thiamin diphosphate biosynthesis (THISYNARA-PWY, βPD = −0.0007, FDR = 0.06), the latter of which was also reported by Boktor.12 Adenosylcobalamin salvage from cobinamide was instead reduced (COBALSYN-PWY, βPD = −0.003, FDR = 0.06) in PD, suggesting a reduced uptake of the bioactive form of vitamin B12 in individuals with PD. Overall, our taxa and pathway results were consistent with prior studies, and we also identified additional taxa and pathways associated with PD. A key contribution of our study is demonstrating that that these pathways were associated with PPS in the same manner as during clinical disease.
The microbiome is modifiable, and several studies have demonstrated proof-of-concept that microbiota manipulation could contribute to PD prevention and treatment. Identification of PD-specific microbial features could lead to future therapies to treat PD or slow its progression. Fecal microbiota transplantation (FMT) studies in mice showed that in the MPTP and rotenone-induced PD mouse models, FMT reduced neuroinflammation and dopaminergic loss.79, 80 Probiotic therapies have also been attempted as a mode of microbiome modulation in PD, and have shown promise for inhibiting α-synuclein aggregation81 and improvement in motor and gastrointestinal function82 in PD. Administration of beneficial microbial-derived metabolites is another potentially beneficial route to PD treatment, as demonstrated by the impact of sodium butyrate on promotion of aSyn degradation.83 In addition to their potential for preventing or slowing the progression of PD, microbiome-derived therapies could also serve to improve response to existing PD treatment. Bacteria play an important role in metabolism of levodopa,84, 85 the key medication used to treat PD symptoms, suggesting that altering this metabolism may impact drug efficacy.
A key strength and unique contribution of our study is its employment of rigorously designed prospective cohorts. Drawing cases and controls from the same population substantially reduces the potential for selection bias in our comparisons. This is in contrast to most previous studies, which relied on a traditional case–control study design that is prone to this type of bias. Furthermore, to our knowledge, ours is the first study that focuses on the microbiome in a well-defined group of patients with prodromal PD. Only one study to date has examined the microbiome of iRBD in addition to patients with PD and healthy controls; analogously to our study, it found substantial overlap and consistent direction in taxa differential in iRBD and PD as compared to healthy controls.14 Our study also included a control group with constipation, allowing us to account for this important aspect of PD and prodromal PD directly. Another key advantage is the use of shotgun metagenomic profiling, allowing us to easily assess both species' taxonomic composition and functional potential.
Our study has several limitations. Although it was substantially larger than most prior investigations of the microbiome and PD, our findings suggest that the microbial changes observed in PD are subtle, and larger studies may be needed to consistently characterize these differences. Our investigation also uniquely included constipated control and PPS groups, but due to the observational nature of the study, it is difficult to distinguish the extent to which the observed dysbiosis gradient can be attributed to changes in transit time versus other factors. It is feasible that microbial changes associated with transit time could have explained some of the observed gradient across our phenotype groups, and the extent to which this is the case should be examined in detail in future studies. However, such effects would have been completely missed in earlier studies without such controls. It is also possible that relatively small changes in the gut microbiome could lead to larger systematic effects when spread over a long period of time, or that they are reflective of as-yet-uncharacterized changes in community function. Further, we identified individuals with probable prodromal PD using low-cost and noninvasive methods, which have the advantage of being scalable to large populations, but are not specific for PD. This could explain the fact the difference in microbiota composition between PPS and controls was attenuated as compared to that observed for clinically diagnosed PD. Finally, the chronic nature and long prodromal phase of PD makes it challenging to differentiate potentially causal microbiome changes from those that may be the result of the disease and associated lifestyle modifications and treatment. To address this, our study included groups with constipation and prodromal PD, allowing us to examine microbiome variation across the spectrum from health to constipation to prodromal to recently onset PD. Despite this advantage, the comparisons are still conducted across and not within participants, and other confounding factors that vary across groups cannot be ruled out. Larger, longitudinal studies that follow healthy participants through the prodromal phase to PD onset are needed to address these limitations and more definitively confirm the findings of our study.
In summary, in this rigorously designed study nested within two prospective epidemiological cohorts, the NHS and the HPFS, we identified overall shifts in the gut microbiome, as well as several specific bacterial taxa and pathways, associated in PD and PPS, and demonstrated that microbiome alterations characteristic of recently onset PD are similar to those in PPS.
Acknowledgments
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work was funded by: R01NS097723 (N. Palacios PI), UM1 CA186107 and U01 CA167552.
Author Contributions
N.P., A.A., and C.H. contributed to the conception and design of the study. N.P., A.A., C.H., K.B., J.W., M.S., and L.M. contributed to the acquisition and analysis of data. N.P., A.A., C.H., and K.B. contributed to drafting the text or preparing the figures.
Potential Conflicts of Interest
C.H. serves on the Scientific Advisory Board for Seres Therapeutics and Empress Therapeutics, who manufacture products that may be affected by this study. The remaining authors have nothing to report.
Open Research
Data Availability
Further information including the procedures to obtain and access data from the Nurses' Health Studies and Health Professionals Follow-up Study is described at https://www.nurseshealthstudy.org/researchers (contact email: [email protected]) and https://sites.sph.harvard.edu/hpfs/for-collaborators/. Metagenomic sequencing data and metadata for the study are available through dbGap (phs002193.v1.p1). Additional detailed statistical model outputs are available through Harvard Dataverse https://doi.org/10.7910/DVN/ZOAWNF.




