Defining a therapeutic target for pallidal deep brain stimulation for dystonia
Tyler Cheung MD
Cedars Sinai Medical Center, Department of Neurology, Los Angeles, CA
Search for more papers by this authorAngela M. Noecker
Department of Biomedical Engineering, Case Western Reserve University, Cleveland, OH
Search for more papers by this authorRon L. Alterman MD
Beth Israel–Deaconess Medical Center, Department of Neurosurgery, Boston, MA
Search for more papers by this authorCameron C. McIntyre PhD
Department of Biomedical Engineering, Case Western Reserve University, Cleveland, OH
Search for more papers by this authorCorresponding Author
Michele Tagliati MD
Cedars Sinai Medical Center, Department of Neurology, Los Angeles, CA
Address correspondence to Dr Tagliati, Department of Neurology, Cedars Sinai Medical Center, 127 South San Vincente Blvd, AHSP Suite A6318, Los Angeles, CA 90048. E-mail: [email protected]Search for more papers by this authorTyler Cheung MD
Cedars Sinai Medical Center, Department of Neurology, Los Angeles, CA
Search for more papers by this authorAngela M. Noecker
Department of Biomedical Engineering, Case Western Reserve University, Cleveland, OH
Search for more papers by this authorRon L. Alterman MD
Beth Israel–Deaconess Medical Center, Department of Neurosurgery, Boston, MA
Search for more papers by this authorCameron C. McIntyre PhD
Department of Biomedical Engineering, Case Western Reserve University, Cleveland, OH
Search for more papers by this authorCorresponding Author
Michele Tagliati MD
Cedars Sinai Medical Center, Department of Neurology, Los Angeles, CA
Address correspondence to Dr Tagliati, Department of Neurology, Cedars Sinai Medical Center, 127 South San Vincente Blvd, AHSP Suite A6318, Los Angeles, CA 90048. E-mail: [email protected]Search for more papers by this authorAbstract
Objective
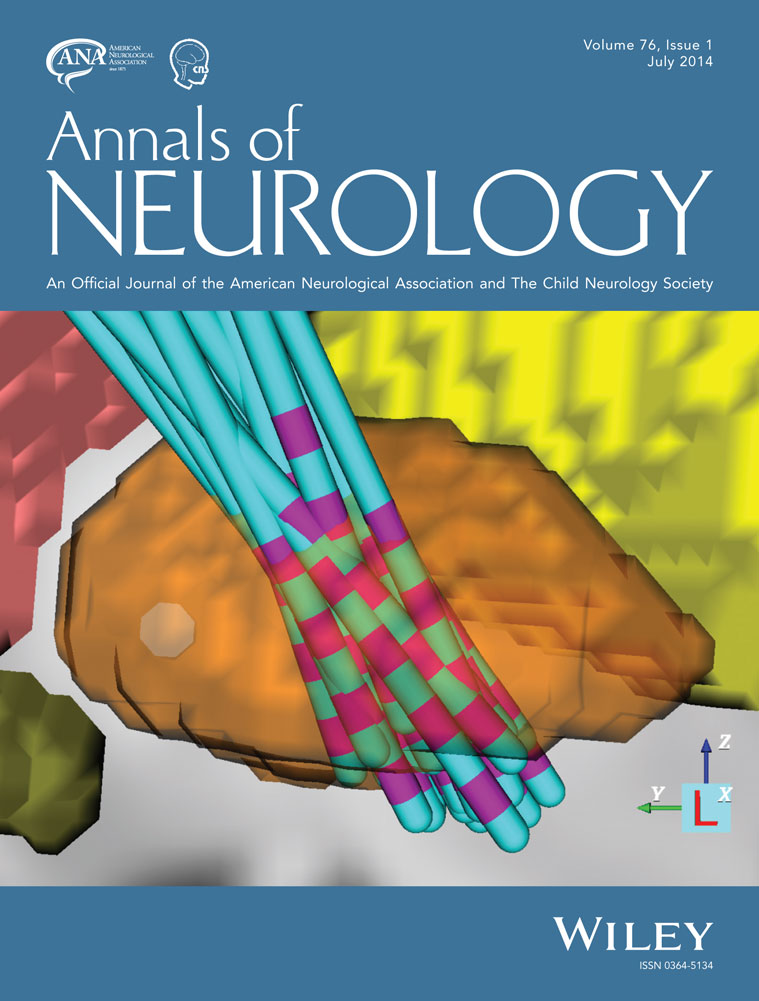
To create a data-driven computational model that identifies brain regions most frequently influenced by successful deep brain stimulation (DBS) of the globus pallidus (GP) for advanced, medication-resistant, generalized dystonia.
Methods
We studied a retrospective cohort of 21 DYT1 primary dystonia patients treated for at least 1 year with bilateral pallidal DBS. We first created individual volume of tissue activation (VTA) models utilizing neuroimaging and postoperative stimulation and clinical data. These models were then combined into a standardized probabilistic dystonia stimulation atlas (DSA). Finally, we constructed a candidate target volume from electrodes demonstrating at least 75% improvement in contralateral symptoms, utilizing voxels stimulated by least 75% of these electrodes.
Results
Pallidal DBS resulted in a median contralateral hemibody improvement of 90% (mean = 83%, standard deviation [SD] = 20) after 1 year of treatment. Individual VTA models of the 42 active electrodes included in the study demonstrated a mean stimulation volume of 501mm3 ([SD] = 284). The resulting DSA showed that areas most frequently stimulated were located squarely in the middle of the posterior GP, with a common target volume measuring 153mm3.
Interpretation
Our results provide a map of the region of influence of therapeutic DBS for dystonia and represent a potential target to refine current methods of surgical planning and stimulation parameters selection. Based on their role in alleviating symptoms, these regions may also provide anatomical and physiological information relevant to disease models of dystonia. Further experimental and clinical studies will be needed to validate their importance. Ann Neurol 2014;76:22–30
References
- 1Ozelius LJ, Bressman SB. Genetic and clinical features of primary torsion dystonia. Neurobiol Dis 2011; 42: 127–135.
- 2Tarsy D, Simon DK. Dystonia. N Engl J Med 2006; 355: 818–829.
- 3Vidailhet M, Vercueil L, Houeto J-L, et al. Bilateral deep-brain stimulation of the globus pallidus in primary generalized dystonia. N Engl J Med 2005; 352: 459–467.
- 4Kupsch A, Benecke R, Müller J, et al. Pallidal deep-brain stimulation in primary generalized or segmental dystonia. N Engl J Med 2006; 355: 1978–1990.
- 5Isaias IU, Alterman RL, Tagliati M. Deep brain stimulation for primary generalized dystonia: long-term outcomes. Arch Neurol 2009; 66: 465–470.
- 6Volkmann J, Wolters A, Kupsch A, et al. Pallidal deep brain stimulation in patients with primary generalised or segmental dystonia: 5-year follow-up of a randomised trial. Lancet Neurol 2012; 11: 1029–1038.
- 7Kupsch A, Tagliati M, Vidailhet M, et al. Early postoperative management of DBS in dystonia: programming, response to stimulation, adverse events, medication changes, evaluations, and troubleshooting. Mov Disord 2011; 26(suppl 1): S37–S53.
- 8Tagliati M, Krack P, Volkmann J, et al. Long-Term management of DBS in dystonia: response to stimulation, adverse events, battery changes, and special considerations. Mov Disord 2011; 26(suppl 1): S54–S62.
- 9Starr PA, Turner RS, Rau G, et al. Microelectrode-guided implantation of deep brain stimulators into the globus pallidus internus for dystonia: techniques, electrode locations, and outcomes. J Neurosurg 2006; 104: 488–501.
- 10Vayssiere N, van der Gaag N, Cif L, et al. Deep brain stimulation for dystonia confirming a somatotopic organization in the globus pallidus internus. J Neurosurg 2004; 101: 181–188.
- 11Hamani C, Moro E, Zadikoff C, et al. Location of active contacts in patients with primary dystonia treated with globus pallidus deep brain stimulation. Neurosurgery 2008; 62(3 suppl 1): 217–223; discussion 223–225.
- 12Tisch S, Zrinzo L, Limousin P, et al. Effect of electrode contact location on clinical efficacy of pallidal deep brain stimulation in primary generalised dystonia. J Neurol Neurosurg Psychiatry 2007; 78: 1314–1319.
- 13Vasques X, Cif L, Hess O, et al. Prognostic value of globus pallidus internus volume in primary dystonia treated by deep brain stimulation. J Neurosurg 2009; 110: 220–228.
- 14Hashimoto T, Elder CM, Okun MS, et al. Stimulation of the subthalamic nucleus changes the firing pattern of pallidal neurons. J Neurosci 2003; 23: 1916–1923.
- 15Miocinovic S, Parent M, Butson CR, et al. Computational analysis of subthalamic nucleus and lenticular fasciculus activation during therapeutic deep brain stimulation. J Neurophysiol 2006; 96: 1569–1580.
- 16Johnson MD, Zhang J, Ghosh D, et al. Neural targets for relieving parkinsonian rigidity and bradykinesia with pallidal deep brain stimulation. J Neurophysiol 2012; 108: 567–577.
- 17Johnson MD, McIntyre CC. Quantifying the neural elements activated and inhibited by globus pallidus deep brain stimulation. J Neurophysiol 2008; 100: 2549–2563.
- 18Dietz J, Noecker AM, McIntyre CC, et al. Stimulation region within the globus pallidus does not affect verbal fluency performance. Brain Stimul 2013; 6: 248–253.
- 19Frankemolle AMM, Wu J, Noecker AM, et al. Reversing cognitive-motor impairments in Parkinson's disease patients using a computational modelling approach to deep brain stimulation programming. Brain 2010; 133(pt 3): 746–761.
- 20Butson CR, Cooper SE, Henderson JM, et al. Probabilistic analysis of activation volumes generated during deep brain stimulation. Neuroimage 2011; 54: 2096–2104.
- 21Alterman RL, Tagliati M. Deep brain stimulation for torsion dystonia in children. Childs Nerv Syst 2007; 23: 1033–1040.
- 22Tagliati M, Shils J, Sun C, Alterman R. Deep brain stimulation for dystonia. Expert Rev Med Devices 2004; 1: 33–41.
- 23Burke RE, Fahn S, Marsden CD, et al. Validity and reliability of a rating scale for the primary torsion dystonias. Neurology 1985; 35: 73–77.
- 24Isaias IU, Alterman RL, Tagliati M. Outcome predictors of pallidal stimulation in patients with primary dystonia: the role of disease duration. Brain 2008; 131(pt 7): 1895–1902.
- 25Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 2002; 17: 825–841.
- 26Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal 2001; 5: 143–156.
- 27Viola P, Wells WM III. Alignment by maximization of mutual information. Int J Comput Vision 1997; 24: 137–154.
- 28Makris N, Goldstein JM, Kennedy D, et al. Decreased volume of left and total anterior insular lobule in schizophrenia. Schizophr Res 2006; 83: 155–171.
- 29Goldstein JM, Seidman LJ, Makris N, et al. Hypothalamic abnormalities in schizophrenia: sex effects and genetic vulnerability. Biol Psychiatry 2007; 61: 935–945.
- 30Frazier JA, Chiu S, Breeze JL, et al. Structural brain magnetic resonance imaging of limbic and thalamic volumes in pediatric bipolar disorder. Am J Psychiatry 2005; 162: 1256–1265.
- 31Desikan RS, Ségonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006; 31: 968–980.
- 32Yelnik J, Damier P, Demeret S, et al. Localization of stimulating electrodes in patients with Parkinson disease by using a three-dimensional atlas-magnetic resonance imaging coregistration method. J Neurosurg 2003; 99: 89–99.
- 33Butson CR, Cooper SE, Henderson JM, McIntyre CC. Patient-specific analysis of the volume of tissue activated during deep brain stimulation. Neuroimage 2007; 34: 661–670.
- 34Nowinski WL, Belov D, Benabid A-L. An algorithm for rapid calculation of a probabilistic functional atlas of subcortical structures from electrophysiological data collected during functional neurosurgery procedures. Neuroimage 2003; 18: 143–155.
- 35Grabner G, Janke AL, Budge MM, et al. Symmetric atlasing and model based segmentation: an application to the hippocampus in older adults. Med Image Comput Comput Assist Interv 2006; 9(pt 2): 58–66.
- 36Coubes P, Cif L, El Fertit H, et al. Electrical stimulation of the globus pallidus internus in patients with primary generalized dystonia: long-term results. J Neurosurg 2004; 101: 189–194.
- 37Moro E, Piboolnurak P, Arenovich T, et al. Pallidal stimulation in cervical dystonia: clinical implications of acute changes in stimulation parameters. Eur J Neurol 2009; 16: 506–512.
- 38Alterman RL, Shils JL, Miravite J, Tagliati M. Lower stimulation frequency can enhance tolerability and efficacy of pallidal deep brain stimulation for dystonia. Mov Disord 2007; 22: 366–368.
- 39Alterman RL, Miravite J, Weisz D, et al. Sixty hertz pallidal deep brain stimulation for primary torsion dystonia. Neurology 2007; 69: 681–688.
- 40Goto S, Mita S, Ushio Y. Bilateral pallidal stimulation for cervical dystonia. An optimal paradigm from our experiences. Stereotact Funct Neurosurg 2002; 79: 221–227.
- 41Kumar R, Dagher A, Hutchison WD, et al. Globus pallidus deep brain stimulation for generalized dystonia: clinical and PET investigation. Neurology 1999; 53: 871–874.
- 42Velez-Lago FMk, Oyama G, Foote KD, et al. Low-frequency deep brain stimulation for dystonia: lower is not always better. Tremor Other Hyperkinet Mov (N Y) 2012; 2.
- 43Koss AM, Alterman RL, Tagliati M, Shils JL. Calculating total electrical energy delivered by deep brain stimulation systems. Ann Neurol 2005; 58: 168; author reply 168–169.
- 44Cif L, Ruge D, Gonzalez V, et al. The influence of deep brain stimulation intensity and duration on symptoms evolution in an OFF stimulation dystonia study. Brain Stimul 2012; 6: 500–505.
- 45Ruge D, Cif L, Limousin P, et al. Shaping reversibility? Long-term deep brain stimulation in dystonia: the relationship between effects on electrophysiology and clinical symptoms. Brain 2011; 134(pt 7): 2106–2115.
- 46Isaias IU, Volkmann J, Kupsch A, et al. Factors predicting protracted improvement after pallidal DBS for primary dystonia: the role of age and disease duration. J Neurol 2011; 258: 1469–1476.
- 47Cheung T, Zhang C, Rudolph J, et al. Sustained relief of generalized dystonia despite prolonged interruption of deep brain stimulation. Mov Disord 2013; 28: 1431–1434.
- 48McIntyre CC, Grill WM, Sherman DL, Thakor NV. Cellular effects of deep brain stimulation: model-based analysis of activation and inhibition. J Neurophysiol 2004; 91: 1457–1469.
- 49Hendrix CM, Vitek JL. Toward a network model of dystonia. Ann N Y Acad Sci 2012; 1265: 46–55.
- 50Johnson MD, Miocinovic S, McIntyre CC, Vitek JL. Mechanisms and targets of deep brain stimulation in movement disorders. Neurotherapeutics 2008; 5: 294–308.
- 51Ranck JB Jr. Which elements are excited in electrical stimulation of mammalian central nervous system: a review. Brain Res 1975; 98: 417–440.
- 52Lenglet C, Abosch A, Yacoub E, et al. Comprehensive in vivo mapping of the human basal ganglia and thalamic connectome in individuals using 7T MRI. PLoS One 2012; 7: e29153.