Anatomical distribution of endometriosis: A cross-sectional analysis of transvaginal ultrasound in symptomatic patients
Abstract
Purpose
The anatomical distribution of deep endometriosis (DE) is essential in treating patients with symptoms associated with the disease. There is an evidence gap in correlating clinical features and symptoms with disease patterns. The study aimed at determining DE anatomic distribution based on advanced transvaginal ultrasound and describe the relationship with symptoms obtained with the World Endometriosis Foundation Questionnaire.
Methods
A cross-sectional study included 549 ultrasound results and 370 questionnaire responses between July 2018 and January 2021. Descriptive statistics are presented. Continuous variables were compared by a simple t-test and ANOVA and categorical variables by the chi-squared test. Logistic regression and R2 values summarised the relationship between positive ultrasound and possible predictor variables (software SAS version 9.4).
Results
The anatomical locations with signs of endometriosis on ultrasound were the right uterosacral ligament (USL) 23.3% (n = 128), left USL 21.3% (n = 117) and bowel 19.1% (n = 105). Endometriomas in the right and left ovaries (14%, n = 77, and 14.7%, n = 81 respectively), superficial endometriosis in 15.5% (n = 85), torus uterinus in 11.7% (n = 64), Pouch of Douglas (POD) in 9.7% (n = 53), rectovaginal septum in 4.2% (n = 23), vaginal fornix in 3.5% (n = 19). A negative ‘sliding-sign’ was noted in 25.3% (n = 139), and ovarian medial immobility was noted frequently (left 20.2%, n = 111 and right 16.9%, n = 93). Dyspareunia, dysmenorrhoea, infertility and family history were associated with endometriosis lesions (P < 0.05). Prediction models based on symptomatology presented low discriminatory power.
Discussion
This large real-life cohort associating the description of the anatomical distribution of endometriosis as seen on advanced TVS in symptomatic patients confirmed that uterosacral ligaments, torus uterinus, ovaries and bowel represent the most common anatomical sites of endometriosis. Also, the dynamic abnormalities elicited via ultrasound, such as the uterus ‘sliding-sign’ and ovarian mobility, remain common. The knowledge of the general locations of identifiable endometriosis on ultrasound and the dynamic abnormalities is essential to sonologists and sonographers in implementing advanced TVS protocols to detect endometriosis. In addition, the different presentations of dyspareunia can be associated with USL and bowel endometriosis. Subfertility might also be associated with USL, ovarian and bowel endometriosis. Nevertheless, prediction models showed suboptimal results.
Conclusions
Endometriosis is mainly distributed in USLs, bowel and ovaries. POD obliteration is frequent. Symptoms can be associated with anatomic locations; however, prediction models showed low clinical applicability.
Introduction
The definition of endometriosis from the World Endometriosis Society shows that it is an inflammatory disease process characterised by the presence of endometrial-like tissue outside the usual site in the uterus, which can be associated with many forms of symptoms, such as pelvic pain and infertility.1 The need for tissue sampling solely for diagnosing purposes might obstruct the care pathway; therefore, less invasive diagnostic alternatives are preferable to reduce the excessive diagnostic delay on endometriosis, which is still unacceptable for 8 years, helping the patients expedite their treatments.2
Establishing the diagnosis of endometriosis via ultrasound has been significantly improved with advanced transvaginal ultrasound (TVS) standardised protocols as per the International Deep Endometriosis Analysis (IDEA) consensus, and the anatomical distribution of deep endometriosis (DE) was found to be a crucial aspect to be addressed because it can influence the diagnostic accuracy of the ultrasound.3-5 Moreover, when surgery to excise endometriosis is considered during the patient's treatment pathway, understanding the affected sites directly impacts surgical planning and outcomes.6 However, information regarding the detailed anatomical distribution of DE through non-invasive methods such as ultrasound is still scarce.
Attempts have been made to identify the similarities and differences of the anatomical distribution; nevertheless, the conclusions lack a clear justification and were primarily taken after surgical procedures and did not use ultrasound as the main diagnostic tool.7
The association of the vast clinical presentations of endometriosis with the actual prevalence of pelvic lesions is a challenging obstacle, remaining a current research priority.8 In that sense, the World Endometriosis Research Foundation (WERF) proposed within the Endometriosis Phenome (and Biobanking) Harmonisation Project (EPHect) the use of the WERF EPHect Clinical Questionnaire as an effort to lead research centres towards the production of better-quality evidence.9 Furthermore, the knowledge and the description of the anatomy and the anatomical distribution of the disease also play an essential role in the learning curve for detecting endometriosis via ultrasound, particularly regarding smaller lesions.10
The primary objective of this study was to determine the anatomical distribution of endometriosis as seen on advanced TVS following the IDEA consensus in an experienced specialised service. The secondary objective is to create prediction models of the lesions’ distribution based on clinical symptoms obtained using the ‘WERF EPHect Clinical Questionnaire’.
Methodology
The study consists of a cross-sectional study of women who attended a specialist tertiary centre, the Acute Gynaecology, Early Pregnancy and Advanced Endoscopy Surgery Unit, at the Nepean Hospital, and OMNI Gynaecological Ultrasound and Care, St Leonards, NSW, Australia, between July 2018 and January 2021. The patients were referred for endometriosis-related symptoms, including dysmenorrhoea, dyschezia, dyspareunia, non-cyclical pelvic pain and chronic pelvic pain (CPP). This study defined CPP as lower abdominal or pelvic pain lasting longer than 6 months, following a continuous or intermittent course, and not necessarily related to menstruation or sexual intercourse.11 Exclusion criteria were women with malignancies, undiagnosed adnexal lesions other than endometrioma, pregnancy and ovarian insufficiency. All patients underwent an advanced TVS, which was performed by experienced sonologists (GC and ML), in accordance with the IDEA consensus statement.5
- Routine or basic evaluation of the uterus and adnexa, including signs of adenomyosis and endometriomas;
- Evaluation of the ultrasound ‘soft markers’, including site-specific tenderness and ovarian mobility;
- Assessment of Pouch of Douglas (POD) status with real-time ultrasound-based ‘sliding-sign’;
- Assessment of DE nodules in the anterior and posterior pelvic compartments.
The experienced sonologist examination was considered as the reference standard for endometriosis identification via ultrasound.12 In addition, all the patients were asked to complete the recommended tool of clinical symptoms assessment, consisting of the standard version of the clinical WERF EPhect questionnaire that comprehends the fundamental and detailed aspects of the patients' history and possible symptomatology. The WERF questionnaire was the data collection instrument of choice, in line with WERF recommendations for endometriosis research standardisation.13 Responses were collected via the SurveyMonkey® platform. The full version of the questionnaire is available at: https://endometriosisfoundation.org/. No initial sample size was calculated as this is a cross-sectional study.
Data descriptions were by mean and standard deviation (SD); median, 25th and 75th percentiles (interquartile range – IQR); minimum to maximum, for continuous variables; and counts and proportions expressed as percentages for categorical variables. Comparison of continuous variables was by simple t-test and ANOVA and categorical variables by the chi-squared test. P < 0.05 was considered statistically significant. The relationship between positive ultrasound and possible predictor variables was summarised by logistic regression and R2 values. Within the prediction modelling, the discrimination for the clinical variables of interest associated with positive ultrasound was by logistic regression, with results summarised by box plots, and the area under the curve for the receiver operating characteristic (ROC) curve. The software SAS version 9.4 was used.
Results
It was possible to retrieve 549 complete advanced TVS data during the study period. One hundred thirty-five patients presented with ovarian and DE; 107 patients with lesions identified but with no ovarian or bowel disease and the ‘sliding sign’ positive; and 307 patients with no lesions seen on ultrasound. The rationale for this grouping follows the proposal of considering endometriosis in different presentations.12 It was possible to retrieve questionnaire responses from 370 patients. The mean age of the patients in this cohort was 33.44 years (SD 8.24), and patients with ovarian and DE are significantly older than patients with posterior compartment positive for lesions but with the ‘sliding-sign’ positive. Other demographic characteristics are depicted in Table 1.
| Ovarian and deep endometriosis | Posterior compartment positive but sliding sign positive | Negative ultrasound | P | |
|---|---|---|---|---|
| Age | ||||
| N | 135 | 107 | 307 | <0.0001 |
| Mean (SD) | 39.4 (7.1) | 33.6 (7.5) | 34.5 (8.2) | |
| Median (IQR) | 39 (33 to 44) | 32 (28 to 39) | 34 (28 to 41) | |
| Min to max | 23 to 56 | 19 to 54 | 18 to 58 | |
| BMI | ||||
| N | 85 | 67 | 186 | 0.22 |
| Mean (SD) | 26.9 (6.4) | 25.3 (5.8) | 25.5 (7.0) | |
| Median (IQR) | 25.7 (22.3 to 30.8) | 24.3 (20.8 to 28.2) | 23.1 (20.6 to 28.7) | |
| Min to max | 16.4 to 45.9 | 16.9 to 46.5 | 16.4 to 55.5 | |
| BMI category N (%) | ||||
| N | 85 | 67 | 186 | 0.59 |
| Normal weight | 37 (43.5) | 39 (58.2) | 100 (53.8) | |
| Obesity 1 | 14 (16.5) | 9 (13.4) | 24 (12.9) | |
| Obesity 2 | 7 (8.2) | 4 (6.0) | 7 (3.8) | |
| Obesity 3 | 4 (4.7) | 1 (1.5) | 10 (5.4) | |
| Overweight | 20 (23.5) | 12 (17.9) | 34 (18.3) | |
| Under weight | 3 (3.5) | 2 (3.0) | 11 (5.9) | |
| Ethnicity | ||||
| N | 85 | 67 | 190 | 0.28 |
| Asian/Pacific/Oriental | 19 (22.4) | 7 (10.4) | 22 (11.6) | |
| Indigenous | 3 (3.5) | 1 (1.5) | 6 (3.2) | |
| Mixed race | 7 (8.2) | 3 (4.5) | 10 (5.3) | |
| Other | 6 (7.1) | 4 (6.0) | 14 (7.4) | |
| White | 50 (58.8) | 52 (77.6) | 138 (72.6) | |
| Education level | ||||
| N | 86 | 67 | 194 | 0.34 |
| College | 7 (8.1) | 3 (4.5) | 11 (5.7) | |
| High school | 16 (18.6) | 19 (28.4) | 34 (17.5) | |
| Other | 0 (0.0) | 2 (3.0) | 5 (2.6) | |
| Postgraduate | 21 (24.4) | 14 (20.9) | 34 (17.5) | |
| Primary school | 0 (0.0) | 0 (0.0) | 3 (1.5) | |
| TAFE | 14 (16.3) | 13 (19.4) | 33 (17.0) | |
| University | 28 (32.6) | 16 (23.9) | 74 (38.1) | |
- BMI, body mass index; IQR, interquartile range; SD, standard deviation.
Anatomical distribution of sonographic signs of endometriosis
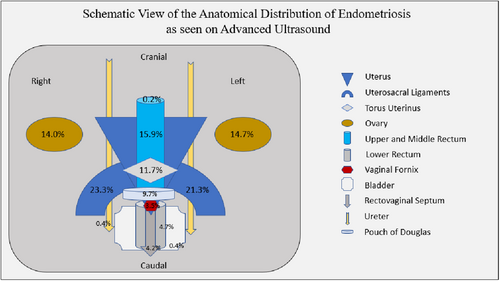
The most prevalent anatomical locations with signs of endometriosis on ultrasound were right uterosacral ligament (USL) 23.3% (n = 128), left USL 21.3% (n = 117) and bowel 19.1% (n = 105). Multifocal USL disease was not observed in this cohort. Signs of torus uterinus endometriosis were noted in 11.7% (n = 64). An anteverted and retroflexed uterus was identified in 7.1% (n = 39) of the patients. Signs of vaginal fornix endometriosis were noted in 3.5% (n = 19) of the patients. POD nodules were found in 9.7% of the patients (n = 53).
Upper and middle rectum nodules were more common than lower rectum nodules, 15.9% (n = 85) against 4.7% (n = 26), respectively, and the rectovaginal septum (RVS) harboured lesions in only 4.2% (n = 23) of the patients. The mean size of bowel nodules located in the upper rectum was 20.42 mm (SD 12.6), 8.49 mm (SD 4.84), 12.19 mm (5.66), and in the lower rectum it was 13.81 mm (SD 6.24), 8.31 mm (SD 3.84) and 10.73 mm (SD 6.67) for sagittal (SG), anteroposterior (AP) and transverse (Tr) measurements respectively. Multifocal bowel disease was found in 2.4% (n = 13) of the cases. Rectum–sigmoid junction endometriosis was observed in 0.2% (n = 1) of the case.
Also common was the presence of endometriomas in the right and left ovaries (14%, n = 77, and 14.7%, n = 81 respectively). The mean size of the cysts in the right ovary was 30.17 mm (SD 24.36), 26.82 mm (SD 23.25) and 29.35 mm (SD 22.15) for SG, AP and Tr measurements, respectively, and in the left ovary it was 32.19 mm (SD 32.29), 27.41 mm (SD 22.2) and 30.12 mm (SD 25.19) for SG, AP and Tr measurements respectively. In addition, signs of superficial endometriosis were observed in 15.5% (n = 85) of the patients.
The most common abnormal dynamic feature observed was a negative sliding sign in 25.3% (n = 139). Ovarian medial immobility was also common (left 20.2%, n = 111 and right 16.9%, n = 93).
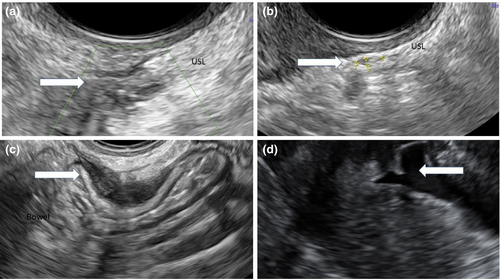
The anatomical distribution is summarised in Figure 1, and examples of the sonographic appearance of lesions are shown in Figure 2.
WERF EPHect questionnaire responses and association analysis
Responses to questions related to the clinical domains dysmenorrhoea, dyspareunia, general pelvic pain, dysuria, dyschezia, infertility and family history were selected for analysis. A summary of the results with the statistical association and the number of answers to the selected questions is shown in Table 2 Therefore, we carefully selected the most meaningful and complete answers with the potential for associations with endometriosis lesion detection. To identify possible associations between the anatomical distribution of the lesions with the responses to the clinical questionnaire, we chose to test the statistical association of the most frequent anatomical locations of sonographic signs of endometriosis (USL left and right, endometriomas left and right and bowel nodules) with the selection of the clinical features divided into the different domains.
| Variable | Mean (SD) | Mean (SD) | N/N (%) | N/N (%) | Simple t-test – Difference (95% CI) | |||
|---|---|---|---|---|---|---|---|---|
| Anatomical location | Clinical domain | aTVS endo signs present | aTVS endo signs absent |
aTVS endo signs present |
aTVS endo signs absent |
|||
| LUSL | Infertility | Longest period of trying to get pregnant (months) |
32.6 (50.0) N = 41 |
17.0 (27.9) N = 142 |
15.60 (3.69 to 27.52) P = 0.01 | |||
| Tried for >6 months to get pregnant |
29 (38.7) N = 75 |
69 (24.1) N = 286 |
P = 0.01 | |||||
| RUSL | Infertility | Longest period of trying to get pregnant (months) |
33.4 (54.5) N = 54 |
15.1 (19.3) N = 129 |
18.23 (7.46 to 28.99) P = 0.001 |
|||
| Tried for >6 months to get pregnant |
32 (36.8) N = 87 |
66 (24.1) N = 274 |
P = 0.02 | |||||
| Dyspareunia | Age pelvic pain during intercourse started |
28.0 (6.8) N = 65 |
24.9 (7.5) N = 208 |
3.16 (1.11 to 5.20) P = 0.003 |
||||
| Age pelvic pain during intercourse was worst |
31.3 (8.3) N = 38 |
28.1 (7.8) N = 151 |
3.29 (0.47 to 6.11) P = 0.02 |
|||||
| Severity of pelvic pain with intercourse at its worst |
5.7 (2.6) N = 70 |
6.6 (2.5) N = 212 |
−0.89 (−1.57 to −0.21) P = 0.01 |
|||||
| LO endometrioma | Dyspareunia | Severity of pelvic pain with intercourse at its worst |
5.6 (2.6) N = 35 |
6.5 (2.5) N = 246 |
−0.94 (−1.84 to 0.05) P = 0.04 |
|||
| RO endometrioma | Infertility | Longest period of trying to get pregnant (months) |
40.9 (66.4) N = 29 |
16.7 (22.8) N = 154 |
24.28 (10.89 to 37.66) P = 0.0004 |
|||
| Tried for >6 months to get pregnant |
22 (47.8) N = 46 |
76 (24.1) N = 315 |
P = 0.0007 | |||||
| Dysmenorrhoea | Age (years) of worst pelvic period pain |
28.3 (9.5) N = 44 |
25.3 (8.6) N = 299 |
2.93 (0.15 to 5.71) P = 0.04 |
||||
| Dyspareunia | Age pelvic pain during intercourse started |
28.6 (7.2) N = 35 |
25.2 (7.4) N = 238 |
3.39 (0.78 to 6.01) P = 0.01 |
||||
| Age pelvic pain during intercourse was worst |
32.6 (6.7) N = 23 |
28.2 (8.0) N = 166 |
4.43 (0.99 to 7.88) P = 0.01 |
|||||
| Bowel nodules | Demographic – Family history | Mother diagnosed with CPP |
16 (34.0) N = 47 |
43 (18.7) N = 228 |
P = 0.02 | |||
| Infertility | Longest period of trying to get pregnant (months) |
37.5 (58.7) N = 39 |
15.9 (22.5) N = 144 |
21.58 (9.65 to 33.52) P = 0.001 |
||||
| Tried for >6 months to get pregnant |
26 (40.6) N = 64 |
72 (24.2) N = 297 |
P = 0.008 | |||||
| Dysmenorrhoea | Age (years) of worst pelvic period pain |
28.1 (9.1) N = 61 |
25.2 (8.6) N = 282 |
2.93 (0.51 to 5.36) P = 0.02 |
||||
| Dyspareunia | Age pelvic pain during intercourse was worst |
32.5 (7.6) N = 28 |
28.1 (7.9) N = 161 |
4.44 (1.28 to 7.61) P = 0.006 |
||||
| Interrupted vaginal intercourse/penetration because of pelvic pain in the last 12 months |
20 (62.5) N = 32 |
137 (78.7) N = 174 |
P = 0.047 | |||||
| Avoided vaginal intercourse/penetration because of pelvic pain in the last 12 months |
19 (59.4) N = 32 |
138 (79.3) N = 174 |
P = 0.01 |
- aTVS, advanced transvaginal ultrasound; CI, confidence interval; CPP, chronic pelvic pain; LO, left ovary; LUSL, left uterosacral ligament; RO, right ovary; RUSL, right uterosacral ligament; SD, standard deviation.
Dyspareunia
There is evidence of a possible association between bowel nodules and both ‘interrupted’ and ‘avoided’ vaginal intercourse/penetration because of pelvic pain (P = 0.05 and P = 0.01 respectively). Higher age is possibly associated with ‘when pelvic pain during intercourse started’ for those with USL lesions (right, P = 0.003) and ovarian endometriomas (right, P = 0.01). The same higher age possible association can be noted with ‘when pelvic pain during intercourse was worst’ for those with USL lesions (right,P = 0.02), ovarian endometriomas (left, P = 0.04; right, P = 0.01) or bowel nodules (P < 0.001). For those with USL lesions (right, P = 0.01) and ovarian endometriomas (left, P = 0.04), the ‘severity of pelvic pain with intercourse as its worse’ was potentially lower considering a subjective visual analogue scale. The same phenomenon occurred for those with ovarian endometriomas (left, P = 0.04).
Dysmenorrhoea
There is evidence that the age of ‘worst pelvic period pain’ is possibly higher for those with ovarian endometriomas (right, P = 0.04) or bowel nodules (P = 0.02).
Family history
There is evidence of an association between bowel nodules and family history (mother) diagnosed with CPP (P = 0.02).
Infertility
There is evidence of a possible association between USL lesions (left and right), ovarian endometriomas (right) and bowel nodules and ‘trying to get pregnant for >6 months’ (P = 0.01, P = 0.02, P = 0.007, P = 0.008 respectively). In addition, there is evidence of a possibly more extended period of trying to get pregnant for those with USL lesions (left and right), ovarian endometriomas (right) or bowel nodules (P = 0.01, P = 0.001, P < 0.001, P = 0.001 respectively).
Prediction modelling for ultrasound detection of lesions based on clinical symptoms
Given the significant number of available variables from the advanced TVS and the WERF EPHect questionnaire, it would not be possible to develop prediction models individually using all the available features. Therefore, we proposed analysis with the groups ovarian and DE, positive ultrasound (except for the first group) and negative ultrasound. From the clinical symptom questionnaire, we selected the responses to the clinical features that were statistically associated with detecting lesions via ultrasound.
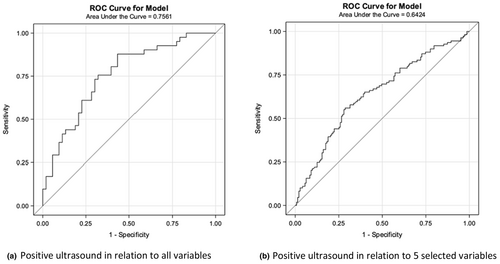
In the univariate analysis of nine potential clinical predictors, none were associated with a positive ultrasound (Table S1). Therefore, the stepwise selection was used to identify the prognostic factors of a positive ultrasound. A significance level of 0.3 was required for a variable to enter the model and a significance level of 0.35 to stay in the model. This resulted in a model with five predictors (Table S2). With all other above variables in the model, ‘increased age when pelvic pain during intercourse or in the 24 hours following vaginal sexual intercourse/penetration started’ and ‘decreased severity of pelvic pain with vaginal intercourse/penetration at its worst ever’ are associated with positive ultrasound. However, even though the model with all nine predictors was the best predictor of a positive ultrasound, all models have low discriminatory power. The ROC curves are shown in Figure 3.
Discussion
To the best of our knowledge, our work is one of the largest real-life cohorts associating the accurate description of the anatomical distribution of endometriosis as seen on advanced TVS with patients' symptoms to date.
Uterosacral ligaments, torus uterinus, ovaries and bowel represent the most common anatomical sites of endometriosis in our study, in line with previous publications associating lesions’ location and ultrasound-based diagnosis.14, 15 Also, the dynamic abnormalities elicited via ultrasound, such as the uterus ‘sliding-sign’ and ovarian mobility, remain notably common.16 The knowledge of the general locations of identifiable endometriosis on ultrasound is essential to sonologists and sonographers in implementing advanced TVS protocols to detect endometriosis. The site and number of lesions can potentially influence the diagnostic accuracy of the ultrasound examination.4
In comparison with the advanced TVS, when performing a general gynaecological ultrasound is expected to detect, most likely, only the conditions involving ovarian endometriosis in the form of endometriomas, representing 14.7% in the presented cohort. For example, applying the standardised advanced TVS technique allowed the detection of a negative ‘sliding-sign’ in 25.3% and of bowel involvement in 19.1% of the patients in our cohort. This discrepancy brings attention to the potentially significant number of patients with a complex assessment of the pelvic posterior compartment disease that could be misdiagnosed and potentially undertreated if they have undergone surgical treatments without the preoperative knowledge of the bowel disease or obliteration of the POD. It has also been demonstrated in other settings that the detection of DE could increase significantly with the recognition of dynamic ultrasonographic abnormalities.17 Therefore, we advocate using the advanced TVS technique based on the IDEA consensus for every patient with suspected endometriosis, also in line with the notion that the ultrasound should be the first-line imaging modality for the diagnosis of endometriosis.12
Our results also agree with other publications about the low prevalence of RVS and anterior compartment lesions.18 The presence of objective nodules in the anterior compartment was rare and absent in the ureter in this cohort. However, it is vital to highlight the presence of signs of hydronephrosis even without demonstrating measurable nodularity, increasing the importance of following the systematic scanning protocol even in the absence of evident lesions. Therefore, special attention should be given to the description of the lesions when endometriosis is suspected in less prevalent anatomical sites.14
When considering the symptomatology retrieved via the WERF EPHect questionnaire, it was possible to identify that the different presentations of dyspareunia can be associated with USL and bowel endometriosis. Subfertility might also be associated with USL, ovarian and bowel endometriosis. Moreover, a family history of the mother diagnosed with CPP might be associated with bowel endometriosis. The higher age associated with pain symptoms might indicate that endometriosis is a progressive inflammatory condition.19 However, in our study, the heterogeneity of the patients' responses and the magnitude of the statistical associations were small. Consequently, it was impossible to build any symptom-based prediction model for the lesions' anatomical location that could be clinically useful. Our results add to the notion that clinical diagnosis, although potentially promising, has not yet achieved optimal performance as a ‘stand-alone’ tool to predict the location and extension of the disease, possibly related to the heterogeneity of the disease itself.20
It is imperative for imaging providers to be mindful that pelvic pain, in general, is not a normal condition and that the detection of any pelvic ultrasound abnormalities was noted in more than a quarter of the patients with pelvic pain in our cohort. Also essential is to remind the patients and other clinicians that the absence of ultrasound-measurable disease does not exclude the possibility of endometriosis. Moreover, endometriosis cannot be ruled out even after a negative diagnostic laparoscopy.21
One of the significant limitations found in our study involves the application of a detailed self-responding questionnaire based on the WERF recommendations, where patients may be exact in describing symptoms when present; however, they may underestimate the importance of actively responding ‘no’ to the symptoms that are not impacting their daily activities. Our cohort shows that the number of patients that responded to some of the questions is much smaller than the total number of patients evaluated via ultrasound. That created a systematic error, making unfeasible the assumption that ‘no answer’ definitively means ‘no symptoms’. We would like to point out the high level of detail proportioned by the WERF EPHect questionnaire and the source of unbiased answers when not involving health professionals directly collecting the data. However, researchers should be mindful that the use of this tool might require specific attention on how to mitigate the consequences of possible missing data. In addition, the retrospective collection of the ultrasound data and the cohort representing an experienced service performing advanced TVS, although showing the real-life application of the IDEA consensus, is not suitable for cause-and-effect assumptions and might not be entirely reproducible in all settings. Moreover, this cohort did not undergo systematic tissue collection for pathological identification of endometriosis. However, we strongly believe that advanced TVS should be the primary diagnostic tool in endometriosis, consequently avoiding unnecessary procedures.12
In conclusion, the present study presented the anatomical distribution of endometriosis as seen on advanced TVS as a clear picture of the real-life experience of diagnosing endometriosis via ultrasound. USLs, bowel and ovaries harbour most of the lesions, and POD obliteration and ovarian immobility are frequent. Dyspareunia and family history of endometriosis are essential aspects associated with identifiable lesions; however, symptomatology was not predictive of the anatomical location of the disease. Using more precise and automated symptomatology data collection might be helpful in future to build symptom-based prediction models of endometriosis lesions. This study provides additional evidence that may facilitate the dissemination of awareness of the role of the advanced TVS between endometriosis diagnostic centres, aiming to promote the journey of individuals designated female at birth towards effective management of endometriosis and CPP.
Acknowledgements
None.
Authorship statement
RMR: Conceptualisation; Data curation; Formal analysis; Writing – Original Draft Preparation; Writing – Review & Editing; ML: Conceptualisation; Formal analysis; Writing – Review & Editing; AE: Formal analysis; Writing – Review & Editing. MA: Writing – Review & Editing; GC: Conceptualisation; Formal analysis; Writing – Review & Editing; Supervision. All authors are in agreement with the content of the submitted manuscript.
Conflict of Interest
Rodrigo Manieri Rocha and Allie Eathorne report nothing to disclose. Mathew Leonardi reports grants from the Australian Women and Children's Research Foundation, outside the submitted work. George Condous reports personal fees from Roche, personal fees from GE Healthcare and grants from the Australian Women and Children's Research Foundation, outside the submitted work. Mike Armour reports grants from Metagenics and Spectrum outside the submitted work.
Ethical Approval
The project was approved by the Nepean Blue Mountains Local Health District Human Research Ethics Committee (ETH 11470).




