Sex differences in scent-marking in captive red-ruffed lemurs
Abstract
Primate chemical communication remains underappreciated, as primates are considered to rely on other sensory modalities. However, various lines of evidence suggest that olfaction plays an important role in primate societies, including the conspicuous scent-marking behavior of many strepsirrhines and callitrichines. Although lemurs typically show scent-marking, little is known about this behavior in red-ruffed lemurs (Varecia variegata rubra). We combined behavioral observations and semiochemistry analyses to improve our understanding of scent-marking in two captive troops housed at Dudley and Twycross zoos (UK). We collected olfactory behavioral observations by focusing on two family troops (N = 7) for 132 hr. We investigated the volatile compounds of ano-genital scent-marks using solid-phase microextraction and gas chromatography-mass spectrometry and compared volatile chemical profiles with features of the signaller. Males scent-marked most frequently and predominantly in specific meaningful areas of the enclosure, while within females the occurrence of scent-marking was related to their age. We found behavioral sexual dimorphism, with male predominantly depositing secretions via neck and mandible glands and females via ano-genital glands. We identified a total of 32 volatile components of ano-genital gland secretion, including compounds that have already been found in other mammals as sex pheromones and cues to fitness, in ano-genital scent-marks spontaneously left on filter paper by adult females. Our findings suggest that red-ruffed lemurs might use scent-marking to convey information about sex and female age, with male neck-marking behavior playing defensive territorial functions and ano-genital marking related to socio-sexual communication.
1 INTRODUCTION
Communication plays a fundamental role within animal societies, especially for species displaying complex social systems. The ultimate goal of communication is to spread information that influences the behaviors of receivers (Seyfarth & Cheney, 2003). Animals can use various sensory modalities to transfer their messages to other individuals. In particular, olfactory communication is exhibited by several mammal species (reviewed in Scordato & Drea, 2007), such as rodents (e.g., Hurst, Robertson, Tolladay, & Beynon, 1998; Roberts, 2007), but also by reptiles (e.g., Müller-Schwarze, 2006) and birds (e.g., Leclaire, Strandh, Mardon, Westerdahl, & Bonadonna, 2017). Odor secretions are directly linked to the physiological conditions of senders (Harris, Boulet, Grogan, & Drea, 2018) and can be costly to produce (Scordato & Drea, 2007), thus they are expected to deliver a more honest signal compared to other forms of communication (Hasson, 1997).
Primates have traditionally been considered to be “microsmatic,” relying more upon other sensory modalities than olfaction (Dulac & Torello, 2003). Since vision and acoustics are considered to be the main sensory modalities in most primate species little is known about the chemical signals used by non-human primates (Walker, 1998). However, several studies support the hypothesis that chemical communication is crucial also for primates (e.g., Geissman & Hulftegger, 1994; Hayes, Morelli, & Wright, 2004, 2006; Heymann, 2006; Jacob, McClintock, Zelano, & Ober, 2002; Knapp, Robson, & Waterhouse, 2006; Laidre, 2009; Porter & Moore, 1971; Scordato, Dubay, & Drea, 2007; Setchell et al., 2010, 2011; Smith, 2006; Smith, Tomlinson, Mlotkiewicz, & Abbott, 2001; Vaglio et al., 2016; Wedekind & Füri, 1997; Wedekind, Seebeck, Bettens, & Paepke, 1995). Particularly, it is established that some primates also rely heavily on olfaction in addition to vision and auditory senses; for instance, this is the case of squirrel monkeys (Laska, Seibt, & Weber, 2000) and several lemurs (Gould & Overdorff, 2002; Scordato & Drea, 2007). Strepsirrhines have retained their olfactory complexity (reviewed in Hayes, Morelli, and Wright (2006)) due to morphological constraints that limit the visual signals produced by senders (Scordato & Drea, 2007).
Lemur behavioral repertoire comprises both olfactory investigative and scent-marking behaviors. Lemurs show both direct and indirect olfactory investigative behaviors (Drea, 2015); direct investigations may include behaviors such as sniffing and/or licking a conspecific's skin (palms, soles, eyelids, or nostrils) or genitals, and self-sniffing genitals, while indirect behaviors consist of sniffing and/or licking scent-marks deposited by the signaller. Scent-marking behavior is shown by several terrestrial vertebrates, including mammal and reptile species (Müller-Schwarze, 2006; Roberts, 2007). Scent-marks may include species-wide pheromones (i.e., chemical substances released by an animal or insect which can affect a conspecific individual; for further details see Vaglio, Bartels-Hardege, and Hardege (2018)) as well as highly individual odors. Scent-marking is a very effective form of communication within habitats that make difficult the detection of visual and auditory signals (Gould & Overdorff, 2002), which is the case with forests inhabited by lemurs (Sussman, Green, Porton, Andrianasolondraibe, & Ratsirarson, 2003). In particular, this behavior is reasonably common in lemurs and New World monkeys—among which may play several functions, including the reproductive suppression of subordinate females, advertisement of individual “quality,” preparing males to assist in the delivery and care of newborn infants, and territorial defence (e.g., Gould & Overdorff, 2002; Heymann, 2006; Pochron, Morelli, Scirbona, & Wright, 2005)—while is less commonly reported in Old World monkeys and apes (e.g., Freeman, Pasternak, Rubi, Barrett, & Henzi, 2012). Especially, among strepsirrhines, social complexity may have selected for olfactory complexity in lemurs (delBarco-Trillo et al., 2012).
Mammals have a common pattern of scent-marking: glandular secretions, if not feces or urine, are placed at meaningful places such as along paths and territorial boundaries (Gosling & Roberts, 2001). Scent-glands have been observed in various lemur species, including all Eulemur species (delBarco-Trillo et al., 2012), ring-tailed lemurs (Scordato & Drea, 2007), red-bellied lemurs (Gould & Overdorff, 2002), red-fronted lemurs (Hayes et al., 2006), Milne-Edward's sifakas (Hayes et al., 2004), black-and-white and red-ruffed lemurs (Gould & Overdorff, 2002). In particular, red-ruffed lemurs have multiple scent-glands (Gould & Overdorff, 2002), composed of neck and mandible glands (male), and anogenital glands (male and female) (Pereira, Seeligson, & Macedonia, 1988); indicating that olfactory communication should be significant for this species (Elisa, Bracchi, & Federico, 2004).
The red-ruffed lemur is a large, frugivorous lemur species, which inhabit the residual primary forests of the Masoala Peninsula (Andriaholinirina et al., 2014). Red-ruffed lemurs have a variable social system; in smaller home ranges their group size is usually between 2 and 5 individuals, whereas larger home ranges have been known to support between 18 and 32 individuals (Rigamonti, 1993). Although red-ruffed lemur communities are not cohesive units, the home range is communally defended. In addition, only females participate in communal home range defense against females from other groups, which includes agonistic behaviors such as chasing, scent-marking, vocalizing, and even physical contact with members of neighboring communities (Vasey, 2005; 2007). Females are dominant to males, winning almost all agonistic encounters with them and rarely showing submissive behavior toward them (Meyer, Gallo, & Schultz, 1999; Raps & White, 1995). Communication is commonly observed as vocalizations, emitting species-specific calls which serve several functions and are transmittable between groups; however, also chemical communication is thought to play a crucial role in group dynamics (Elisa et al., 2004).
The overarching aim of this study is to improve our understanding of the role played by chemical communication, particularly focusing on scent-marking behavior, in red-ruffed lemurs. We predict that red-ruffed lemurs advertise information about their sex, age, and rank by using scent-marking. We also anticipate that this study may contribute to further exploring the connection between functional and mechanistic levels of lemur scent-marking.
2 MATERIALS AND METHODS
2.1 Subjects and housing
We studied two captive troops of red-ruffed lemurs (n = 7) housed at Dudley and Twycross zoos (UK). The troop housed at Dudley Zoological Gardens consisted of two related (brothers) adult males (13 years old) and one unrelated adult female (12 years old). The troop housed at Twycross Zoo consisted of one adult male (11 years old), one adult female (12 years old) and their offspring (two 1.5 years old females). Red-ruffed lemurs are considered sexually mature at 2 years old, with first conception approximately 1 year later (Vasey, 2007). Adult females were contracepted, and all individuals in non-breeding season (i.e., regarding red-ruffed lemurs in captivity in the Northern Hemisphere breeding usually occurs in December-January with births in April-May; Brockman, Willis, & Karesh, 1987).
We carried out behavioral observations and odor sampling from September to November 2016 (Twycross Zoo) and from July to September 2018 (Dudley Zoological Gardens). In both institutions, the troops lived in an indoor enclosure (heated to 28 °C) with access to an outdoor enclosure (“visitor walktrough” enclosures).
2.2 Ethics statement
This study followed the guidelines for the care and use of captive animals in the UK, involving non-invasive methods for obtaining both behavioral data and odor samples from red-ruffed lemurs. Moreover, the study was conducted in compliance with the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES) and approved by the Life Sciences Ethics committee at the University of Wolverhampton (UK) and the Ethics committees at Dudley Zoological Gardens and Twycross Zoo (UK).
2.3 Behavioral data collection and analysis
We collected behavioral data by instantaneous scan sampling (Altmann, 1974), with behaviors recorded at 30-sec intervals over the duration of an hour in three time periods (two during the morning, and one during the afternoon), 2 days per week, over 3 months. Alongside the scan sampling we also used ad libitum sampling for recording olfactory behaviors (Table 1), including both scent-marking (ano-genital marking, neck-marking—that is scent-marking via neck and mandible glands) behavior and locations (comprising of “hatches,” “tree branch,” “indoor enclosure,” “wooden hut,” and “climbing frame”). We recorded a total of 132 hr of observations throughout the study period, including 360 scan samples each sampling day on the entire group.
| Behavior | Description |
|---|---|
| Scent-Marking; Neck/Mandible | Individual rubs neck region against substrate or upon an item within the enclosure |
| Scent-Marking; Ano-genital | Individual rubs genital region against substrate or upon an item within the enclosure |
| Sniffing/Licking; Environment | Individual deliberately places nostrils or tongue within 3 cm from substrate or an item within the enclosure and sniffs/licks |
| Sniffing/Licking; Conspecific | Individual deliberately places nostrils or tongue within 3 cm from a conspecific and sniffs/licks |
| Self-Licking | Individual uses tongue to lick an area near a scent gland on their own body |
We investigated the relationships between individuals and scent-marking behavior in relation to sex and age of senders. We also investigated the role played by different types of scent-marking behavior, and locations of scent-marks within the enclosure. We tested all variables through Shapiro-Wilk test for normality. As data were not normally distributed, we performed non-parametric Kruskall-Walis tests followed by pairwise Mann-Whitney U post-hoc tests. All tests were carried out using SPSS v.23, and a significance level of p < 0.05 was applied.
2.4 Odor sampling and analysis
We collected odor secretions spontaneously released via scent-marking by red-ruffed lemurs on brand-new filter paper fixed on hatches, climbing equipment, and tree trunks and branches (Figure 1). Unfortunately, we were not able to collect any odor sample from males, while we collected scent-marks deriving from ano-genital marking by all the females (14 samples, 3–4 replicates per individual). In addition, we placed control filter paper in the environment to control for the contact with wood (where there may be chemical compounds deriving from the wood, but also algae, microorganisms, etc.) and we exposed control filter paper also to the air during sampling in order to detect any chemical compounds which did not derive from the red-ruffed lemurs. We collected odor samples immediately after scent-mark deposition by red-ruffed lemurs in the outdoor enclosure. We placed all samples and controls into brand-new sterile vials (Supelco) and immediately stored them at −20 °C. We used 10-ml screw-capped clear glass vials (thread: 18O.D. 22.5 × H 46 mm) closed by teflon-faced rubber septa and seals (1.3-mm thick).

We conducted laboratory analyses at the Rosalind Franklin Science Centre, University of Wolverhampton (UK). We investigated the volatile components of odor secretions using established solid-phase microextraction (SPME) and gas chromatography-mass spectrometry (GC-MS) and applying the same methods used in our previous work on mandrill odor signals (Setchell et al., 2010; Vaglio et al., 2016).
We introduced a 65-μm polydimethylsiloxane/divinylbenzene SPME syringe needle through the vial septum and then we exposed the fibre to the headspace above the sample in the vial for 15 min at 40 °C. We analyzed the adsorbed volatile analytes of all samples by using a 5975C mass spectrometer (Agilent Technologies, Santa Clara, CA) EI, 70 eV, coupled directly to a 7890B gas chromatograph (Agilent Technologies) equipped with a fused silica HP5-MS UI capillary column (Agilent Technologies) 30 m × 0.25 mm crossbonded 5%-phenyl-95%-dimethylpolysiloxane, film thickness 0.25 μm. We maintained the injector and transfer line temperatures at 270 and 280 °C, respectively. We made injections in splitless mode (purge valve opened after 1 min) with a constant flow of helium carrier gas of 1 ml min-1. We started the oven temperature program at 45 °C for 2 min, then raised it by 4 °C min-1 to 170 °C, and finally by 20 °C min-1 to 300 °C.
We assessed potential contamination due to the lab environment through blank analyses of an empty 10-ml vial (Supelco) following the same procedure as for the samples. In addition, we conditioned the fibre at 260 °C pre- and post- injection, for 5 and 20 min respectively in order to avoid any possible carry-over effects.
We tentatively identified eluted compounds by comparing the experimental spectra with the spectra provided by the mass-spectral library in ChemStation (Agilent Technologies) and NIST (National Institute of Standards and Technology) Database, version MSD F.01.01.2317 (Agilent Technologies). We accepted a putative identification when the minimum matching factor was higher than 80%. If more than one compound was a good match for the same GC peak then we considered the chromatographic retention time and compared it with the retention time reported in the literature for the same chromatographic column type (El-Sayed, 2016) in order to minimize the chance of misidentification. We created a data matrix using the peak area relative to each identified compound by using the integrated signal of the deconvoluted total ion current (TIC). We analyzed all samples in a short period of time (approximately 24 hr) to minimize interassay variability. We removed all the contaminants (i.e., any compounds that appeared in the “environmental controls” and “lab blanks”) from the scent-mark results.
3 RESULTS
3.1 Behavioral observations
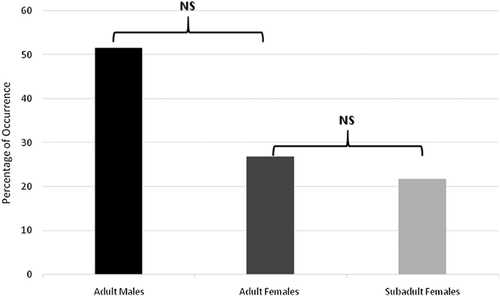
During the study period olfactory behaviors were exhibited predominantly by males (40.00%), followed by adult females (34.48%) and subadult females (25.52%). These behaviors included both scent-marking (ano-genital marking and neck-marking) and investigative behaviors (sniffing and/or licking an area within the enclosure, sniffing and/or licking a conspecific, self-licking of the ano-genital glands). Scent-marks were most commonly deposited by males (51.55%), followed by adult females (26.80%) and subadult females (21.65%) (Figure 2), although differences were not significant between the sexes (U = 137.5; p = 0.688).
We observed males (N = 3) scent-marking significantly more via neck glands rather than via ano-genital glands (U = 12.5; p < 0.001). Females (N = 4) displayed the opposite behavioral pattern; showing a significantly higher frequency of ano-genital marking rather than neck marking (U = 41.5; p = 0.022). We found significant differences in neck-marking behavior between individuals, and particularly between males and females (N = 7; U = 12.5; p = 0.003).
We found a significant difference (N = 7; Z = −5.675, p < 0.001) in scent-marking behaviors between inside and outside locations, with 81.73% of scent-marks occurring in the outdoor enclosure. Moreover, scent-marks were most commonly deposited near, or upon, the hatches leading to the indoor and off-show enclosure (18.27%). However, scent-marks were also deposited on tree branches next to the path of the walk-through, all climbing frames in the walk-through, a hunt providing shelter within the walk-through, and upon furniture in the indoor enclosure.
We also found a preference of location when considering the type of scent-marking performed; “hatches” were used most commonly overall for neck-marking (81.82%) compared to ano-genital marking (18.18%), whereas “climbing frame” was used more for ano-genital marking (71.43%) than neck-marking (28.57%).
We found significant differences in areas used for neck-marking, most commonly upon “hatches” (X2 = 23.152; p < 0.001), and upon “tree branches” (X2 = 9.456; p = 0.009). Deposition of neck scent-marks upon “hatches” was significantly different between males and females (U = 04.5; p < 0.001). Neck-marking on “tree branches” showed a difference between males and females (U = 30.0; p = 0.029), and between adult and subadult females (U = 30.0; p = 0.029).
We found significant differences in areas used for ano-genital marking, with most frequent occurrences upon “hatches” (X2 = 11.748; p = 0.003) and “climbing frame” (X2 = 13.119; p < 0.001). Deposition of ano-genital marks upon “hatches” was significantly different between adult and subadult females (U = 84.0; p = 0.037). Ano-genital marks upon “climbing frames” were also significantly different between adult and subadult females (U = 35.0; p < 0.001).
3.2 Odor secretions
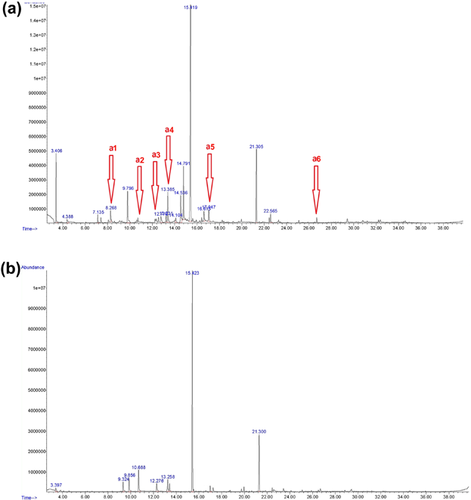
We identified a total of 32 individual compounds from the analysis of 14 filter paper samples of female ano-genital secretions. These compounds included a range of naturally occurring odorous volatile compounds such as hydrocarbons, terpenes, terpene alcohols, and ketones. Tentative identifications are listed in Table 2, while typical chromatograms (1 from the blank control and 1 from a female lemur ano-genital scent-mark) are shown in Figure 3. There was variation in the number and abundance of the compounds observed from sample to sample across different individuals. However, six compounds (benzaldehyde, 2-ethyl-1-hexanol, p-cresol, cis-p-mentha-2,8-dien-1-ol, 2-pinen-4-one, pentadecane) were present in all samples. We were not able to analyze the ratios of individual components in order to compare the volatile profiles with features of the signaller (for instance, adult vs. subadult females) due to the small amount of filter paper samples.
| Retention time (mins) | Tentative compound ID | Molecular weight |
|---|---|---|
| 3.906 | Hexanal | 100 |
| 6.057 | 5-methyl-3-hexanone | 114 |
| 7.413 | Alpha-pinene | 136 |
| 8.077 | 1-isopropyl-4-methylenebicyclo[3.1.0]hex-2-one | 134 |
| 8.268 | Benzaldehyde | 106 |
| 8.623 | 3,7,7-trimethyl-1,3,5-cycloheptatriene | 134 |
| 9.096 | Phenol | 94 |
| 9.269 | 6-methoxy-5-hepten-2-one | 126 |
| 10.720 | 2-ethyl-1-hexanol | 130 |
| 12.362 | p-Cresol | 108 |
| 12.553 | cis-Verbenol | 152 |
| 13.385 | cis-p-Mentha-2,8-dien-1-ol | 152 |
| 14.104 | 1,7,7-Trimethylbicyclo[2.2.1]hepta-2-one | 152 |
| 14.536 | L-Pinocarveol | 152 |
| 14.791 | trans-Verbenol | 152 |
| 15.605 | p-Ethyl-phenol | 122 |
| 15.928 | Terpinen-4-ol | 154 |
| 16.415 | Alpha-Terpineol | 154 |
| 16.615 | Myrtenol | 152 |
| 17.047 | 2-Pinen-4-one | 150 |
| 18.252 | Carvone | 150 |
| 19.217 | p-Mentha-1,8-dien-3-one | 150 |
| 23.283 | 4,7,7-Trimethylbicyclo[4.1.0]hept-3-ene-2-one | 150 |
| 23.443 | Tetradecane | 198 |
| 25.094 | Geranylacetone | 194 |
| 25.899 | Isomethylionone | 206 |
| 26.513 | Pentadecane | 212 |
| 30.871 | 2,6,10-Trimethylpentadecane | 254 |
| 32.208 | Heptadecane | 240 |
| 32.372 | 2,6,10-Trimethylhexadecane | 268 |
| 34.446 | n-Tetracosane | 338 |
| 34.591 | 2,6,10,14-Tetramethylhexadecane | 282 |
4 DISCUSSION
Primates rely on olfactory communication in several contexts, including foraging, territorial defense, individual and family recognition, mate choice, and mother-offspring bonding (Zeller, 1987). Although very little is known about Old World primates, research has been accumulating on chemical communication in strephsirrhines and New World monkeys; particularly, semiochemical data are accessible for few non-human primate species, including various strepsirrhines [galago (Crewe, Burger, Le Roux, & Katsir, 1979); lemurs (delBarco-Trillo et al., 2011, 2012; Hayes et al., 2004, 2006; Palagi & Dapporto, 2006; Scordato et al., 2007), owl monkeys (Macdonald, Fernandez-Duque, Evans, & Hagey, 2008), marmosets and tamarins (Epple et al., 1993; Smith et al., 2001), macaques (Curtis, Ballantine, Keverne, Bonsall, & Michael, 1971) and mandrills (Setchell et al., 2010, 2011; Vaglio et al., 2016)]. In this study we focused on scent-marking behavior, via both behavioral and chemical approaches, in two troops of zoo-managed red-ruffed lemurs.
Red-ruffed lemurs, as the well-studied ring-tailed lemurs, are characterized by a female-dominated society. In ring-tailed lemurs males scent-mark more than females (Pochron et al., 2005) and female age is positively correlated to scent-marking frequency (Gould & Overdorff, 2002; Kappeler, 1990; Pochron et al., 2005). Similarly, in our study, males scent-marked significantly more than any female and adult females showed the highest frequency of scent-marking within females. Therefore, our findings support the prediction that scent-marking would differ between individuals based on their sex, as found in other lemur species such as sifakas (Pochron et al., 2005), and age, as found in ring-tailed lemurs (Gould & Overdorff, 2002; Kappeler, 1990; Pochron et al., 2005).
Neck-marking was exhibited by all study subjects, but significantly more by males. In contrast, females exhibited ano-genital scent-marking significantly more than males. This supports the hypothesis of sexual dimorphism in red-ruffed lemur scent-marking, as already observed by Vasey (2003). The preferences shown by males for neck-marking “hatches” and ano-genital marking “tree branches,” and by adult females for ano-genital marking “climbing frames,” confirm behavioral sexual dimorphism. These observable preferences also suggest that scent-marking behaviors might play different roles in males and females, as observed in other primates, including ring-tailed lemurs (Scordato & Drea, 2007), black-and-white ruffed lemurs (Pereira et al., 1988), and mandrills (Vaglio et al., 2016).
Our results also support the hypothesis that scent-marking might have a territorial function in this species (Pereira et al., 1988). In particular, males scent-marked mostly specific meaningful places, by using neck-marking for hatches (small openings allowing access from outdoor to indoor enclosure; i.e., potential role of territorial defense) and ano-genital marking for tree branches and climbing equipment (areas of frequent transit by individuals; i.e., potential role of social communication). This also implies that scent-marks released via neck-marking and ano-genital marking might communicate different messages to the receivers by conveying information about distinct features of the senders. Previous studies have indicated information regarding sex to be conveyed in scent-marks from ring-tailed lemurs (Hayes et al., 2004, Scordato et al., 2007), but absent in odorants from sifakas (Hayes et al., 2004, 2006).
Although scent-marking behavior is observable, it is challenging to decipher the message which is chemically communicated. Therefore, the chemical investigation of odor secretions released by scent-marking is critical to understand the message transferred by this behavior. Since we used spontaneously released scent-marks, we were able to investigate odor secretions released by scent-marking and thus corresponding to the exact message sent by red-ruffed lemurs.
A total of 32 compounds were tentatively identified within the ano-genital secretions released by female study subjects (excluding environmental and lab contaminants as well as co-eluted compounds). This low amount of volatile compounds in comparison to other female lemur ano-genital marks (for example, ring-tailed lemurs and sifakas; Hayes et al., 2004, Scordato et al., 2007) might be explained by the fact that breeding versus non-breeding season (Scordato & Drea, 2007) and chemical contraception (Crawford, Boulet, & Drea, 2011) can have significant impacts on semiochemical signals in lemurs. For instance, in ring-tailed lemurs (Crawford et al., 2011) genital odorants of adult contracepted females were proved altered, including decreased richness, modified relative abundances, and minimized individual chemical distinctiveness of their volatile chemical profiles.
Volatile hydrocarbons have previously been identified in odorants deriving from ring-tailed lemurs and sifakas (Hayes et al., 2004; Scordato et al., 2007) as well as from Old World monkeys such as mandrills (Setchell et al., 2010; Vaglio et al., 2016) and olive baboons (Vaglio et al., in preparation). In particular, high-molecular weight volatile hydrocarbons might act as a fixative which slows the release of more volatile compounds, as suggested for major urinary proteins in mice (Greene et al., 2016; Hurst et al., 1998). The compounds benzaldehyde, p-cresol (also known as p-methylphenol), hexanal, and geranylacetone are commonly encountered in mammal scent markings (e.g., lions, wild dogs, wolves, mice, red foxes) (Osada, Miyazono, & Kashiwayanagi, 2015; Roberts et al., 2010; Soso & Koziel, 2016). The compound benzaldehyde has already been found in gland secretions released by marmosets (Smith et al., 2001), and functions as sex pheromone in other mammals (reviewed in El-Sayed, 2016) and also as cue to genetic quality (reviewed in Wyatt, 2014) in other vertebrates (e.g., in the crested auklet, a seabird with citrus scent based on decanal and octanal produced by both sexes during the breeding season, concentration correlates with rank in males). Thus, benzaldehyde might serve as pheromone and play a role in signalling individual quality also in red-ruffed lemurs. In addition, ethyl-phenol occurs in rat urine as mate attraction signal and also in beaver urine as part of a multicomponent signal of range occupation (reviewed in Apps, Weldon, and Kramer (2015)). The compound 2-pine-4-one (also known as verbenone) is a bark beetle antiaggregation pheromone (Lindgren & Miller, 2002), which has similarly been found in other insects (i.e., bees and butterflies) and is also naturally occurring in plants (reviewed in Bakthavatsalam (2016)). 2-ethyl-1-hexanol and cis-p-metha-2,8-dien-1-ol both appear to be associated with fragrancies. Finally, other compounds, such as α-pinene, are known to derive from plants; therefore, they might be a by-product and potentially vary with the environmental context but could also contribute to the message communicated by red-ruffed lemurs through scent-marking (for instance, convey information about group identity).
5 CONCLUSION
In conclusion, the present study supports the hypotheses (Smith et al., 2015) of sexual dimorphism and of more than one function served by scent-marking in red-ruffed lemurs. We suggest that scent marking could serve a function in intergroup spacing and intrasexual competition for both sexes, as might be expected in a female-dominant species.
In particular, male neck-marking might have a defensive territorial function while ano-genital marking might play a role in socio-sexual communication in this lemur species. Furthermore, our findings suggest that odor secretions released via ano-genital-marking might convey information about the age of female signallers. Additionally, the similarity of red-ruffed lemur's volatile chemical profiles to those found in other vertebrates would support our previous suggestion (Setchell et al., 2010) that non-human primates are not as microsmatic as traditionally considered.
Since this study is based on seven animals living in two captive family troops it can only be considered a preliminary work for the red-ruffed lemur species. Future research work should focus on a larger sample size, record behaviors consistently throughout the day, and investigate the odor secretions released by adult non-contracepted females and also by male scent-marks. In addition, it would be crucial to study the perception by the recipient, for instance looking for evidence of behavioral or physiological responses facilitated by scent-marks via bioassay tests (Wyatt, 2014). Also, more detailed analysis of the ratios of individual components could form the basis of further studies. Finally, although we focused on the volatile profile of red-ruffed lemur odor, we also recognize the significance of non-volatile components of odor secretions, as high-molecular weight compounds may extend the persistence of volatile signals in scent-marks (Alborne, 1984; Belcher et al., 1990; Hurst & Beynon, 2004).
ACKNOWLEDGMENTS
We are grateful to Dudley Zoological Gardens (especially David Beeston, Chris Leeson, Pat Stevens, and Lemur Wood keepers) and Twycross Zoo (especially Mat Liptovszky, Manuela Townsend, and Zak Showell) for their support to the project and assistance with sample collection. We also thank Keith Holding for his assistance with GC-MS chemical analyses at the Rosalind Franklin Science Centre, Wolverhampton, and Clare Everson and Farzana Aslam (Coventry University) for facilitating the Nuffield Research Placement of KLP. Furthermore, we would like to thank Tim Baldwin for his invaluable suggestions. On top of that, we also thank two anonymous reviewers for constructive comments. This research work was supported by the Faculty of Science and Engineering, University of Wolverhampton (sampling & laboratory analysis), and the Nuffield Foundation (Nuffield Research Placement for KLP).
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.




