Real-world effectiveness of intensive chemotherapy with 7&3 versus venetoclax and hypomethylating agent in acute myeloid leukemia
Abstract
Intensive chemotherapy with cytarabine and anthracycline (7&3) remains the standard therapy for patients medically fit for induction, but the assessment of fitness remains controversial. Venetoclax and hypomethylating agent (ven/HMA) combination therapy has improved outcomes in unfit patients but no prospective study has assessed ven/HMA versus 7&3 as initial therapy in older, fit patients. Given no studies and expectation of ven/HMA use in patients outside of trial criteria, we evaluated retrospective outcomes among newly diagnosed patients. A nationwide electronic health record (EHR)-derived database and the University of Pennsylvania EHR identified 312 patients receiving 7&3 and 488 receiving ven/HMA who were 60–75 years old without history of organ failure. Ven/HMA patients were older and more likely to have secondary AML, adverse cytogenetics, and adverse mutations. Median overall survival (OS) for patients receiving intensive chemotherapy was 22 versus 10 months for ven/HMA (HR 0.53, 95% CI 0.40–0.60). Controlling for measured baseline characteristic imbalances reduced survival advantage by half (HR 0.71, 95% CI 0.53–0.94). A sub-group of patients with equipoise, likelihood at least 30%–70% of receiving either treatment, had similar OS outcomes (HR 1.10, 95% CI 0.75–1.6). Regarding safety outcomes, 60-day mortality was higher for ven/HMA (15% vs. 6% at 60 days) despite higher documented infections and febrile neutropenia for 7&3. In this multicenter real-word dataset, patients selected for intensive chemotherapy had superior OS but a large group had similar outcomes with ven/HMA. Prospective randomized studies, controlling for both measured and unmeasured confounders, must confirm this outcome.
1 INTRODUCTION
Since the approval of venetoclax combined with a hypomethylating agent in 2018, clinicians have had an unprecedented amount of choice in treating older adults with acute myeloid leukemia. Patients too frail for traditional intensive chemotherapy—most commonly 7&3 consisting of seven days of cytarabine and three days of anthracycline infusions1—have achieved high response rates to venetoclax and hypomethylating agent combination (ven/HMA).2 This group of patients has historically been underserved. Many adults have multiple comorbidities and are marginal candidates for such intensive therapy with historical trials showing low response rates, overall survival and high early mortality often exceeding 20%.3-5 This has led to low treatment rates in historical national series, often as low as 30%–40% in elderly patients.6-8
Given this success among older patients, clinicians have expanded the use of ven/HMA to a different clinically challenging set of patients: younger, less frail patients with multiple comorbidities who are marginal candidates for 7&3. It may be that ven/HMA can offer similar efficacy with lower toxicity and less healthcare utilization. But to date there are no results available from prospective trials comparing traditional intensive 7&3 to ven/HMA. Larger, older landmark retrospective studies have examined intensive chemotherapy versus non-intensive treatments such as HMA alone. While HMA monotherapy provided an important option for patients unfit for intensive induction chemotherapy, overall survival has typically been <7–8 months.4, 9 However, prior studies do not include ven/HMA.4, 9-11 While these studies demonstrate an important principle—carefully selected older patients receiving intensive chemotherapy can achieve good outcomes—they do not guide the daily choice of initial therapy for AML patients between 60 and 75.
More recent retrospective studies have compared 7&3 and ven/HMA.12-15 That said, they are frequently limited to single centers and only examine outcomes for patients with full information available for all baseline characteristics. They focus on a small selection of patients, ignoring large sections of the populations of interest. These studies have consistently identified a subgroup of patients where outcomes appear similar, confirming the difficult choice of initial therapy selection. They provide limited information for clinicians deciding on initial treatment. Because they do not look at the broad range of patients in the clinics or the wards, these studies do not describe the magnitude of the potential benefit or risk of choosing intensive chemotherapy.
We sought to examine not only if 7&3 led to superior outcomes overall but also if we could identify fit patients who saw no obvious benefit of intensive chemotherapy and could have reasonably instead received ven/HMA with similar efficacy, less adverse events, and less healthcare utilization. Our study starts by describing outcomes for a large group of patients from a national sample. We focus on patients for whom clinicians are likely to have hesitations about treating with ven/HMA versus intensive chemotherapy: patients age 60–75 without diagnosed organ failure or low-performance status. Although some older patients may be eligible for intensive chemotherapy at certain centers and some younger patients may be viewed as ineligible for intensive 7&3 therapy, unmeasured confounding would likely drive therapy choice in those under age 60 receiving ven/HMA and those over age 75 receiving intensive chemotherapy. We conduct a series of sensitivity analyses to further probe how large a difference in outcomes may exist between treatment groups after accounting for measured and unmeasured characteristics at time of diagnosis.
2 METHODS
2.1 Study design and data sources
This report follows the Strengthening the Reporting of Observational Studies in Epidemiology Statement guidelines.16, 17 Two sources were used for patient cohorts in this retrospective study. First, we queried the University of Pennsylvania Health System (UPHS) Electronic Medical Records database which provides longitudinal data spanning both inpatient and outpatient settings at five hospitals including two academic medical centers in Philadelphia, a rural hospital in Lancaster, PA, and two community hospitals in urban/suburban Philadelphia.
The second source was Flatiron Health, a nationwide electronic health record (EHR)-derived de-identified database. The Flatiron Health database is a longitudinal database, comprising de-identified patient-level, structured and unstructured data, curated via technology-enabled abstraction.18, 19 During the study period, the de-identified data originated from ~280 cancer clinics (~800 sites of care).
Institutional Review Board approval of the study protocol was obtained before study conduct, and included a waiver of informed consent.
2.2 Eligibility
For both data sources, all patients who received 7&3 (7 days of cytarabine and 3 days of an anthracycline, either daunorubicin or idarubicin) or ven/HMA (either azacitidine or decitabine) as initial therapy (primary exposure) and had a diagnosis of acute myeloid leukemia (ICD-9 codes 205.0, 205.2, 205.3, 205.8, 205.9 or ICD-10 codes C92.0, C92.3, C92.5, C92.Z) with documented 20% blasts in bone marrow or peripheral blood were included. Patients receiving midostaurin and 7&3 were included. UPHS charts were manually reviewed to confirm that patients received 7&3 or ven/HMA as initial therapy. Any patients with acute promyelocytic leukemia, mixed phenotype or ambiguous lineage acute leukemia, or did not receive 7&3 or ven/HMA as initial therapy were excluded. Patients were excluded from analysis if they had a documented diagnosis of congestive heart failure, a recorded ejection fraction of <45% or an estimated glomerular filtration rate of <31 mL/min/1.73 m2 or ECOG performance status >2 or age <60 or >75 years old. The time period for inclusion was a diagnosis made from January 2017 to January 2021 with data cut-off on June 30, 2022.
2.3 Outcomes
The primary outcome was overall survival, defined as time from diagnosis to death or censored at the end of study period on June 30, 2022. Safety outcomes were early (30- and 60-day) mortality as well as documented infections. UPHS data allowed chart review for all patients to examine febrile neutropenia, culture positive infections, laboratory abnormalities and total inpatient and outpatient hospital days before cycle 2 of therapy (“length of stay”) were exploratory outcomes. Additionally total number of “home days”; that is days without any scheduled lab visits, infusions, clinic visits, or inpatient hospitalizations were assessed. Anticipated potential effect modifiers or confounders were collected as covariates including, at time of diagnosis: demographic factors (age, race, ethnicity, practice setting), clinical factors (Hematopoietic Comorbidity Index [HCT-CI], see Tables S2c,d,20 including albumin and lactate dehydrogenase [LDH]21 and other comorbidities including history of myelodysplastic syndrome and prior HMA treatment), disease severity (baseline blood counts, bone marrow blast counts, European LeukemiaNet—ELN—2022 risk groups22 see Table S2a, myelodysplastic syndrome related cytogenetics see Table S2b, and FLT3, IDH1, IDH2, TP53, ASXL1, RUNX1, TET2, and NPM1 mutational status).
2.4 Power
Before initial data collection, power calculation showed that 693 cases, allocated 2:1 between ven/HMA and 7&3 would have 80% power to detect a hazard ratio of 0.70 for improved OS from diagnosis to death or censoring at end of study period for 7&3, assuming an event rate of 40% at 1 year2, 23 with the use of a log-rank test at a two-sided significance level of 0.05 and 10% administrative censoring. By post hoc power calculation with 800 cases allocated 3:2, the minimally detected hazard ratio was 0.76.
2.5 Statistical methods
Descriptive statistics were calculated for baseline characteristics for the two treatment groups. For continuous variables, means and standard deviations for symmetric distributions, medians and inter-quartile ranges for skewed distributions, and frequencies for categorical or ordinal variables with t-test and chi-squared test to compare continuous and categorical variables are reported. Primary outcome of OS was assessed using the Kaplan–Meier method for median OS. Log rank test was used to assess survival differences between groups. Multivariable Cox proportional hazards regression analysis was used to estimate association between covariates and OS with hazard ratios and 95% confidence intervals (95% CIs). Patients were further stratified by age and cytogenetics to assess for effect modification given prior work.5, 24 Proportional hazards assumption was assessed using Schoenfeld residuals. Subgroups were examined in univariate analyses. Loss to follow-up, or last visit more than 3 months before data-cut-off without documented death, was assumed to be missing at random.25 All tests were 2-sided, with a significance level of .05. All statistical analyses were performed in Stata.26
2.6 Sensitivity analyses
This study used several sensitivity analyses to address common pitfalls of cohort studies. Missing data can severely limit multivariable Cox proportional hazards regression analysis which relies on complete cases, thus introducing bias if observations are not missing at random and limiting power. We, therefore, also conducted multiple imputation (MI), filling in missing values in multiple variables iteratively by using chained equations, a sequence of univariate imputation methods with fully conditional specification of prediction equations. Thirty imputations were used, with regression for continuous lab values, logistic regression for binary variables and ordinal logistic regression for ordinal variables.27, 28 Furthermore, to address confounding by indication, baseline differences between treatment groups were adjusted for, after multiple imputation, with inverse probability treatment weighting (IPTW), a propensity score-based approach to estimate marginal treatment effects. The treatment effect estimates from each imputed dataset were then combined to obtain an overall estimate.29, 30 In an additional sensitivity analysis a preference score, a standardizing transformation of the propensity score (logit of the propensity score – logit of Treatment A prevalence divided by Treatment B prevalence), was calculated and only patients with “equipoise” or 30%–70% chance of receiving either treatment were included as previously described.31 That is all patients who had less than a 30% chance of receiving either treatment based on their baseline characteristics at diagnosis were excluded in this sensitivity analysis. Additionally, to further assess the potential impact of unmeasured confounding E-values32 were calculated for the primary causal question of initial treatment after controlling for other baseline factors with MI and IPTW. E-values show the minimum strength of association on the risk-ratio scale that an unmeasured confounder would need to have with both the treatment assignment and the outcome to fully explain away a specific treatment-outcome association, conditional on the measured covariables.
3 RESULTS
3.1 Patient population
A total of 488 patients received ven/HMA (94 from UPHS, 394 from Flatiron Health) and 312 received 7&3 (85 from UPHS, 227 from Flatiron Health; Figures 1 and S1a). Median follow-up duration was 13.0 months at end of study period and median time to administrative censoring was 23.5 months. Loss to follow-up with >90 days from last visit, lab, or treatment before data-cut-off without documented death, was 5.6% overall (and similar in both groups at 7.1% for 7&3 and 4.7% for ven/HMA p = .208). Median time from diagnosis to treatment was 4.5 days for 7&3 group and 13 days for ven/HMA. There were a total of 444 death events (280 in ven/HMA and 164 in 7&3 groups). Febrile neutropenia, transfusion support, hospital length of stay and total clinic visits and infusion days were evaluable only for UPHS patients.

Patient baseline characteristics (Table 1, Figure S1) differed greatly between patients selected to receive ven/HMA versus 7&3. Patients receiving ven/HMA were older (median age 71 vs. 67 years), more likely to be treated in the community rather than an academic center (64% vs. 46% treated in the community setting), and they had more comorbidities by HCT-CI score (20% vs. 14% with a score of ≥3, see Table S2c), lower blood counts at time of diagnosis and higher rates of secondary AML (64% vs. 45%). They were also more likely to have adverse risk disease by ELN 2022 criteria (52% vs. 29%), fewer NPM1 mutations (7% vs. 25%), fewer FLT-3 ITD or FLT-3 TKD mutations (10% vs. 26%) and more high-risk mutations (including 20% vs. 4% with TP53 mutations).
| Ven/HMA | 7&3 | p-value | |
|---|---|---|---|
| N = 488 | N = 312 | ||
| Age | 71 (60–75) | 67 (60–70) | <.001 |
| Gender | .560 | ||
| Female | 204 (42%) | 137 (44%) | |
| Male | 284 (58%) | 175 (56%) | |
| Practice type | .004 | ||
| Academic | 175 (36%) | 168 (54%) | |
| Community | 313 (64%) | 144 (46%) | |
| Insurance | .690 | ||
| Commercial | 223 (46%) | 156 (50%) | |
| Medicare | 195 (40%) | 121 (39%) | |
| Other | 60 (14%) | 38 (12%) | |
| ECOG performance status | .012 | ||
| 0 | 112 (23%) | 71 (23%) | |
| 1–2 | 269 (55%) | 146 (47%) | |
| Missing | 107 (22%) | 95 (30%) | |
| HCT-comorbidity index | .008 | ||
| 0 | 198 (41%) | 138 (44%) | |
| 1–2 | 74 (15%) | 70 (22%) | |
| ≥3 | 98 (20%) | 45 (14% | |
| Missing | 118 (24%) | 59 (19%) | |
| Type | <.001 | ||
| De novo | 104 (21%) | 159 (51%) | |
| Secondary AMLa | 312 (64%) | 140 (45%) | |
| Prior MDS diagnosis | 153 (31%) | 42 (13%) | |
| Prior MPNb diagnosis | 58 (12%) | 25 (8%) | |
| Post-cytotoxic therapy | 72 (15%) | 13 (4%) | |
| European LeukemiaNet (ELN) 2022 Risk Groupc | <.001 | ||
| Favorable | 45 (9%) | 59 (19%) | |
| Intermediate | 62 (13%) | 89 (29%) | |
| Adverse | 258 (53%) | 99 (31%) | |
| Missing | 123 (25%) | 65 (21%) | |
| Baseline marrow blasts | <.001 | ||
| <30% | 240 (35%) | 83 (27%) | |
| 31%–50% | 110 (31%) | 60 (19%) | |
| >50% | 138 (29%) | 169 (54%) | |
| NPM1 mutation | <.001 | ||
| Positive | 33 (7%) | 79 (25%) | |
| Negative | 283 (58%) | 137 (44%) | |
| Missing | 172 (35%) | 96 (31%) | |
| IDH 1 or 2 mutation | .007 | ||
| Positive | 69 (14%) | 61 (20%) | |
| Negative | 310 (64%) | 206 (66%) | |
| Missing | 109 (22%) | 45 (14%) | |
| FLT-3 mutation | <.001 | ||
| Positive | 49 (10%) | 80 (26%) | |
| Negative | 310 (64%) | 169 (54%) | |
| Missing | 129 (26%) | 63 (20%) | |
| High risk mutations | <.001 | ||
| TP53 | 98 (20%) | 12 (4%) | |
| ASXL1 | 61 (13%) | 36 (12%) | |
| RUNX1 | 37 (8%) | 24 (8%) | |
| Negatived | 107 (22%) | 97 (31%) | |
| Missing | 185 (38%) | 143 (46%) | |
| Grade 3 Anemia | .012 | ||
| Negative | 323 (66%) | 197 (63%) | |
| Positive | 70 (14%) | 30 (10%) | |
| Missing | 95 (19%) | 85 (27%) | |
| Grade 3 Thrombocytopenia | .025 | ||
| Negative | 242 (66%) | 133 (43%) | |
| Positive | 152 (14%) | 94 (30%) | |
| Missing | 94 (19%) | 85 (27%) | |
| Albumin | .073 | ||
| >3.5 | 232 (48%) | 131 (22%) | |
| <3.5 | 223 (46%) | 147 (34%) | |
| Missing | 33 (6%) | 34 (11%) | |
| LDH | <.001 | ||
| Below ULN | 199 (41%) | 21 (28%) | |
| ULN—5 times ULN | 159 (33%) | 109 (35%) | |
| Over 5 times ULN | 6 (1%) | 4 (1%) | |
| Missing | 124 (25%) | 113 (36%) |
- Note: Data are presented as median (range) for continuous measures, and n (%) for categorical measures. Grade 3 anemia, thrombocytopenia according to the Common Terminology Criteria for Adverse Events version 5.
- Abbreviations: ECOG, Eastern Cooperative Oncology Group; HCT-CI, hematopoietic cell transplantation-specific comorbidity index; ULN, upper limit of normal.
- a Includes AML-MR (myelodysplasia related) by WHO 2022 mutations or cytogenetic changes regardless of prior diagnosis of myelodysplastic syndrome (MDS)/myeloproliferative neoplasm (MPN; see Tables S2a,b).
- b MPN includes MDS/MPN chronic myelomonocytic leukemia as well as polycythemia vera, essential thrombocytosis, myelofibrosis, and chronic myeloid leukemia.
- c Presence of any adverse risk feature sufficient for classification as adverse risk, intermediate or favorable risk classification required negative karyotype and mutation analysis for adverse risk features (Table S2a).
- d Negative for high-risk mutation refers to tested and negative for all three mutations listed, if any of these three not assessed, listed as missing.
Missing values in the UPHS dataset were <5% for all baseline covariates except for performance status; Flatiron Health dataset missing values ranged from 0% to 36% for covariates (e.g., sex and baseline LDH, respectively).
3.2 Overall survival
Median OS (mOS) was 14 months for all patients, 22 months for 7&3 versus 10 months for ven/HMA (HR 0.53, 95% CI 0.40–0.60, p < .0005, Figure 2A). There were 144 deaths in the 7&3 group (164/312, 39%) and 236 deaths in the ven/HMA group (280/488, 61%) by the end of study period. One year OS was 70% (95% CI 0.64–0.75) versus 44% (95% CI 0.39–0.49), and 2-year OS was 48% (95% CI 0.41–0.54) versus 25% (95% CI 0.20–0.30) for 7&3 and ven/HMA, respectively.
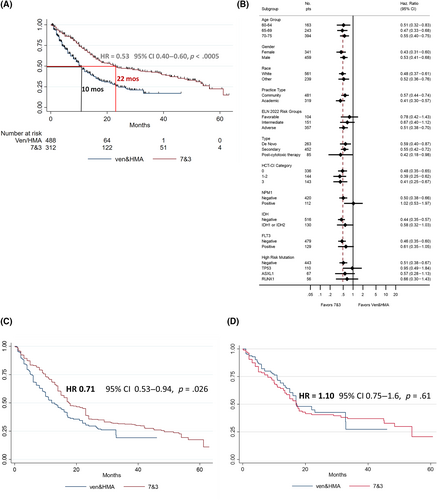
There was no clear subset with improved OS with either 7&3 or ven/HMA (Figures 2B and S8–S10) by univariate analysis. Patients selected to receive 7&3 had statistically significant superior overall survival in ELN adverse risk groups but similar overall survival in favorable and intermediate risk groups (Figure S7). Similar to data presented from the phase 3 VIALE-A study of venetoclax and azacitidine, there was no significant difference between favorable and intermediate risk groups by ELN 2022 criteria (Figure S7). However, there were small number of patients with core binding factor AML (11 and 8 for ven/HMA and 7&3 groups, respectively), it may not be possible to apply these findings to general populations from which ELN criteria were derived. Among patients with FLT3 mutations (n = 49 in ven/HMA group and n = 80 in 7&3 group), 81% of 7&3 patients received midostaurin, excluding these patients did not significantly change the hazard ratio between patients selected to receive 7&3 versus ven/HMA (HR 0.49–0.52).
In multivariable analysis controlling for all covariates with univariate p-value <.20 (sex, insurance, ECOG performance status, ELN risk category, type, HCT-CI score at diagnosis, mutations in ASXL1, DNMT3A, IDH, NF1, NPM1, RUNX1, TET2, TP53 and baseline white blood cell count, hemoglobin, platelets), induction choice did not affect overall survival when limited to complete cases (n = 140, HR 0.76, p-value 0.41, Table S1b).
3.3 Effect of allogeneic hematopoietic cell transplant on OS
More patients receiving 7&3 as initial therapy went on to receive transplant (31% vs. 15%, p ≤ .005). Among patients who underwent transplant, mOS was 42.3 months (Figure S2). Baseline imbalances were reflected in the two groups of patients who went on to receive transplant (e.g., 33% of ven/HMA patients who underwent transplant had TP53 mutations versus 0% of 7&3 patient who underwent transplant). Transplant status as a time-varying covariate showed that transplant improves OS as expected (HR 0.44, p < .005), and 7&3 showed higher OS compared with ven/HMA when controlling for transplant status (HR 0.52 for 7&3 vs. ven/HMA p < .005).
3.4 Sensitivity Analyses
To address missingness, after multiple imputation (MI) allowed use of all 800 patients, OS remained improved with 7&3 in multivariate cox regression analysis controlling for all covariates with univariate p-value <.20 (n = 800, HR 0.66, p-value <.001, Table S1a). To address imbalances in measured baseline covariates while accounting for missingness, we undertook a separate analysis using MI and inverse probability of treatment weighting (IPTW). After MI and IPTW, baseline covariates were well balanced with <10% absolute standardized differences between groups (Figure S3). The HR for OS, while about half as large as the unadjusted analysis, remained improved with 7&3 after balancing covariates (HR 0.71, 95% CI 0.53–0.94, p-value .026, Figure 2C). An E-value was calculated at 1.85 (E-values for upper and lower bounds of the 95% CI of hazard ratio found in MI and IPTW analysis were 1.26–2.47). An unmeasured confounder, or group of confounders, beyond all of the measured baseline co-variates balanced by MI and IPTW, with a HR of 1.85 associated with ven/HMA and death could explain away the observed HR of 0.71, but weaker confounding could not.
Given lack of overlap of probability density functions for receiving 7&3 and ven/HMA (Figure S4) there may have also been unmeasured confounding. An initially unplanned sensitivity analysis was employed to ensure the assumption of positivity was met, only patients with “equipoise” or 30%–70% chance of receiving either treatment by preference score were included as previously described.31 In this subgroup analysis limited to 120 out of the 488 ven/HMA (25%) and only 135 out of 312 (43%) of the 7&3 patients OS was similar at 17.5 months (HR = 1.10, 95% CI 0.75–1.6, Figure 2D).
Event free survival (EFS) was also assessed (Figure S5 and Table S2e). EFS was defined as time from the date of diagnosis to the date of induction failure, or relapse from CR, or CRi, or death from any cause; patients not known to have any of these events are censored on the date they were last examined.33 EFS was greater in patients selected for 7&3 (HR 0.67, 95% CI 0.57–0.81, p-value <.0005), after adjusting for missingness and imbalance in baseline covariates with MI and IPTW this difference was not statistically significant (HR 0.93, 95% CI 0.75–1.17, p-value .56).
3.5 Adverse events and healthcare utilization
Thirty-day all-cause mortality was similar at 3% and 5% for 7&3 and ven/HMA, respectively (p = .20); 60-day mortality was higher for ven/HMA (6% vs. 15% p < .001 for 7&3 and ven/HMA, respectively). However, documented diagnosed infections were higher in 7&3 patients and, within the UPHS cohort where full charts could be reviewed, rates of febrile neutropenia, median transfusions during induction, and grade 3–4 electrolyte abnormalities were also higher (Table 2). Length of stay, including any admission before the next cycle of therapy, was more than twice as long for 7&3 in the UPHS cohort (31.5 vs. 15.5 days, p-value <.001). For the UPHS cohort this time reflects both readmission, prolonged admission, as well as the practice of administering 7&3 inpatient for the UPHS cohort and remaining inpatient until count recovery.
| Ven/HMA, n = 488 | 7&3, n = 312 | p-value | |
|---|---|---|---|
| 30 day mortality | 5% | 3% | .20 |
| 60 day mortality | 15% | 6% | <.001 |
| Febrile neutropenia %a | 47% | 93% | <.001 |
| Documented infection %b | 21% | 44% | .004 |
| Median days inpatient induction (range)a,c | 15.5 (0–90) | 31.5 (6–82) | <.001 |
| Grade 3–4 Adverse Events by Common Terminology Criteria for Induction Adverse Eventsa,c | |||
|---|---|---|---|
| UPHS only (n = 179) | Ven/HMA, n = 94 | 7&3, n = 85b | p-value |
| Hypokalemia | 6% | 25% | <.001 |
| Alanine aminotransferase increased | 7% | 6% | .77 |
| Aspartate aminotransferase increased | 8% | 8% | 1.00 |
| Blood bilirubin increased | 6% | 2% | .44 |
| Anemia | 89% | 99% | .018 |
| Mean transfusions in induction | 12 | 18 | .006 |
| Platelet count decreased | 86% | 99% | <.001 |
| Mean transfusions in induction | 6 | 20 | <.001 |
- a UPHS only, confirmed with manual chart review.
- b Documented infection in Flatiron limited to use of intravenous antibiotics, diagnosis code of infection. Within UPHS data all charts manually reviewed for culture-positive infections including urine cultures, blood cultures, sputum cultures or c. diff positivity between treatment initiation and next cycle of consolidation therapy.
- c Includes readmission before second cycle of therapy. The p-values by Fisher's exact test and two sample t-test for means.
However, we also examined healthcare utilization over the entire course of therapy. “Home days” were days on which patients did not have a lab draw, infusion, office visit and were not hospitalized. The median percentage of home days was 68% for 7&3 compared with 54% for ven/HMA (Figure S6). Similarly, the total percent of days alive spent in the hospital was similar between the two groups at 10% and 12% over the entire course of treatment.
4 DISCUSSION
In older adults with AML, patients selected to receive 7&3 had an OS twice as long as those selected to receive ven/HMA. This greater OS persisted when controlling for measured baseline characteristics with multiple imputation and inverse probability of treatment weighting. However, there was likely additional unmeasured confounding, given this large difference in baseline characteristics. In overall survival analysis restricted to a smaller group of patients with greater clinical equipoise among physicians, defined by an observed high probability of receiving either treatment, OS was similar. This likely reflects clinical practice where some patients are felt to be clearly “unfit,” others are “fit” for intensive chemotherapy. There is a sizable plurality with unclear ability to tolerate and benefit from intensive chemotherapy. While our results confirm this, they also highlight a large group of older patients in which ven/HMA leads to similar efficacy outcomes as 7&3. This fits with other recent retrospective series,13, 15, 34 and together requires a prospective randomized trial to unequivocally ensure baseline outcome distributions would be balanced between groups.
Our study also confirms the high unmet need among older adults with AML. Transplant remains critical for long-term benefit. OS without transplant is poor and consistent with other studies. Other real-world series have found a mOS with ven/HMA ranging from 9.8 to 12.5 months35-37 versus the 14.7 months seen in the VIALE-A study.2 There are not many contemporaneous benchmark studies for older adults receiving intensive chemotherapy. Our national study shows lower early mortality, higher transplant rates, and greater OS for patients receiving 7&3 than in studies such as E2906,38 the UK NCRI AML16 trial,39 or AZA-AML-001 trial,40 likely reflecting selection of more fit patients, improved therapy options for second-line and post-transplant therapy. Finally, while this study's results are consistent with others regarding the higher rates of febrile neutropenia, infection and length of stay during initial treatment for intensive chemotherapy, the superior percentage of “home-days” over the entire treatment course in patients receiving 7&3 offers an important challenge to an otherwise more straightforward discussion of tolerability and outpatient management with patients. Our finding of close to half of total days alive spent interacting with the healthcare system is similar to recent work exploring time toxicity in AML patients and should be considered in future studies.41
There are several important limitations to this study. The most critical are confounding by indication and selection bias. This is not a prospective randomized trial or even an instrumental variable analysis. The two groups comprise patients selected by their physicians to receive intensive treatment with 7&3 or venetoclax and HMA. Differences in these two groups are shown in baseline characteristics (e.g., baseline rate of TP53 mutations 20% vs. 4%), the percentage of patients receiving transplant (15% vs. 31%) and cross-over rates (5% vs. 20%). The availability of CPX-351 during this study period may also have further exacerbated the differences between these two groups as patients with high-risk AML may have received alternative intensive chemotherapy. This study is also not powered to definitively assess the impact of key mutations such as NPM1, subsets of mutations (e.g., monoallelic vs. biallelic TP53 mutations), or the impact of targeted therapy options like FLT3 or IDH.
Our sensitivity analyses cannot fully correct for unmeasured confounding. Furthermore, while unmeasured confounding and confounding by indication impact overall survival as we have described above with several sensitivity analyses, they also impact transplantation rates, days spent at home, and other outcome measures. These measures must also be assessed in future prospective work where patient groups are balanced for similar baseline characteristics. Finally, this study incorporates patients from both Flatiron and the University of Pennsylvania Health System ages 60–75 years old without baseline organ failure to attempt to incorporate a broad geographic range of patients in community and academic settings across the United States eligible for intensive treatment and transplant. Results may be different for particular subgroups such as younger patients with multiple comorbidities, favorable risk patients, or community only practices.
This large, multicenter, national, well-powered, real-world retrospective series shows that patients selected to receive intensive induction therapy with 7&3 live more than twice as long as patients selected for ven/HMA. Others have repeatedly shown two related points: carefully selected older patients can benefit from intensive chemotherapy and a large group of older patients do not benefit from intensive chemotherapy. Our results suggest that in everyday practice clinicians can often identify patients likely to benefit from intensive chemotherapy at a population level. However, risk prediction tools for individual patients choosing between treatment options are not yet available and require further studies with validation cohorts. Furthermore, as we have noted previously, until randomized comparative data or individual risk prediction tools emerge, shared decision-making regarding risks and benefits will remain critical. A prospective randomized trial, such as NCT04801797 which randomizes newly diagnosed patients without favorable risk features by ELN criteria or FLT3 mutations to either venetoclax and azacitadine or intensive chemotherapy with 7&3 or CPX-351, is necessary to address whether superior OS with 7&3 persists in the setting of balanced measured and unmeasured baseline characteristics.
AUTHOR CONTRIBUTION
Andrew H. Matthews and Keith W. Pratz conceived of the study. Andrew H. Matthews and Keith W. Pratz reviewed the charts. Andrew H. Matthews conducted all analyses. Andrew H. Matthews drafted the manuscript with contributions from Keith W. Pratz, Alexander E. Perl, Sarah Skuli. All authors read, edited and approved the final manuscript.
ACKNOWLEDGMENTS
Andrew H. Matthews appreciates numerous discussions with Dr. William Chapin MD, MSCE regarding sensitivity analyses.
CONFLICT OF INTEREST STATEMENT
Matthews: none. Perl: Fujifilm: Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Sumitomo Dainippon: Consultancy; Astellas: Consultancy, Research Funding; Arog: Research Funding; BMS/Celgene: Consultancy; Genentech: Consultancy; Loxo: Consultancy; Onconova: Consultancy; Syndax: Consultancy; Forma: Consultancy; Actinium: Consultancy; Roche: Consultancy; AbbVie: Consultancy, Research Funding. Luger: Syros: Honoraria; Agios: Honoraria; Daiichi Sankyo: Honoraria; Jazz Pharmaceuticals: Honoraria; Brystol Myers Squibb: Honoraria; Acceleron: Honoraria; Astellas: Honoraria; Pfizer: Honoraria; Onconova: Research Funding; Celgene: Research Funding; Biosight: Research Funding; Hoffman LaRoche: Research Funding; Kura: Research Funding. Frey: Sana Biotechnology: Consultancy; Novartis: Research Funding; Kite Pharma: Consultancy; Syndax Pharmaceuticals: Consultancy. Gill: Novartis: Other: licensed intellectual property, Research Funding; Carisma Therapeutics: Current holder of stock options in a privately-held company, Research Funding; Interius Biotherapeutics: Current holder of stock options in a privately-held company, Research Funding. Hexner: Tmunity Therapeutics: Research Funding; Blueprint medicines: Membership on an entity's Board of Directors or advisory committees, Research Funding; PharmaEssentia: Membership on an entity's Board of Directors or advisory committees. Porter: American Society for Transplantation and Cellular Therapy: Honoraria; ASH: Membership on an entity's Board of Directors or advisory committees; DeCart: Membership on an entity's Board of Directors or advisory committees; Genentech: Current equity holder in publicly-traded company; Incyte: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Kite/Gilead: Membership on an entity's Board of Directors or advisory committees; National Marrow Donor Program: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees, Patents & Royalties, Research Funding; Unity: Patents & Royalties; Wiley and Sons Publishing: Honoraria. Stadtmauer: Oncopeptides: Consultancy; Amgen: Consultancy; BMS Celgene: Consultancy; GSK: Consultancy; Janssen: Consultancy; AbbVie: Consultancy, Research Funding. Maillard: Genentech: Research Funding; Regeneron: Research Funding; Garuda Therapeutics: Membership on an entity's Board of Directors or advisory committees. Pratz: Abbvie: Consultancy, Honoraria, Research Funding; Agios: Consultancy; BMS: Consultancy, Honoraria; Novartis: Consultancy; Astellas: Consultancy, Honoraria, Research Funding; Cellgene: Consultancy, Honoraria; Millenium: Research Funding; University of Pennsylvania: Current Employment.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions. Please contact [email protected] for original data. Flatiron data can be obtained through agreement; please contact [email protected].




