FKBP12 inhibits hepcidin expression by modulating BMP receptors interaction and ligand responsiveness in hepatocytes
Mariateresa Pettinato and Alessandro Dulja are considered as co-first authors.
Antonella Nai, Alessia Pagani, and Laura Silvestri are considered as co-last authors.
Abstract
The expression of the iron regulatory hormone hepcidin in hepatocytes is regulated by the BMP-SMAD pathway through the type I receptors ALK2 and ALK3, the type II receptors ACVR2A and BMPR2, and the ligands BMP2 and BMP6. We previously identified the immunophilin FKBP12 as a new hepcidin inhibitor that acts by blocking ALK2. Both the physiologic ALK2 ligand BMP6 and the immunosuppressive drug Tacrolimus (TAC) displace FKBP12 from ALK2 and activate the signaling. However, the molecular mechanism whereby FKBP12 regulates BMP-SMAD pathway activity and thus hepcidin expression remains unclear. Here, we show that FKBP12 acts by modulating BMP receptor interactions and ligand responsiveness. We first demonstrate that in primary murine hepatocytes TAC regulates hepcidin expression exclusively via FKBP12. Downregulation of the BMP receptors reveals that ALK2, to a lesser extent ALK3, and ACVR2A are required for hepcidin upregulation in response to both BMP6 and TAC. Mechanistically, TAC and BMP6 increase ALK2 homo-oligomerization and ALK2–ALK3 hetero-oligomerization and the interaction between ALK2 and the type II receptors. By acting on the same receptors, TAC and BMP6 cooperate in BMP pathway activation and hepcidin expression both in vitro and in vivo. Interestingly, the activation state of ALK3 modulates its interaction with FKBP12, which may explain the cell-specific activity of FKBP12. Overall, our results identify the mechanism whereby FKBP12 regulates the BMP-SMAD pathway and hepcidin expression in hepatocytes, and suggest that FKBP12–ALK2 interaction is a potential pharmacologic target in disorders caused by defective BMP-SMAD signaling and characterized by low hepcidin and high BMP6 expression.
1 INTRODUCTION
Maintaining adequate levels of iron in the body is critical for optimal health. Since excess iron cannot be actively excreted from the body, it is important to closely regulate its entry into the bloodstream to avoid harmful accumulation. Iron is released into the circulation through the cellular iron exporter ferroportin mainly by two cell types: duodenal enterocytes (that absorb iron from the diet) and reticuloendothelial macrophages (that recycle iron from senescent red blood cells). The key regulator of iron homeostasis is hepcidin, a liver peptide hormone that controls systemic iron levels by degrading and/or occluding ferroportin and thus modulating iron release into the circulation.1, 2 Defective hepcidin synthesis, such as in hereditary hemochromatosis and in thalassemia,3 causes iron accumulation in parenchymal organs, prevalently the liver.
Hepcidin is mainly released by hepatocytes. Its expression is regulated in response to iron levels through the activation of the bone morphogenetic protein (BMP)-small mothers against decapentaplegic (SMAD) signaling pathway.4 The activation of the BMP-SMAD pathway requires the formation of a multiprotein complex composed of transmembrane BMP type I and type II receptors (BMPRIs and BMPRIIs) and BMP ligands. Upon binding of dimeric BMPs, a dimer of constitutively active BMPRIIs phosphorylates the intracellular glycine/serine (GS)-rich domain of a dimer of BMPRIs, unlocking their kinase activity. In turn, BMPRIs phosphorylate the cytosolic SMAD1/5/8 that translocate together with SMAD4 into the nucleus to induce the expression of BMP target genes, including hepcidin in hepatocytes.5
In the liver, the crosstalk between different cell types adjusts hepcidin expression according to iron availability and demand. Liver sinusoidal endothelial cells (LSECs) produce the ligands BMP2 and BMP6, which activate the BMP-SMAD pathway in hepatocytes in a paracrine manner. BMP2 is mainly responsible for basal hepcidin transcription. BMP6, in contrast, is strongly upregulated in response to increased liver iron levels, and boosts hepcidin production to prevent further iron release into the circulation.6 Consistently, Bmp6 KO and LSEC-specific Bmp2 KO mice7 develop iron overload.8
Once released by LSECs, the ligands activate the BMP pathway in hepatocytes by signaling through the BMP type I receptors ALK2 and ALK39 and the type II receptors ACVR2A and BMPR2.10 While ACVR2A and BMPR2 have redundant roles in hepcidin regulation in vivo,10 hepatocyte-specific Alk3 knockdown (KD) mice9 develop severe iron overload. On the contrary, hepatocyte-specific Alk2 KD animals are characterized by a mild iron accumulation,9 excluding that ALK2 has a major role in iron-balanced conditions. However, the evidence that Alk2 deletion worsens the phenotype of Alk3 KD mice11 suggests that it plays a role in iron overload, likely in response to the iron-responsive ligand BMP6.
These genetic studies in mice, along with in vitro data showing that BMP2 has a high affinity for ALK3 and that BMP6 has a higher affinity for ALK2 than for ALK3,12 suggest a model where BMP2-ALK3 signaling guarantees basal hepcidin levels, while BMP6 signals through both ALK2 and ALK3 mainly in response to increased iron levels. The possibility that the ligands also act as BMP2–BMP6 heterodimers13 adds further complexity to the system. How the different type I and type II receptors combine to form active complexes, how this process is controlled by the receptors' regulators and ligands, and how the receptor combinations respond to the different ligands are still open questions.
We previously reported that the cytosolic protein FKBP12 (FK506 binding protein 12) limits hepcidin expression by binding to the GS-rich domain of ALK2. We found that its pharmacological displacement from the receptor by the immunosuppressive drug tacrolimus (TAC) increases hepcidin expression both ex vivo in primary hepatocytes and in vivo in wild-type (WT) mice in an acute setting.14 However, the molecular mechanism by which FKBP12 modulates BMP-SMAD pathway activity and therefore hepcidin expression remains unknown.
To address this question, we explored the role of FKBP12 in regulating ALK2 interaction with other BMP receptors and the subsequent response to the ligand BMP6. Our study reveals that FKBP12 controls hepcidin expression mainly through the BMP type I receptor ALK2 and the type II receptor ACVR2A (both recruited by BMP6), and to a lesser extent through ALK3. Mechanistically, FKBP12—that in hepatocytes interacts exclusively with ALK2—regulates ALK2 homo-oligomerization and ALK2–ALK3 hetero-oligomerization, and ALK2 interaction with the BMP type II receptors. TAC and BMP6, which share the same mechanism of ALK2 activation, cooperate in increasing hepcidin expression. Interestingly, we show that the lack of binding between FKBP12 and ALK3 in hepatic cells can be rescued by mutagenizing ALK3 or by chemically inhibiting its activity, explaining the cell type-specific activity of FKBP12 on BMPRIs.
Overall, these data shed light on the molecular mechanism through which FKBP12 modulates the BMP-SMAD pathway in hepatocytes and suggest that releasing FKBP12 from ALK2 might be tested as a therapeutic approach to increase hepcidin in conditions characterized by increased BMP6 levels due to iron overload, as in hereditary hemochromatosis and β-thalassemia.
2 MATERIALS AND METHODS
2.1 Mammalian expressing vectors
The pCMV6-ALK2MYC-FLAG, pCMV6-ALK3MYC-FLAG, pCMV6-FKBP12MYC-FLAG, pCMV6-BMPR2MYC-FLAG, and pCMV6-ACVR2AMYC-FLAG expressing vectors were from OriGene (Rockville, Maryland). The MYC-tag version of these constructs was obtained by site-directed mutagenesis through the insertion of a STOP codon after the MYC sequence.
pCMV6-ALK3FLAG, pCMV6-ALK2FLAG, pCMV6-BMPR2FLAG, and pCMV6-FKBP12FLAG were obtained by introducing a second XhoI restriction site between the MYC and FLAG tag sequences by mutagenesis using the QuickChange site-directed mutagenesis kit (Stratagene, La Jolla, California), according to the manufacturer's protocol. The resulting mutant constructs were then digested with XhoI to excise the MYC sequence and subsequently ligated.
The pCMV6-FKBP12no tag was generated from the pCMV6-FKBP12MYC-FLAG as previously described.15 The ALK3 mutants T229V and K232R were generated by mutagenesis using the QuickChange site-directed mutagenesis kit (Stratagene, La Jolla, California), according to the manufacturer's protocol. Mutagenesis was verified by DNA sequencing.
Primer sequences for mutagenesis are listed below:
Myc-stop.
Fw: 5′-TCAGAAGAGGATCTGTAAGCAAATGATATCCTG-3′.
Rev: 5′-AGTCTTCTCCTAGACATTCTGTTACTATAGGAC-3′.
XhoI.
Fw: 5′-GGATCTGGCAGCAAATGATCTCGAGGATTACAAGGATGACG-3′.
Rev: 5′- CGTCATCCTTGTAATCCTCGAGATCATTTGCTGCCAGATCC-3′.
ALK3-T229V.
Fw: 5′-CCATCTGAATCTGTTTGGCAATAACTCGCTGAACCAATAAAGGTAGTC-3′.
Rev: 5′-GACTACCTTTATTGGTTCAGCGAGTTATTGCCAAACAGATTCAGATGG-3′.
ALK3-K232R.
Fw: 5′-GGACCATCTGAATCTGTCTGGCAATAGTTCGCTGAAC-3′.
Rev: 5′-GTTCAGCGAACTATTGCCAGACAGATTCAGATGGTCC-3′.
2.2 Cell culture
The human hepatoma-derived (HuH7) or the Human Embryonic Kidney (HEK293) cell lines were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 2 mM L-glutamine, 200 U/mL penicillin, 200 mg/mL streptomycin, and 10% heat-inactivated fetal bovine serum (FBS) at standard culture conditions (37°C in 95% humidified air and 5% CO2). Cell culture media were purchased from Thermo Fisher Scientific (Waltham, Massachusetts).
2.3 Coimmunoprecipitation
HuH7 or HEK293 cells at 70%–80% confluency were transiently transfected with FKBP12, ALK2, ALK3, BMPR2, ACVR2A (MYC or FLAG-tagged) expressing vectors using Metafectene, according to manufacturer's instructions (Biontex Laboratories GmbH, Germany). When indicated, cells were treated with 1 μg/mL TAC-FK506 (Cayman Chemicals, Michigan), 100 ng/mL BMP6 (R&D Systems, Minneapolis, Minnesota), 1 μg/mL CsA (Tocris Bioscience, Bristol, UK), a combination of TAC and BMP6 for 1 h, or with the BMP-SMAD inhibitor LDN193189 (400 ng/mL; Sigma-Aldrich) for 18 h. Cells were then lysed in NET/Triton buffer plus protease inhibitor cocktail (Sigma-Aldrich). Cell lysates (containing 500 μg of total proteins) were incubated with the anti-FLAG M2 affinity gel (Sigma Aldrich, Missouri) at 4°C for 2 h. After affinity gel washing, samples were eluted with 20 μL of 2× LaemmLi sample buffer (without β-mercaptoethanol) and incubated at 95°C for 10 min. After centrifugation, β-mercaptoethanol was added to supernatants. Samples were then subjected to 12% Sodium Dodecyl Sulphate - PolyAcrylamide Gel Electrophoresis (SDS-PAGE) electrophoresis, transferred to Hybond C membrane (Amersham Bioscience Europe GmbH, Germany) and immunodetection performed by standard western blot techniques.
2.4 Western blot analysis
Blots were incubated with anti-phospho-SMAD1/5/8 (1:1000, Cell Signaling, Danvers, Massachusetts), anti-SMAD1 (1:1000, Cell Signaling), anti-FLAG (1:1000, Sigma-Aldrich), anti-MYC (1:1000, Cell Signaling), anti-vinculin (1:10 000; Sigma-Aldrich), anti-actin (1:10 000, Sigma-Aldrich) or anti-FKBP12 (1:2000, Abcam, Cambridge, UK) according to standard procedures. Blots were incubated with the relevant HRP-conjugated antisera (1:50 000; Sigma-Aldrich) and developed using a chemiluminescence detection kit (LiteAblot TURBO; Euroclone, Italy).
2.5 Luciferase assay
HuH7 or HEK293 cells were seeded in a 48-well plate and were transiently transfected at 70%–80% confluency with pGL3-BRE-Luc plasmid (200 ng)14 and 15 ng of pRL-TK Renilla luciferase vector (Promega, Madison, Wisconsin) using Metafectene (Biontex Laboratories GmbH). Eighteen hours after transfection cells were serum-starved (DMEM +2% FBS) for 4 h and, when indicated, incubated overnight with TAC (1 μg/mL) or BMP6 (0.1, 1, 10, or 100 ng/mL). Twenty-four hours later cells were lysed and luciferase activity was determined according to manufacturer's protocol (Dual Luciferase Reporter Assay, Promega). Relative luciferase activity was calculated as the ratio of Firefly (reporter) to Renilla luciferase activity and expressed as a multiple of the activity of cells transfected with the reporter alone.
2.6 Isolation of primary murine hepatocytes
In situ liver perfusion of 8-week-old C57BL/6N WT or mutant mice (Alk2flox/flox; Alk3flox/flox; Alk2flox/flox; Alb-Cre; Alk3flox/flox-Alb-Cre) was performed with Liver Perfusion Medium and Liver Digest Medium (Thermo Fisher Scientific; pump flux: 5 mL/min) as previously described.14 After liver digestion, debris and membranes were filtered through a 100 μm cell strainer. Hepatocytes were separated from nonparenchymal cells through low speed centrifugation (50 g for 3 min), resuspended in Williams-E medium (4% FBS, 1% P/S, Glutamax; Thermo Fisher Scientific) and plated into collagen-coated 12-well (2.5–3 × 105 cells/well; Corning-Thermo Fisher Scientific).
2.7 RNAi silencing and treatment of primary murine hepatocytes
Six hours after seeding, primary hepatocytes were transfected with 30 nM of siRNA targeting Fkbp12, Alk2, Alk3, Bmpr2, Acvr2a, or siRNA control (Thermo Fisher Scientific) using lipofectamine RNAiMAX (Thermo Fisher Scientific) according to manufacturer's instructions. Eighteen hours after transfection hepatocytes were serum starved (in Williams-E medium 0% FBS, 1% P/S) for 2 h and then treated with TAC (1 μg/mL) or BMP6 (100 ng/mL) for 8 h.
Catalog number and ID of siRNA from Silencer Select are tabled below:
| 4390847 | siRNA negative control 2 |
| 4390771, ID: s66082 | siRNA Fkbp12 (Fkbp1a) |
| 4390771, ID: s61924 | siRNA Alk2 (Acvr1) |
| 4390771, ID: s201097 | siRNA Alk3 (Bmpr1a) |
| 4390771, ID: s63049 | siRNA Bmpr2 |
| 4390771, ID: s61931 | siRNA Acvr2a |
To simulate iron overload in primary murine hepatocytes (mHCs), cells isolated from 8-week-old WT mice were seeded and starved for 2 h in serum-free media, and treated with Ferric Ammonium Citrate (100 μM), or vehicle, in serum-free media for 18 h. TAC (1 μg/mL) or BMP6 (100 ng/mL) was added and cells were incubated for 8 additional hours.
2.8 TAC treatment in mice
Twelve-week-old WT and Hjv KO male mice on an inbred 129S6/SvEvTac background were subcutaneously implanted with mini osmotic pumps (ALZET Osmotic Pumps, Cupertino, California) by surgical approach. Mice were treated with vehicle or 0.37 μg/h TAC (Cayman Chemical) for 28 days. Mice were then euthanized and tissues were collected for subsequent examinations.
Liver and spleen were harvested for tissue iron concentration. Liver was also collected for RNA isolation.
2.9 RNA isolation and gene expression analysis
Total RNA was isolated from hepatocytes or mouse tissues using TRIFAST (Euroclone) according to manufacturer's instruction. cDNA was synthetized with the High Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific). Gene expression levels were measured by quantitative reverse transcription polymerase chain reaction (qRT-PCR) using the SybrGreen or the TaqMan Gene Expression Master Mix (Thermo Fisher Scientific). Hypoxanthine Phosphoribosyltransferase 1 (Hprt1) and 18 S9 were used as housekeeping genes. Primers for qRT-PCR are in listed in Tables 1 and 2.
| Gene | Forward primer (5′➔3′) | Reverse primer (5′➔3′) |
|---|---|---|
| Hprt1 | TCCTCCTCAGACCGCTTTT | AACCTGGTTCATCATCGCTAA |
| Hamp | ATACCAATGCAGAAGAGAAGG | AACAGATACCACACTGGGAA |
| Id1 | ACCCTGAACGGCGAGATCA | TCGTCGGCTGGAACACATG |
| Alk2 (Acvr1) | ATTGAAGGGCTCATCACCAC | AAGACCGGAGCCACTTCC |
| Alk3 (Bmpr1a) | GCTCATTTCCATGGCTGTCT | CGACCCCTGCTTGAGATACT |
| Bmpr2 | GAGCACAGAGGCCCAATTCT | GCCATCTTGTGTTGACTCACCT |
| Acvr2a | CCCTCCTGTACTTGTTCCTAC | GCAATGGCTTCAACCCTAGT |
| Fkbp12 | ATGGGAGTGCAGGTGGAG | TCTTTCCATCTTCAAGCATCC |
| NFATC1 | CCAGATGCAGAGAGAGGCTG | TGCATTCGGCTCTTCTTGGT |
| IL4 | GCCTCACAGAGCAGAAGACTC | GACATGCATGCTGCTTGGAG |
- Abbreviation: RT-qPCR, quantitative reverse transcription polymerase chain reaction.
| Gene | Transcription assay ID |
|---|---|
| Hprt1 | Mm01318743_m1 |
| Hamp | Mm00519025_m1 |
| Id1 | Mm00775963_m1 |
| Smad7 | Mm03023958_m1 |
| Bmp2 | Mm01340178_m1 |
| Bmp6 | Mm01332882_m1 |
| Saa1 | Mm00656927_g1 |
| A2m | Mm00558642_m1 |
- Abbreviation: qRT-PCR, quantitative reverse transcription polymerase chain reaction.
2.10 Quantification of liver and spleen iron content
Liver and spleen samples were dried at 110°C and then digested in 1 mL of acid solution (3 M HCl, 0.6 M trichloroacetic acid) for 20 h at 65°C. Twenty microliters of acid extract were added to 1 mL of working chromogen reagent (1 volume of 0.1% bathophenanthroline sulfate and 1% thioglycolic acid solution, 5 volumes of water, and 5 volumes of saturated sodium acetate), incubated at room temperature for 30 min and the absorbance measured at 535 nm. The standard curve was prepared by adding an increasing amount of iron ammonium sulfate (Merck, Darmstadt, Germany) to the acid solution.
2.11 Statistics
Data are presented as mean ± SEM. Unpaired 2-tailed Student's t-test or 2-way analysis of variance was performed using GraphPad Prism 7.0 (GraphPad). A p < .05 was considered statistically significant.
3 RESULTS
3.1 Fkbp12 silencing in primary mHCs impairs TAC-mediated hepcidin upregulation
We previously reported that in human hepatoma cell lines FKBP12 limits hepcidin expression by binding to and inhibiting the BMP type I receptor ALK2.14 To investigate the role of FKBP12 in hepcidin regulation in a more physiological ex vivo setting, we silenced it in primary murine hepatocytes (mHCs). The efficiency of Fkbp12 downregulation was about 90% and did not affect the expression of BMPRIs and BMPRIIs (Figure S1A,B). The mRNA expression levels of the BMP-SMAD target genes Hepcidin and Id1 were higher in Fkbp12-silenced cells compared with controls (Figure S1C,D). This indicates that FKBP12 maintains low the basal BMP pathway activity also in primary cells, and its downregulation is sufficient to activate the signaling pathway.
We previously reported that Tacrolimus (TAC or FK506)—an immunosuppressive drug that binds to FKBP12 and, in complex with it, inhibits calcineurin—displaces FKBP12 from ALK2 and increases hepcidin expression both in hepatoma cell lines and in mHCs.14 To exclude the involvement of calcineurin in ALK2–FKBP12 interaction, we transfected human hepatoma HuH7 cells with ALK2 and FKBP12 and treated them with TAC or Cyclosporin A (CsA), which inhibits calcineurin independently of FKBP12. CsA, by inhibiting calcineurin, decreases the nuclear translocation of the Nuclear Factor of Activated T cells (NFAT) and indeed downregulated the NFAT target genes IL4 and NFATC1 (Figure S2A). Only TAC, but not CsA, abrogated the interaction between FKBP12 and ALK2 (Figure S2B) and increased SMAD1/5/8 phosphorylation (Figure S2C), showing that calcineurin does not modulate the BMP-SMAD signaling and suggesting that the effect of TAC on hepcidin depends only on FKBP12. To test this hypothesis, we treated Fkbp12-silenced mHCs with TAC. TAC significantly upregulated Hepcidin and Id1 in control mHCs but not in Fkbp12-silenced cells (Figure S2D,E). Overall these results demonstrate that TAC increases hepcidin expression in hepatocytes exclusively via FKBP12.
3.2 ALK2 and ACVR2A are essential for hepcidin regulation by FKBP12 in primary mHCs
Both BMPRIs and BMPRIIs are required to activate the BMP pathway. In hepatocytes, the type I receptors ALK2 and ALK39 and the type II receptors ACVR2A and BMPR210 play a role in hepcidin expression in vivo. Which of these receptors are modulated by FKBP12 and are responsible for hepcidin upregulation in response to TAC is unknown.
To investigate this, we isolated mHCs from hepatocyte-specific Alk2-deficient and Alk3-deficient mice (KD)9 and treated them with TAC to sequester FKBP12. Alk2 KD and Alk3 KD mice are characterized by mild and severe iron overload, respectively, as previously published.9 Alk2 and Alk3 expression was greatly reduced in hepatocytes (Figure S3A), and Hepcidin and Id1 more strongly downregulated in Alk3 KD than in Alk2 KD cells, as expected (Figure S3B,C). TAC upregulated both Hepcidin and Id1 expression in control mHCs, but not in Alk2 KD and Alk3 KD mHCs (Figure S3D,E), suggesting that ALK2 and possibly ALK3 are needed for BMP-SMAD pathway modulation following FKBP12 sequestration. However, Alk3 KD hepatocytes are more severely iron loaded, which could differentially affect hepcidin upregulation by TAC and BMP6 (Figure S4A,B), as already reported.16 To exclude the influence of intracellular iron accumulation, we silenced Alk2 and Alk3 in WT mHCs. The KD efficiency was about 90% for both genes (Figure S5A) and siRNA knockdown specificity was verified (data not shown). Downregulation of Alk2 only mildly inhibited Hepcidin and Id1 expression, whereas Alk3 silencing strongly reduced the expression of both genes (Figure S5B,C), in agreement with the corresponding KD mouse models.9 When we treated Alk2-silenced and Alk3-silenced mHCs with TAC, Hepcidin (Figures 1A and S6A) and Id1 mRNA expression (Figures 1B and S6B) was still upregulated in Alk3-silenced hepatocytes. On the other hand, Alk2 silencing completely abrogated Hepcidin (Figures 1A and S6A) and Id1 mRNA expression (Figures 1B and S6B) in response to TAC. We conclude that FKBP12 sequestration increases BMP-SMAD pathway activation and hepcidin expression mainly through ALK2.
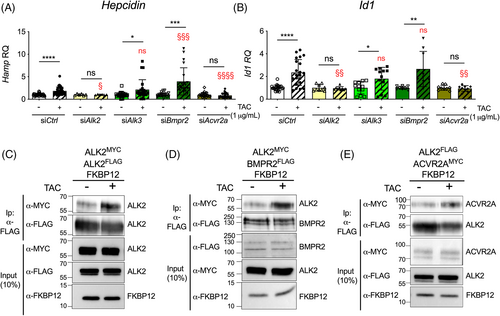
We then silenced the type II receptors BMPR2 and ACVR2A in primary mHCs with 80%–85% efficiency (Figure S5A) and verified siRNA specificity (data not shown). It has been demonstrated that BMPR2 and ACVR2A redundantly regulate hepcidin in vivo.10 However, we observed that Bmpr2 silencing mildly but significantly decreased Hepcidin expression, whereas Id1 mRNA levels remained comparable to control cells (Figure S5B,C). Surprisingly, Acvr2a KD upregulated both Hepcidin and Id1 (Figure S5B,C). Since ACVR2A can form nonsignaling complexes with type I receptors in the absence of ligands,17 we hypothesize that its silencing activates the BMP-SMAD pathway by releasing ALK2 and/or ALK3 and making them available for the formation of active receptor complexes, likely with BMPR2. Upon FKBP12 sequestration by TAC, Hepcidin (Figures 1A and S6A) and Id1 (Figures 1B and S6B) upregulation was preserved in Bmpr2-silenced cells but abolished in Acvr2a-silenced mHCs.
Overall, these data demonstrate that ALK2 and ACVR2A are indispensable for TAC-mediated Hepcidin and Id1 upregulation. Since FKBP12 interacts neither with ALK3 (Figure 4)14 nor with ACVR2A and BMPR2 (Figure S7A) in hepatic cells, we hypothesize that the effect of their silencing on the response to TAC is indirect and likely caused by changes in ALK2 interactions following FKBP12 sequestration.
3.3 FKBP12 and BMP6 regulate the BMP-SMAD pathway through the same BMP receptors
An oligomeric receptor complex composed of type I and type II receptors is required for BMP signaling initiation in response to the binding of the ligand. How FKBP12 regulates this process is at present unknown. Since there are no validated commercial antibodies against BMP receptors, we overexpressed tagged receptors in hepatoma cell lines. HuH7 cells transfected with MYC-tagged and FLAG-tagged ALK2 together with FKBP12 were treated with TAC for 1 h, a timeframe sufficient to completely displace FKBP12 from ALK2 (Figure S2B). The rapid FKBP12 sequestration increased ALK2–ALK2 interaction (Figure 1C) and boosted SMAD1/5/8 phosphorylation (Figure S2C). Interestingly, it was previously shown that ALK2 may also form hetero-oligomers with ALK3.11 Although ALK3 does not interact with FKBP12 in hepatic cells (Figure 4),14 its downregulation impaired the activation of the SMAD signaling pathway by TAC treatment (Figure 1A,B). Thus, we asked whether FKBP12 displacement from ALK2 could affect the ALK2-ALK3 interaction. As shown in Figure S7B, the rapid FKBP12 sequestration by TAC raised ALK2 interaction with ALK3, explaining why Alk3 silencing reduced the BMP-SMAD pathway activation in response to TAC in mHCs.
Overall, our results show that the pharmacological sequestration of FKBP12 increases both ALK2–ALK2 homo-oligomers and ALK2–ALK3 hetero-oligomers.
To activate downstream SMAD1/5/8 proteins, type I receptors have to interact with and be phosphorylated by type II receptors. To investigate whether FKBP12 sequestration by TAC favors the formation of ALK2/BMPRIIs complexes, HuH7 cells were transiently transfected with ALK2, FKBP12, and BMPR2 or ACVR2A, and receptor interaction was investigated by coimmunoprecipitation in the presence or absence of TAC. The interaction between ALK2 and both BMPRIIs, already detectable in control cells, increased after TAC treatment (Figure 1D,E). Since we could not detect any binding between the immunophilin and the BMPRIIs (Figure S7A), we conclude that the increased interaction is due to FKBP12 displacement from ALK2, likely because the receptor becomes more accessible to BMPRII.
Overall, these results indicate that ALK2, when free from FKBP12, interacts more efficiently with both type I and type II receptors to activate the signaling.
BMP6 binds preferentially to ALK212 and decreases ALK2–FKBP12 interaction in hepatoma cell lines.14 When we studied the response to BMP6, we found that BMP6 failed to upregulate Hepcidin in Alk2-silenced and Acvr2a-silenced cells (Figures 2A and S6C), and in hepatocytes isolated from the Alk2-liver conditional KD mice (Figure S8A). This was due to impaired BMP-SMAD pathway activation, as demonstrated by the blunted Id1 expression in silenced primary hepatocytes (Figures 2B and S6D) and in Alk2-KD cells (Figure S8B). Interestingly, the enhanced expression of Hepcidin and Id1 in response to BMP6 was reduced in Alk3-silenced mHCs (Figures 2A,B) and comparable to the Cre-negative control cells in Alk3-KD hepatocytes (Figure S8A,B). BMP6 strongly increased Hepcidin and Id1 expression also in Bmpr2-silenced hepatocytes (Figures 2A and S6C; Figures 2B and S6D). These results indicate that ALK2 and ACVR2A are indispensable for Hepcidin upregulation by BMP6.
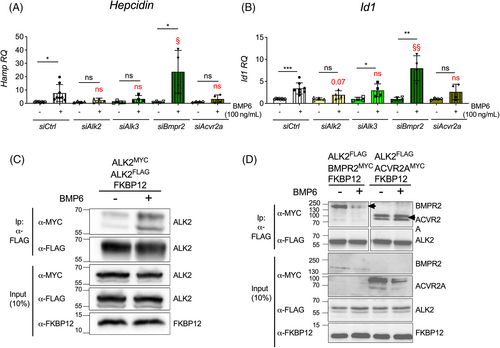
When we analyzed the effect of BMP6 on the ALK2 interaction with BMPRIs and BMPRIIs, we found that 1 h BMP6 treatment increased both ALK2–ALK2 and ALK2–ALK3 binding (Figures 2C and S7B). However, at difference with TAC, BMP6 favored ACVR2A–ALK2 binding and reduced BMPR2–ALK2 interaction (Figure 2D). This finding is consistent with a role for the ligand in the recruitment of a subset of BMPRIIs by the FKBP12-free ALK2.
Overall, these data demonstrate that both BMP6 and FKBP12 predominantly act on the type I receptor ALK2 and prompted us to investigate whether TAC and BMP6 synergize to activate the BMP-SMAD pathway and increase hepcidin expression.
3.4 BMP6 and TAC cooperate in BMP-SMAD pathway activation and hepcidin upregulation both in vitro and in vivo
By increasing the formation of BMPRIs-BMPRIIs oligomers, FKBP12 sequestration might enhance the response to the ligand BMP6. To test this, HuH7 cells transfected with ALK2 and FKBP12 were pretreated with TAC and then exposed to BMP6. SMAD1/5/8 phosphorylation by BMP6 was greater in TAC-treated cells compared with vehicle-treated cells (Figure 3A) and accordingly, the luciferase activity of HuH7 cells transfected with the BMP-responsive element (BRE)-Luc vector was increased compared with cells treated with TAC or BMP6 alone (Figure 3B). Since the luciferase levels are regulated by the SMAD1/5/8-SMAD4 transcriptional complex we conclude that FKBP12 sequestration sensitized hepatic cells to BMP6, increasing the BMP-SMAD pathway activation in response to lower concentrations of the ligand.
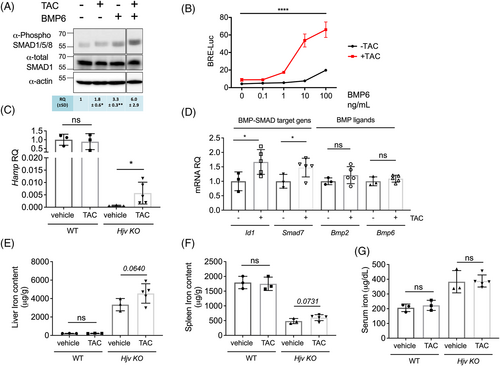
To investigate whether TAC and BMP6 cooperate in enhancing the BMP-SMAD signaling in vivo, we took advantage of the Hjv KO mice, a model of severe hereditary hemochromatosis caused by mutations in the BMP coreceptor hemojuvelin (HJV) and characterized by liver BMP-SMAD pathway inhibition, low hepcidin but increased liver Bmp6 secondary to iron overload.18 Hjv KO and WT mice were treated with a nonimmunosuppressive TAC dosage19 administered through subcutaneously implanted miniosmotic pumps for 28 days. This treatment upregulated hepcidin expression in Hjv KO animals but not in WT mice (Figure 3C) by activating the BMP-SMAD pathway, as shown by the increased Id1 and Smad7 mRNA levels (Figure 3D). The expression of the ligands Bmp2 and Bmp6, (Figure 3D) and of the inflammatory target genes Saa1 and A2m (Figure S9) remained unchanged, excluding that TAC upregulates hepcidin through the inflammatory pathway. However, in this experimental setting, TAC was unable to increase hepcidin to a pharmacologically relevant level able to ameliorate the severe iron overload of Hjv KO mice. In fact, we only observed a trend towards an increase in liver (Figure 3E) and spleen iron content (Figure 3F) following TAC treatment in Hjv KO mice, suggesting tissue iron redistribution. In contrast, serum iron levels remained unchanged (Figure 3G). We previously reported that the absence of Hjv in hepatocytes does not impair the response to TAC ex vivo.14 This led us to speculate that TAC may support the release of FKBP12 from ALK2 in response to BMP6, which is increased in this model, making the receptor available to interact with BMPRIs and IIs. Thus, TAC increases the formation of primed ALK2 complexes that can trigger the signaling cascade and work synergistically with BMP6 to increase pathway activation both in vitro and in vivo. However, we cannot exclude the possibility that in conditions of severe iron overload, iron accumulation in hepatocytes may blunt hepcidin upregulation in response to TAC/BMP6, as observed in iron-loaded primary mHCs (Figure S4).
3.5 ALK3 activity and FKBP12 binding capacity are connected
FKBP12 interacts with both ALK2 and ALK3 in HEK293 but only with ALK2 in HuH7 cells, suggesting the existence of a cell type-specific mechanism in the regulation of their binding, that is disrupted by TAC treatment in both cell lines (Figure 4A). To gain a better understanding of this mechanism, we further analyzed ALK3-mediated activation of the BMP-SMAD pathway. By using a BRE-Luc luciferase assay, we observed that ALK3 activates the pathway more strongly in hepatoma cells than in HEK293 cells (Figure 4B). In contrast, ALK2 — which displays stable interaction with FKBP12 in both cell lines — fails to activate the SMAD signaling pathway (Figure 4A,B). To analyze the correlation between receptor activation and FKBP12 interaction, HuH7 cells were transfected with ALK3 and FKBP12 and treated with the BMPRI inhibitor LDN193189, an ATP competitive inhibitor that prevents receptor activation by blocking the ATP binding pocket.15 Interestingly, we observed a partial rescue of the ability of ALK3 to bind FKBP12 (Figure 4C, left panel), indicating that ALK3 activity and FKBP12 binding are strictly interconnected.
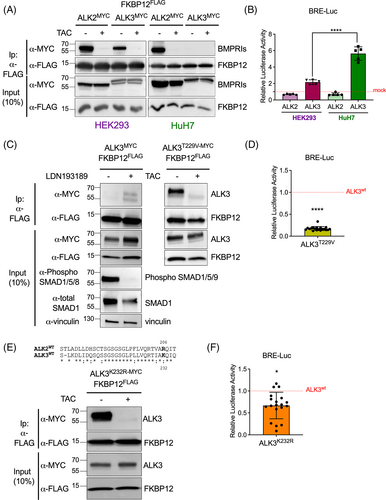
Since the activation of the type I receptors is mediated by the phosphorylation of their intracellular GS-rich domain (the domain recognized by FKBP1215), we generated an ALK3 “dead” mutant by replacing Threonine 229 — a conserved amino acid residue critical for signal transduction in the GS-rich domain — with a Valine (ALK3T229V).20 In contrast with the WT receptor, the activity of the ALK3 variant T229V was reduced (Figure 4D). Consistently with ALK3 inhibition by LDN193189, this ALK3 variant could interact with FKBP12 and their binding was reversed by TAC treatment (Figure 4C, right panel). Therefore, we hypothesize that the T229V mutation, by changing the conformation of the receptor and locking it in an inactive state, stabilizes its interaction with FKBP12.
To better understand the relationship between FKBP12 binding and ALK3 activity, we generated an ALK3 mutant capable of binding FKBP12 while maintaining, in principle, its kinase activity. The GS-rich domain is highly conserved between ALK3 and ALK2 (Figure 4E). One of the few differences is the Lysine residue 232 of ALK3, which corresponds to the Arginine residue 206 in ALK2. The mutation R206H in ALK2 causes Fibrodyslasia Ossificans Progressiva—a disease caused by the over-activation of ALK2 as a result of defective FKBP12 binding15—suggesting that this residue might play a crucial role in regulating the binding affinity with FKBP12. Replacing the Lysine 232 of ALK3 with an Arginine (to make the GS domain more similar to that of ALK2) was sufficient to induce a stable interaction between ALK3 and FKBP12 (Figure 4E) and to reduce the receptor activity (Figure 4F).
Overall, these data suggest that ALK3 has a lower affinity for FKBP12 than ALK2 and that the activation status of ALK3 and its ability to bind to FKBP12 are strictly connected, which might help explain the cell-type specificity. However, the exact relationship between FKBP12 binding and ALK3 inhibition remains unclear: it is possible that FKBP12 binding is the cause of ALK3 inhibition, or vice versa it could be a consequence. Further experiments are needed to determine the exact nature of this relationship and understand how the interaction between type I receptors and FKBP12 is regulated in different cell types, which could reveal new mechanisms underlying the cell type-specific response to various BMP ligands.
4 DISCUSSION
The BMP-SMAD pathway is involved in several biological processes, including the control of systemic iron homeostasis through the regulation of hepcidin expression in hepatocytes. Given the broad function of the BMP-SMAD pathway, its activation has to be titrated according to tissue and organ needs. This is achieved through the formation on the cell surface of a hexameric complex composed of dimeric BMPRIs and BMPRIIs, and dimeric ligands. The composition of the hexameric complex varies according to the cell type and the biological processes in which it is involved. In hepatocytes, the type I receptors ALK2 and ALK3 and the type II receptors ACVR2A and BMPR2 drive hepcidin expression in response to the ligands BMP2 and BMP6.5
The function of ALK2 and ALK3 in hepatocytes is nonredundant and, according to the iron phenotype of in vivo models, they activate the BMP-SMAD pathway in response to different stimuli using different receptor complexes.9 Biochemical studies have shown that ALK2 is mainly involved in iron-mediated hepcidin upregulation through the iron-responsive ligand BMP6, whereas ALK3 controls basal hepcidin levels. In agreement, Alk2 deficiency in mHCs has moderate effects on systemic iron metabolism in steady-state conditions, whereas hepatocyte-specific Alk3 deficiency severely affects hepcidin expression, causing iron overload at baseline.9 Moreover, the combined hepatocyte-specific Alk2 deficiency in Alk3 KD mice further worsens the iron overload.11
Fine-tuning the BMP-SMAD pathway is needed to avoid excessive or defective hepcidin production and thus iron deficiency or overload, respectively. This fine-tuning is mediated by different accessory proteins. The BMP-coreceptor HJV, the second transferrin receptor TFR2, and the hemochromatosis protein HFE positively regulate the BMP-SMAD pathway.5 Interestingly, HJV and HFE signal mainly through ALK3.12, 21 To inhibit the BMP-SMAD pathway, the serine protease TMPRSS6 cleaves HJV22 and other components of the BMP pathway23 (or interferes with the signaling independently of its protease activity)24 and the immunophilin FKBP12 blocks ALK2.14
To gain more insights into the mechanism(s) of FKBP12-mediated hepcidin regulation, we took advantage of the rapid (1 h) FKBP12 sequestration that can be achieved with the immunosuppressive drug TAC. TAC preferentially interacts with FKBP12 to promote calcineurin inhibition14, 25 and activates the BMP-SMAD pathway by displacing FKBP12 from ALK2, independently of the downstream calcineurin inhibition (this manuscript).14, 25 Here, we demonstrate that FKBP12 modulates the BMP-SMAD pathway by binding exclusively ALK2 and by signaling preferentially through the type II receptor ACVR2A in hepatic cells (Figure 5A). Alk2 or Acvr2a silencing in primary mHCs fully abrogates the TAC-mediated pathway activation, whereas Alk3 silencing impairs BMP-SMAD pathway upregulation by TAC, and Bmpr2 downregulation has no effect. Interestingly, when released from FKBP12 inhibition, ALK2 shows enhanced capacity to form both homo-oligomers and hetero-oligomers with ALK3 and to interact with the type II receptors BMPR2 and ACRV2A. Since BMPRI activation requires interaction with BMPRIIs, we hypothesize that sequestering FKBP12 promotes the formation of a functionally active receptor complex (Figure 5A).
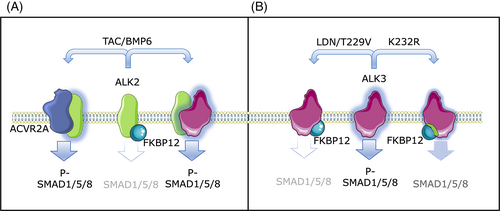
In vitro BMP6 signals preferentially through ALK2.12 BMP6-mediated hepcidin and Id1 upregulation depends on ALK2 and ACVR2A, similar to what is observed in TAC-treated hepatocytes and in agreement with the ability of BMP6 to displace FKBP12 from ALK2 (Figure 5A and14). In keeping with this, TAC and BMP6 cooperate in upregulating hepcidin and Id1 both in vitro and in vivo, as shown in the Hjv KO mice characterized by increased Bmp6 expression due to iron overload. In contrast to the single high-dose treatment,14 chronic treatment with a reduced TAC dosage is not sufficient to upregulate hepcidin in WT mice. We speculate that this is due to the inability of the drug to fully sequester FKBP12 and sensitize ALK2 to BMP6. In agreement, acute TAC treatment14 and the proteolysis-targeting chimera (PROTAC)-mediated FKBP12 degradation26 successfully upregulate hepcidin in WT mice. Since the absence of HJV per se does not impair the TAC-mediated effect on the BMP-SMAD pathway in mHCs,14 our data suggest that TAC contributes to FKBP12 displacement from the receptor and favors further activation of the pathway in vivo when BMP6 levels are increased as in iron overload.
The BMPRIIs play a different role in the modulation of the BMP-SMAD signaling in response to TAC and BMP6. In Bmpr2-silenced hepatocytes, TAC treatment upregulates Hepcidin and Id1 to a higher level compared with TAC-treated control cells. These results suggest that BMPR2 inhibits ALK2-mediated signaling at steady state, likely by decreasing ALK2–ACVR2A oligomerization, reported to be essential for the activation of ALK2 by BMP6.27 In agreement, Acvr2a silencing abrogates the effect of TAC and BMP6 on the BMP-SMAD pathway (Figure 5A). It is also worth noting that BMP6 treatment increases ALK2–ACVR2A interaction but reduces ALK2–BMPR2 binding.
Interestingly, in contrast to the previous publication by Traeger et al.,11 we were able to detect ALK2 homo-oligomers in the absence of exogenous BMP ligands, likely because in our experimental setting FKBP12 is coexpressed with BMP receptors for coimmunoprecipitation studies. The presence of FKBP12 might influence BMP receptor oligomerization through conformational changes due to the binding of FKBP12 with the ALK2 intracellular GS-rich domain. This might be a physiologic mechanism to regulate the assembly of preformed type I and type II complexes independently of the ligands, which might remain inactive until ligand binding displaces FKBP12 and triggers the signaling cascade.28
The existence of inactive preformed complexes has been demonstrated by studies on the ALK2R206H variant, in which the amino acidic substitution renders the receptor constitutively active due to the reduced FKBP12 binding. The overexpression of the Alk2 WT allele in the ALK2R206H mutant mice decreases the BMP-SMAD pathway activation, whereas deletion of the WT allele in the heterozygous ALK2R206H mutant mice worsens the phenotype by upregulating the pathway.29 In agreement with the existence of inactive preformed complexes, the reduced BMP-SMAD signaling induced by the overexpression of the Alk2 WT allele is likely mediated by the formation of ALK2wt–ALK2R206H heterodimers.25
On the other hand, activation of BMP type I receptors can influence the FKBP12 binding by changing the accessibility of the GS-rich domain. We observed that at difference with other cell types, ALK3 does not interact with FKBP12 in hepatoma cells unless the receptor activity is reduced through mutagenesis or treatment with a BMP-SMAD inhibitor (Figure 5B). The evidence that the different activation status of ALK3 influences the ability of the receptor to stably interact with FKBP12 is relevant since drugs sequestering FKBP12, such as TAC and Rapamycin, are commonly used in the clinical practice and may cause a more or less pronounced activation of the BMP-SMAD pathway depending on the cellular context.
In summary, we have shown that FKBP12 modulates the activation of the BMP-SMAD pathway by binding ALK2 and regulating its functional interaction with ALK3, ACVR2A, and BMPR2. The possibility of decreasing the binding of FKBP12–ALK2 is worth being explored as a novel tool to increase hepcidin expression in diseases characterized by elevated BMP6 levels, such as hereditary hemochromatosis or conditions with ineffective erythropoiesis. In support of this hypothesis, FKBP12 degradation in vivo was shown to increase hepcidin and reduce serum iron in WT mice,26 an approach that is worth being tested in preclinical models of iron overload disorders.
This study has potential limitations. First, BMP6, the only ligand whose expression is upregulated in iron overload, may act not only as a homodimer but also as a heterodimer with BMP2. This is particularly intriguing as BMP6 has a high affinity for ALK2, while BMP2 is known to bind ALK3, suggesting that the BMP6–BMP2 heterodimer may interact with the ALK2–ALK3 heterodimeric receptor complex, adding another layer of complexity to the system. Interestingly, our data show that BMP6, by displacing FKBP12 from ALK2, can enhance the formation of the heterodimeric ALK2–ALK3 receptor complex. Second, in vivo data show that the displacement of FKBP12 from ALK2 by TAC can be effective in WT mice when the drug is administered acutely, but not when administered chronically. This may suggest that complete sequestration of FKBP12 is required to activate ALK2 or that alternative mechanisms are activated in the long term to compensate for ALK2 activation and hepcidin increase. Further studies on endogenous proteins will help to fully elucidate the role of FKBP12 in hepcidin regulation and BMP-SMAD signaling in vivo.
AUTHOR CONTRIBUTIONS
Mariateresa Pettinato and Alessandro Dulja performed research, analyzed results, and contributed to article writing. Silvia Colucci performed experiments and contributed to article editing. Valeria Furiosi and Franca Fette performed experiments. Andrea U. Steinbicker contributed to study design of the hepatocyte-specific Alk2 and Alk3 deficient cells, data analysis and article editing. Martina U. Muckenthaler participated to data analysis and article editing. Antonella Nai performed experiments and contributed to critical data analysis and article writing. Laura Silvestri and Alessia Pagani conceived the experiments, analyzed results and wrote the article. All Authors approved the final version of the article.
ACKNOWLEDGMENTS
The authors are indebted to Prof. Clara Camaschella for criticism and invaluable suggestions. This work was supported by the Fondazione Regionale per la Ricerca Biomedica (FRRB) to Alessia Pagani (Grant number: 1749055); by Cariplo Foundation (“Young Investigator” Grant no. 2017-0916), European Hematology Association (Advanced Research Grant 2020) and Italian Ministry of Health (GR-2019-12369583) to Antonella Nai; by the Cariplo Telethon Alliance GJC2021 (GJC21117) to Laura Silvestri; by the German Research Foundation (Deutsche Forschungsgemeinschaft) grant STE 1895/9-1 and STE 1895/10-1 to Andrea U. Steinbicker as part of the German research consortium FerrOS 5146. We acknowledge Boehringer Ingelheim Fonds Travel Grant for supporting Mariateresa Pettinato, SFB1036 and DFG (FerrOs-FOR5146) for providing research funding to Martina U. Muckenthaler. We acknowledge SMART servier medical art (https://smart.servier.com/) for the graphic tools used to generate the figures. Open access funding provided by BIBLIOSAN.
CONFLICT OF INTEREST STATEMENT
Antonella Nai received research funding from Celgene (BMS group). Andrea U. Steinbicker is sponsored by a research grant from Pharmacosmos, Denmark. The other authors declare no conflict of interests.
Open Research
DATA AVAILABILITY STATEMENT
Data available on request from the authors.




