Venous thromboembolism risk in cancer patients receiving first-line immune checkpoint inhibitor versus chemotherapy
Abstract
It remains unclear if immune checkpoint inhibitor (ICI) therapy is associated with higher rate of venous thromboembolism (VTE) compared with cytotoxic chemotherapy (chemo) in patients with comparable cancer type, staging, and comorbidities. Using the national Veterans Affairs healthcare system database from 2016 to 2021, we performed a propensity score (PS)-weighted retrospective cohort study to compare the incidence of VTE in patients with selected stage III/IV cancer receiving first-line ICI versus chemo. The PS model utilized overlap weights to balance age, sex, race, treatment year, VTE history, paralysis/immobilization, prolonged hospitalization, cancer type, staging, time between diagnosis and treatment, and National Cancer Institute comorbidity index. Weighted Cox regressions with robust standard error were used to assess the hazard ratio (HR) and 95% confidence interval (CI). We found that among comparable advanced cancers, first-line ICI (n = 1823) and first-line chemo (n = 6345) had similar rates of VTE (8.49% for ICI and 8.36% for chemo at 6 months). The weighted HR was 1.06 (95% CI 0.88–1.26) for ICI versus chemo. In a subgroup analysis restricted to lung cancers, first-line ICI/chemo (n = 828), ICI monotherapy (n = 428), and chemo monotherapy (n = 4371) had similar rates of VTE (9.60% for ICI/chemo, 10.04% for ICI, and 8.91% for chemo at 6 months). The weighted HR was 1.05 (95% CI 0.77–1.42) for ICI versus chemo, and 1.08 (95% CI 0.83–1.42) for ICI/chemo versus chemo. In conclusion, ICI as a systemic therapy has a similarly elevated risk as cytotoxic chemo for VTE occurrence in cancer patients. This finding can inform future prospective studies exploring thromboprophylaxis strategies.
1 INTRODUCTION
Immune checkpoint inhibitors (ICIs), including monoclonal antibodies targeting programmed cell death 1 (PD-1), programmed cell death ligand 1 (PD-L1), and cytotoxic T lymphocyte antigen 4 (CTLA-4) pathways, have become an essential backbone of antineoplastic treatment over the past decade.1 In different cancer types, such as nonsmall cell lung cancer (NSCLC), melanoma, and renal cell carcinoma, among others, ICI has emerged as the recommended therapy in appropriately selected patients.2-4 In fact, evolving data supporting the survival benefit in patients treated with ICI have not only led to approval in most types of cancers, but they have increasingly moved this treatment modality to the upfront setting.
While ICI is commonly associated with immune-related toxicities, venous thromboembolism (VTE) is not a frequently reported complication in randomized controlled trials (RCTs). A recent meta-analysis that compiled data from 29 861 patients in 50 RCTs found a pooled VTE incidence of only 1.7% in patients treated with ICI.5 In contrast, another recent systematic review found the incidence of VTE reported from observational cohort studies to be much higher at approximately 5–8% at 6 months.6 Interpretation of the findings of these observational studies is complicated by the fact that ICI recipients often have progressive cancers refractory to multiple prior lines of therapy, and prior studies often do not distinguish first-line and subsequent lines of therapy.7-10 Consequently, current hematology and oncology society risk stratification guidelines for pharmacologic thromboprophylaxis do not specify the inclusion of ICIs as systemic therapy type conferring elevated risk of thrombosis.11, 12
In the current study, we assessed the incidence of VTE associated with first-line ICI versus first-line chemo among comparable patients with matched cancer types, staging, and comorbidities. Our goal was to determine if ICI as a systemic therapy type is associated with higher risk of VTE when compared with traditional cytotoxic chemo.
2 METHODS
2.1 Study design, group assignment, and baseline variables
We performed a retrospective cohort study using data from the national Veterans Affairs (VA) healthcare system. Patients with an incident cancer diagnosis during 2016–2021 were identified from the VA Cancer Registry and linked to electronic health record data in the VA Corporate Data Warehouse (CDW). The index date of the study was the time of first-line systemic therapy initiation after cancer diagnosis. Patients were excluded if they had benign histology, in situ stage, nonprimary malignancy, nonprimary VA healthcare user, did not receive systemic or oral anticancer therapy within 1 year after diagnosis, or had missing Khorana score components (Figure 1). We also excluded patients with recent diagnosis of acute VTE within 6 months before index date or who were prescribed anticoagulant therapy within 1 month prior to the index date, as the goal was to assess incident VTE rate after treatment initiation in patients not receiving prophylactic or therapeutic anticoagulant at baseline. Detailed description of data harmonization, variable selection, and outcome validation can be found in our previously published study.13 The study was approved by the Institutional Review Board (IRB) from VA Boston Healthcare System.
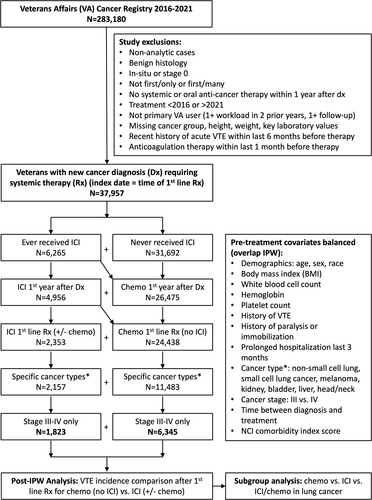
Among those meeting the cohort inclusion criteria, we selected patients receiving ICI (atezolizumab, avelumab, cemiplimab, durvalumab, nivolumab, pembrolizumab, or ipilimumab) (exposed cohort) versus those receiving cytotoxic chemo without ICI (unexposed cohort) as the first-line systemic therapy. To account for multiday regimen, first-line therapy was defined as first-ever systemic antineoplastic medications given within one cycle up to 30 days. Patients receiving first-line targeted or endocrine monotherapies without ICI or chemo were excluded from the study (Table S1). We further restricted patients to cancer types with at least 100 cases of first-line ICI use (NSCLC, small cell lung cancer (SCLC), melanoma, kidney, bladder, liver, and head/neck) and advanced stage (III or IV) disease to facilitate matching (Figure 1). Additional baseline confounders extracted from CDW at the time of first-line ICI or chemo receipt (index date) included age, sex, race, treatment year, history of VTE, history of paralysis or immobilization, recent prolonged hospitalization within the last 3 months, cancer type, cancer stage, time interval between diagnosis and treatment, National Cancer Institute (NCI) comorbidity index (2021 version),14 antiplatelet prescription, and other Khorana Score15 components (body mass index, hemoglobin, white blood cell count, and platelet count). Furthermore, the number of patients from each VA station was used to examine its association with both exposure and outcome to ensure the absence of center-specific confounding.
2.2 VTE outcome
The primary outcome of the study was overall VTE defined as radiologically confirmed symptomatic or incidental pulmonary embolism (PE), proximal or distal lower extremity deep venous thrombosis (LE-DVT), or upper extremity DVT (UE-DVT).16 Secondary outcome included PE/LE-DVT and overall survival. Unusual site thromboses, such as superficial venous thrombosis, cerebral venous thrombosis, and splanchnic venous thrombosis were excluded due to frequent admixture of tumor thrombi. VTE outcomes were evaluated from the index date of systemic therapy initiation until first outcome event, death, loss of follow-up defined as a 90-day gap without any clinical encounters, or administrative censoring on April 1, 2022. Survival outcome was evaluated from index date until known death or last contact date.
The VTE ascertainment algorithm utilized a combination of structured data (international classification of diseases (ICD) codes, medication) and unstructured data (natural language processing (NLP) extracted radiology impressions) described in further detail in a previous publication.17 We retested the final computable phenotype algorithm in the current VA cohort and achieved a sensitivity of 96% and a positive predictive value (PPV) of 91%.13
2.3 Statistical analysis
In addition to the initial cohort exclusion, we used inverse probability of treatment weighting (IPTW) to balance potential residual confounders. Overlap weight, a type of balancing weight to define a subpopulation that receives all treatments under study in substantial proportions, was estimated by fitting propensity score (PS) logistic regression models on treatment assignment using baseline covariates via the PSweight package.18, 19 The two baseline variables not included in the PS estimation were treatment year and antiplatelet prescription—increasing treatment year was strongly associated with ICI assignment but not with VTE outcome (definition of an instrumental variable that should be avoided in PS20) and antiplatelet prescription had no apparent association with VTE outcome though over-the-counter aspirin data could be under-captured. A PS model using two treatment groups (ICI vs. chemo) was estimated for the primary analysis in all relevant cancer types. A separate PS model using three treatment groups (ICI vs. ICI/chemo vs. chemo) was estimated for the secondary analysis in lung cancer patients since this was the only subgroup that received significant number of ICI/chemo combination. Covariate balance after PS weighting was checked via standardized mean difference (SMD) where an SMD of <0.10 was considered adequate balance. Incidence of VTE and PE/LE-DVT was estimated using unweighted and weighted Kaplan–Meier (KM) curves at 6 months. Hazard ratios (HRs) and 95% confidence intervals (CIs) for VTE and PE/LE-DVT as well as overall survival were estimated using unweighted and weighted Cox regression with robust standard errors.21, 22 For sensitivity analysis, multivariable Fine–Gray proportional subhazards model was used to ensure replicable findings. All statistical analyses were performed in R (Vienna, Austria 4.2.2).
3 RESULTS
In the national VA healthcare system, 37 957 patients with new cancer diagnosis from 2016 to 2021 requiring upfront systemic therapy within 1 year of diagnosis met the inclusion criteria (Figure 1). Overall, 6265 (16.5%) patients received ICI at some time point after cancer diagnosis; however, only 4956 (13.1%) patients received ICI within the first year of cancer diagnosis, and only 2353 (6.2%) patients received ICI as the first-line regimen without antecedent chemo. When restricted to selected cancer types (NSCLC, SCLC, melanoma, kidney, bladder, liver, and head/neck) and advanced stage (III-IV), 1823 patients (4.8%) remained in the ICI cohort (972 ICI/chemo, 851 ICI only). The ICI breakdown (non-mutually exclusive) included 1119 pembrolizumab, 344 nivolumab, 311 atezolizumab, 109 ipilimumab, 37 durvalumab, and 2 avelumab. In comparison, 6345 patients met the same selection criteria in the cytotoxic chemo cohort without ICI. There was a clear trend of increasing ICI and decreasing chemo treatment from 2016 to 2021. The odds of receiving first-line chemo over ICI were 43.1 in 2016, 10.9 in 2017, 3.5 in 2018, 1.5 in 2019, and 1.2 in 2020–21. There was no clear change in VTE rate in either group during the 6-year study period.
In the naïve comparison, first-line ICI and chemo cohorts had similar distribution (SMD <0.1) in sex, race/ethnicity, BMI, leukocyte, hemoglobin, platelet count, history of VTE, paralysis, recent hospitalization, and NCI comorbidity index (Table 1). However, the two groups were not balanced (SMD >0.1) in age, cancer type, cancer stage, and time to therapy. When compared with patients in the chemo cohort, those in the ICI cohort were older, more likely to have melanoma (and less likely to have head and neck), and stage IV diagnosis with longer time to therapy initiation. After IPW using the overlap weight, all covariates were balanced with an SMD of 0.
| Pre-weighted variables | Post-weighted variables | |||||
|---|---|---|---|---|---|---|
| ICI (N = 1823) | Chemo (N = 6345) | SMD | ICI (ESS = 1449) | Chemo (ESS = 3178) | SMD | |
| Age in years, mean | 69.4 | 67.8 | 0.20 | 69.3 | 69.3 | 0.00 |
| Male, % | 96.5% | 96.0% | 0.03 | 96.0% | 96.0% | 0.00 |
| Race/ethnicity, % | ||||||
| White | 77.0% | 75.1% | 0.05 | 74.4% | 74.4% | 0.00 |
| Black | 15.7% | 19.1% | 0.09 | 18.6% | 18.6% | 0.00 |
| Hispanic | 3.3% | 3.2% | 0.00 | 3.6% | 3.6% | 0.00 |
| Asian Pacific Islander | 2.2% | 1.6% | 0.05 | 2.0% | 2.0% | 0.00 |
| Unknown | 1.7% | 1.0% | 0.06 | 1.4% | 1.4% | 0.00 |
| Cancer type, % | ||||||
| Non-small cell lung cancer | 54.1% | 48.9% | 0.10 | 62.7% | 62.7% | 0.00 |
| Small cell lung cancer | 14.8% | 20.0% | 0.14 | 20.8% | 20.8% | 0.00 |
| Melanoma | 16.0% | 0.2% | 0.60 | 1.3% | 1.3% | 0.00 |
| Kidney | 6.7% | 0.8% | 0.32 | 3.2% | 3.2% | 0.00 |
| Liver | 3.4% | 2.5% | 0.06 | 3.8% | 3.8% | 0.00 |
| Bladder | 2.1% | 4.4% | 0.13 | 3.2% | 3.2% | 0.00 |
| Head & neck | 2.9% | 23.2% | 0.63 | 5.0% | 5.0% | 0.00 |
| Stage at diagnosis, % | ||||||
| III | 22.4% | 47.6% | 0.55 | 20.7% | 20.7% | 0.00 |
| IV | 77.6% | 52.4% | 0.55 | 79.3% | 79.3% | 0.00 |
| Days to treatment initiation, mean | 71.4 | 58.7 | 0.22 | 62.6 | 62.6 | 0.00 |
| NCI comorbidity index, mean | 0.5 | 0.5 | 0.03 | 0.5 | 0.5 | 0.00 |
| Other pretreatment Khorana score values, mean | ||||||
| Body mass index | 27.4 | 27.5 | 0.01 | 26.9 | 26.9 | 0.00 |
| Leukocyte | 9.4 | 9.0 | 0.09 | 9.6 | 9.6 | 0.00 |
| Hemoglobin | 12.8 | 12.9 | 0.07 | 12.7 | 12.7 | 0.00 |
| Platelet | 283.4 | 274.4 | 0.08 | 286.7 | 286.7 | 0.00 |
| Other relevant VTE predictors, % | ||||||
| History of VTE | 5.1% | 4.5% | 0.03 | 5.3% | 5.3% | 0.00 |
| History of paralysis or immobility | 1.6% | 1.0% | 0.06 | 1.6% | 1.6% | 0.00 |
| History of recent hospitalization >3d | 30.1% | 31.3% | 0.03 | 33.8% | 33.8% | 0.00 |
- Abbreviations: Chemo, cytotoxic chemotherapy; ESS, effective sample size; ICI, immune checkpoint inhibitor; SMD, standardized mean difference; VTE, venous thromboembolism.
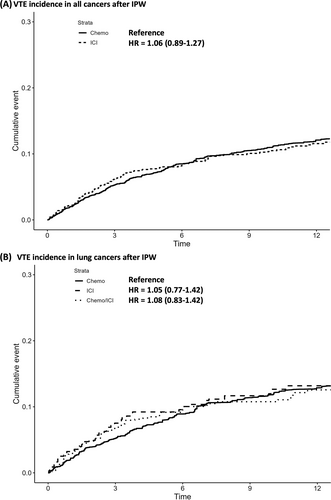
The median continuous follow-up for VTE assessment was 10.5 months (IQR 4.4–22.2) with a median follow-up for mortality assessment of 15.6 months (IQR 7.6–33.2). In the naïve analysis, VTE occurred in 7.71% of the ICI versus 7.54% of the chemo cohorts at 6 months with an unadjusted HR of 1.11 (95% CI 0.95–1.29). Specifically, the 6-month incidence for the four most common cancer subtypes receiving ICI was 9.55% in NSCLC, 8.96% in SCLC, 8.47% in kidney cancer, and 3.63% in melanoma. After IPW, VTE occurred in 8.36% of ICI versus 8.49% of chemo group at 6 months with a weighted HR of 1.06 (95% CI 0.88–1.26) (Figure 2A). PE/LE-DVT outcome followed a similar nonsignificant trend (Table 2). In the sensitivity analysis using multivariable competing model risk to also account for early death, ICI versus chemo had a similar SHR of 1.05 (95% CI 0.88–1.25) for VTE (Table S2). Overall survival post-IPW trended toward improved survival in the ICI group compared with the chemo group with a weighted HR of 0.94 (95% CI 0.88–1.01) (Figure S1A).
| Outcome | Treatment type | Pre-weighted analysis | Post-weighted analysis | |||||
|---|---|---|---|---|---|---|---|---|
| N | Incidence at 6 mo | HR (95% CI) | ESS | Incidence at 6 mo | HR (95% CI) | |||
| All cancers | Overall VTE | First-line chemo (no ICI) | 6345 | 7.54% | 1 | 3178 | 8.49% | 1 |
| First-line ICI (+/− chemo) | 1823 | 7.71% | 1.11 (0.95–1.29) | 1449 | 8.36% | 1.06 (0.88–1.26) | ||
| PE/LE-DVT | First-line chemo (no ICI) | 6345 | 6.22% | 1 | 3178 | 6.80% | 1 | |
| First-line ICI (+/− chemo) | 1823 | 6.37% | 1.14 (0.97–1.35) | 1449 | 6.93% | 1.07 (0.88–1.30) | ||
| Lung cancers | Overall VTE | First-line chemo (no ICI) | 4371 | 8.24% | 1 | 1592 | 8.91% | 1 |
| First-line ICI (no chemo) | 428 | 9.90% | 1.19 (0.91–1.55) | 386 | 10.04% | 1.05 (0.77–1.42) | ||
| First-line ICI/chemo | 828 | 9.20% | 1.14 (0.93–1.41) | 527 | 9.60% | 1.09 (0.83–1.42) | ||
| PE/LE-DVT | First-line chemo (no ICI) | 4371 | 6.82% | 1 | 1592 | 7.02% | 1 | |
| First-line ICI (no chemo) | 428 | 7.72% | 1.09 (0.81–1.48) | 386 | 7.55% | 0.94 (0.67–1.32) | ||
| First-line ICI/chemo | 828 | 7.67% | 1.15 (0.92–1.45) | 527 | 7.32% | 1.03 (0.77–1.39) | ||
- Abbreviations: Chemo, cytotoxic chemotherapy; ESS, effective sample size; ICI, immune checkpoint inhibitor; VTE, venous thromboembolism.
In the subgroup analysis restricted only to lung cancer (NSCLC and SCLC), we compared VTE incidence in the ICI/chemo (n = 828) versus ICI only (n = 428) versus chemo only (n = 4371) cohorts. Both PS estimation and IPW were repeated to ensure adequate balance in residual confounders. In the naïve analysis, VTE occurred in 8.24% of chemo versus 9.90% of ICI versus 9.20% of ICI/chemo cohorts at 6 months. The unadjusted HR was 1.19 (95% CI 0.91–1.55) for ICI only versus chemo and 1.14 (95% CI 0.93–1.41) for ICI/chemo versus chemo only. After IPW, VTE occurred in 8.91% of chemo versus 10.04% of ICI versus 9.60% of ICI/chemo at 6 months (Figure 2B). The weighted HR was 1.05 (95% CI 0.77–1.42) for ICI only versus chemo and 1.09 (95% CI 0.83–1.42) for ICI/chemo versus chemo. The PE/LE-DVT outcome was also not significantly different before or after IPW (Table 2). In contrast, overall survival after IPW was significantly improved for ICI only versus chemo (HR 0.78 [95% CI 0.69–0.89]) as well as ICI/chemo versus chemo (HR 0.85 [95% CI 0.76–0.94]) in comparable patients with lung cancer (Figure S1B).
4 DISCUSSION
In this PS-weighted analysis limited to selected advanced cancers, we found that 1823 patients who received first-line ICI and 6345 patients with first-line chemo treatment had similarly high rates of VTE over time (8.36% vs. 8.49% at 6 months). In a subgroup analysis restricted to lung cancer, we further confirmed that first-line ICI/chemo combination, ICI monotherapy, and chemo monotherapy had similar rates of VTE. In contrast, there was a clear improvement in overall survival for ICI versus chemo in the weighted lung cancer patients. Taken together, our study provides evidence that ICI as a systemic therapy type should be classified in a similar risk category as cytotoxic chemo in future VTE risk stratification models of pharmacologic thromboprophylaxis.
The VTE incidence in the current study is comparable to several previous large retrospective studies. For example, Moik et al., Madison et al., and Roopkumar et al. reported a VTE incidence of 5.0%, 6.3%, and 7.1% at 6 months, respectively.23-25 The distribution of cancer subtypes likely significantly impacts variations in the reported rates of VTE across different studies. For example, 78% of our ICI cohort comprised lung, kidney, or bladder—cancer types that are classified as high risk based on the Khorana score.15 Furthermore, NSCLC and melanoma (two of the common cancer subtypes for ICI) had very different VTE incidence in the unadjusted analyses (9.55% vs. 3.63% at 6 months). Therefore, any comparative study must appropriately balance cancer subtypes. In contrast to previous studies, we specifically excluded patients who received ICI as a second-line therapy. While the introduction of ICI has dramatically changed the treatment landscape in oncology, it remains more common for patients to receive this regimen in subsequent-line settings rather than first-line. For example, while the odds of receiving first-line chemo versus ICI have changed from 43.1 in 2016 to 1.2 in 2021, the overall fraction of patients receiving first-line ICI therapy without prior systemic therapy remained low (37.6% [2353/6265]). This delayed initiation of ICI can cause significant time-varying confounding in retrospective studies as patients who failed prior lines of therapies are oftentimes sicker and have a different risk profile than those receiving first-line systemic therapy. Therefore, we designed the current study to focus on VTE risk for ICI and chemo as first-line treatment within 1 year of diagnosis to isolate the attributable effect of therapy type rather than cancer progression or patient comorbidity. Furthermore, the design and results of our study are of increasing relevance as the oncologic treatment paradigm evolves and ICI is introduced in earlier line settings.
Additionally, ICI/chemo combination as first-line therapy (n = 828) was predominately given to selective patients with advanced lung cancer (68% NSCLC and 32% SCLC). While the absolute rate of VTE was higher for this therapy combination, it is likely driven by the underlying cancer subtype as shown previously. Furthermore, ICI monotherapy and chemo monotherapy were also associated with comparably high rates of VTE in this subgroup after IPW. This result differs from an analysis conducted by Icht et al., where the authors compared VTE incidence in patients with NSCLC who received chemo (n = 169) versus ICI (n = 176) monotherapy and found a 6-month VTE rate of 7.1% versus 4.5%, respectively (HR 1.6 [95% CI 0.6–3.9]). However, the two cohorts in the previous study differed significantly in baseline characteristics without IPW adjustment and included less first-line therapy in the ICI cohort—these could have explained the discrepancy between the studies.26 Another recently published study by Khorana et al. compared patients receiving first-line chemo (n = 1092) versus ICI (n = 605) versus ICI/chemo (n = 602) in patients with stage IV NSCLC using an administrative claims database.27 The authors found that the 6-month VTE incidence was 10.9% for chemo (baseline), 8.1% for ICI (HR 0.74 [95% CI 0.56–0.97]), and 12.8% for ICI/chemo (HR 1.12 [95% CI 0.88–1.42]). Differences in study outcome definition (unadjudicated ICD claims codes vs. adjudicated computable phenotype), statistical methodology (multivariable regression vs. IPW), and adjusted confounders (missing laboratory value vs. complete case analysis) may explain differences in the interpretation from the two studies. However, the unadjusted naïve VTE incidence of 8–10% for chemo and ICI cohorts were more similar than different in both. These findings from our analysis and others serve to support that cancer type has a more significant impact on VTE rates than therapy type. Certainly, further studies are needed to fully delineate if and to what extent treatment-specific mechanisms induce prothrombotic states and whether immune-related toxicities may heighten the risk of VTE further.
We acknowledge the limitations of the current retrospective study. First, we acknowledge inherent limitations associated with the VA database such as male predominance. While sex has not been found as an important predictor of VTE in cancer patients, we cannot generalize our findings to female patients receiving ICI versus chemo. Second, despite the robustness of the IPW statistical method, we cannot account for unmeasured or nonoverlapping covariates to completely prevent confounding by indication. For example, PD-L1 status is an important biomarker to determine whether patients with advanced nonsmall cell lung cancer should receive ICI versus chemo—this cannot be matched in the current study since there would be little to no overlap. However, a prior study did not find an association between PD-L1 expression and VTE.28 Third, to ensure the accuracy of our ICD and NLP algorithms for VTE assessment, we excluded patients with recent diagnosis of acute VTE within 6 months before ICI treatment or those on chronic anticoagulation. By not counting recurrent VTE outcomes, this may lead to lower incidence of VTE but more accurate outcome classification (sensitivity 96% and PPV 91%). Fourth, given the limited literature on how to estimate appropriate standard errors (confidence intervals) while incorporating PS weights into competing risk regression, we used PS-weighted cause-specific Cox regression with robust standard error. We acknowledge Cox regression may overestimate incidence compared with competing risk regressions when dealing with early deaths. Fortunately, our sensitivity analysis using multivariable competing risk regression model demonstrated a similar finding.
In conclusion, the incidence of VTE in patients with advanced staged lung, melanoma, kidney, bladder, liver, and head/neck cancer receiving first-line ICI appears to be variably elevated. This elevated VTE risk is comparable to that conferred by traditional cytotoxic chemo. ICI as a systemic therapy type should be classified in a similar risk category as cytotoxic chemo for VTE. These findings can inform future prospective studies exploring prophylaxis strategies and are needed to delineate how treatment-specific mechanisms induce prothrombotic states.
AUTHOR CONTRIBUTIONS
Ang Li designed the study, performed the statistical analysis, interpreted the data, and wrote the manuscript. Sarah B. May performed the statistical analysis. Jennifer La, Mary T. Brophy, Nhan V. Do, Vipul Chitalia, and John Michael Gaziano created the dataset and critically revised the manuscript. Kylee L. Martens, Christopher I. Amos, and Christopher R. Flowers interpreted the data and critically revised the manuscript. Katya Ravid and Nathanael R. Fillmore designed the study, interpreted the data, and critically revised the manuscript.
FUNDING INFORMATION
AL, a CPRIT Scholar in Cancer Research, was supported by Cancer Prevention and Research Institute of Texas (RR190104), National Heart, Lung, and Blood Institute (K23 HL159271), and National Institute of Health AIM-AHEAD (1OT2-OD032581). CF, a CPRIT Scholar in Cancer Research, was supported by Cancer Prevention and Research Institute of Texas (RR190079). JL, VC, KR, JMG, and NRF were supported by the American Heart Association (857078). JL, NVD, MTB, JMG, and NRF were supported by the VA Cooperative Studies Program. KLM was supported by the ASH Research Training Award for Fellows (RTAF). The views expressed are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the US government.
CONFLICT OF INTEREST STATEMENT
The authors declare no competing financial interests.
Open Research
DATA AVAILABILITY STATEMENT
Data cannot be shared online due to the U.S. government institutional policies pertaining to individual patient data. For specific data access request, please contact the corresponding author to coordinate a collaborative data use agreement with the primary institution.




