Kinetics of cellular and humoral immunogenicity and effectiveness of SARS-CoV-2 booster vaccination in hematologic neoplasms
Marta Crespo, Juliana Esperalba, and Pau Abrisqueta contributed equally to this study.
Abstract
Data on the effect of booster SARS-CoV-2 vaccination are mainly focused on humoral immunogenicity, while the kinetics of vaccine-induced cellular response and its correlation with effectiveness in hematologic patients are less explored. Our aim was to evaluate the longitudinal cellular and humoral immunogenicity induced by two and three doses of the mRNA-1273 SARS-CoV-2 vaccine in 270 patients with hematologic malignancies, and its relationship with the severity of breakthrough SARS-CoV-2 infection. Results indicate that at 23 weeks after the second dose, the seroconversion rate declined from 68.5% to 59.3%, with a reduction in median anti-S titers from 1577 to 456 BAU/mL, mainly in patients over 65 years of age or chronic lymphocytic leukemia (CLL) patients undergoing active therapy. Cellular immunogenicity, however, remained positive in 84.4% of cases. A third vaccine dose seroconverted 42.7% (41/96) and triggered cellular response in 36.7% (11/30) of previously negative patients. Notably, only 7.2% (15/209) of patients failed to develop both humoral and cellular response. Active therapy, anti-CD20 antibodies, lymphopenia, hypogammaglobulinemia, and low CD19+ cell count were associated with poor humoral response, while active disease, GvHD immunosuppressive therapy, lymphopenia, and low CD3+, CD4+, CD56+ cell count determined an impaired cellular response. After 13.8 months of follow-up, the incidence of SARS-CoV-2 infection was 24.8% (67/270), including 6 (9%) severe/critical cases associated with a weaker cellular (median interferon gamma (IFN-γ) 0.19 vs. 0.35 IU/mL) and humoral response (median anti-S titer <4.81 vs. 788 BAU/mL) than asymptomatic/mild cases. In conclusion, SARS-CoV-2 booster vaccination improves humoral response and COVID-19 severity is associated with impaired vaccine-induced immunogenicity.
Abbreviations
-
- Allo-SCT
-
- allogeneic stem cell transplant
-
- CIT
-
- chemoimmunotherapy
-
- CLL
-
- chronic lymphocytic leukemia
-
- MM
-
- multiple myeloma
-
- IMIDs
-
- immunomodulatory drugs
1 INTRODUCTION
Vaccination against SARS-CoV-2 has proven to be an effective strategy to prevent severe coronavirus disease 2019 (COVID-19).1 Nevertheless, despite being vaccinated patients with hematological neoplasms continue to have a higher risk of hospitalization, severe disease, and COVID-19-related death with a mortality rate of 12.4%.2, 3
One of the reasons for the poor clinical outcomes of COVID-19 could be the impaired humoral and cellular immunogenicity obtained by the initial vaccination scheme of two doses. Different studies describe heterogeneous rates of seroconversion (38.1%–99.1%)4-9 and cellular response (26.5%–85.9%)4, 5 depending on the underlying hematologic disease and treatment administered. In addition, there is a reduction over time in the median antibody titers associated with a decreased effectiveness against symptomatic disease and hospitalization.10-12 Therefore, the need to increase immunogenicity after a suboptimal initial response to vaccination and the waning of humoral response over time has led to the authorization to administer vaccine booster doses in this high-risk immunocompromised population.
A third vaccine dose has demonstrated success in the seroconversion of around 23%–35% of seronegative patients with hematologic malignancies after two doses of vaccine.13-15 However, patients diagnosed with lymphoproliferative diseases and under active therapy with B-cell directed agents remain with a poor humoral response,16-18 therefore, cellular immunogenicity may be crucial to provide vaccine effectiveness in these patients.19, 20 We and others described the discordance between cellular and humoral immunogenicity, especially in patients under treatment with anti-CD20 antibodies who preserve a robust vaccine-induced cellular response, as opposite to allogenic hematopoietic stem cell transplantation (HSCT) patients who exhibit a diminished cellular response.21, 22
The kinetics of humoral and cellular immune response and long-term incidence of breakthrough infections by the succession of diverse virus variants remain unclear in the immunocompromised population, including patients with hematologic malignancies.
The objective of this study was to evaluate the longitudinal cellular and humoral immunogenicity of the mRNA-1273 SARS-CoV-2 vaccine and determine the persistence of the initial immune response, the effect of a third vaccine dose and the clinical correlation of achieving immunogenicity with the severity of the infection during a long follow-up (March 2021–May 2022) in patients with hematologic malignancies.
2 METHODS
2.1 Study design and population
This is a prospective, longitudinal, and observational study conducted by Vall d'Hebron University Hospital Campus in Barcelona, Spain. The study cohort included patients diagnosed with hematologic malignancies or who underwent an allogenic HSCT (median time from transplant to vaccination 29.6 months, range 3.7–70.3 months) who received the mRNA-1273 SARS-CoV-2 vaccine. The initial scheme included two vaccine doses administered 28 days apart during the months of March and April 2021 and a posterior third vaccine dose between September 2021 and December 2021 (median of 26 weeks [interquartile range (IQR) 26–31] apart from the second vaccine dose).
Patients who suffered COVID-19 prior or during the study and positive anti-S above the selected threshold or developed T-cell response were also included in the analysis.
Blood draws were obtained at four points: (1) Baseline, immediately before the first vaccine dose (March 2021); (2) post-second dose (post-D2), after a median time of 26 days (IQR 22–28) (April/May 2021); (3) pre-third dose (pre-D3), at a median time of 23 weeks (IQR 22–23) after the second vaccine dose (September/October 2021); and (4) post-third dose (post-D3), at a median time of 11 weeks (IQR 8–15) after the third dose (December 2021/February 2022) (Figure 1).
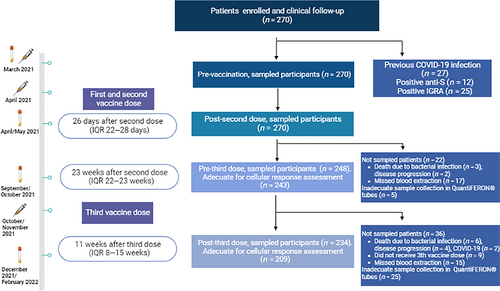
To determine the immunological condition, a blood count test was performed, and total serum immunoglobulin levels were evaluated at baseline and pre-D3. In addition, at the latter time point, T, B, and natural killer cell subpopulations were also assessed.
In addition, to identify individuals with previous or recent SARS-CoV-2 asymptomatic infection, specific antibodies against the nucleocapsid (N) were measured at every time point.
This study was approved by the Institutional Clinical Research Ethics Committee of our center (Study number: 5818) and all patients provided written informed consent in accordance with the Helsinki declaration.
2.2 Assessment of humoral immune response
To evaluate the humoral immune response to the vaccine, at each time point two commercial chemiluminescence immunoassays (CLIA) were used: (1) LIAISON® SARS-CoV-2 TrimericS immunoglobulin G (IgG) (DiaSorin, Stillwater, MN, USA) performed on the LIAISON® XL Analyzer (DiaSorin, Italy) for the determination of IgG antibodies against spike glycoprotein of SARS-CoV-2 (range < 4.81 to >2080 BAU/mL), and (2) Elecsys® Anti-SARS-CoV-2 (Roche Diagnostics, Mannheim, Germany) performed on the Cobas® 8800 system (Roche Diagnostics, Switzerland) for the determination of total antibodies (including IgG, immunoglobulin M [IgM], and immunoglobulin A [IgA]) against nucleocapsid SARS-CoV-2 proteins (cutoff = 1.0 index).
A positive humoral response or seroconversion was defined when an anti-S IgG titer was ≥260 BAU/mL based on the agreement to promote an international common measure unit,23, 24 the correlation of anti-S IgG level ≥ 260 BAU/mL with 80% vaccine efficacy against symptomatic COVID-19,,25 and also on the clinical impact of this threshold in the Spanish health care system that enables prescription of anti-SARS-CoV-2 monoclonal antibodies pre- and post-exposure to patients considered as nonresponders below this antibodies level.
2.3 Assessment of cellular immune response
SARS-CoV-2-specific T-cell response was evaluated at each time point by the whole blood interferon-gamma-release-immuno-assay (IGRA) technology using QuantiFERON® SARS-CoV-2 RUO tubes from Qiagen® (Hilden, Germany), consisting of two tubes coated with a combination of SARS-CoV-2 spike antigens (S1, S2, RBD), a mitogen tube that serves as a positive control and a Nil tube as a negative control.26 This test was conducted following the manufacturer's instructions: venous blood samples were collected directly into the four QuantiFERON® SARS-CoV-2 RUO tubes, incubated at 37°C for 16–24 h and centrifuged to separate plasma. Interferon-gamma (IFN-γ) (IU/mL) was measured by CLIA using LIAISON® QuantiFERON-®TB Gold Plus assay (DiaSorin, Saluggia, VC, Italy) with the LIAISON® XL Analyzer (DiaSorin, Italy). Experimentally established cut-off values (Ag1 = 0.0617527 and Ag2 = 0.05135 [unpublished data]) were used for the interpretation of the results.
2.4 Assessment of vaccine effectiveness
The incidence of COVID-19 was evaluated during a median follow-up of 13.8 months (95% confidence interval [CI] 13.7–14 months) since the administration of the first vaccine dose. Patients with compatible COVID-19 symptomatology underwent a screening with a SARS-CoV-2 RT-PCR or an antigen test. Viral variants were genetically characterized in the RT-PCR confirmed cases and clinical data about the severity of COVID-19 (World Health Organization [WHO] classification), rate of hospitalization, ICU admission, need for supplemental oxygen or COVID-19 specific therapy (steroids, antivirals, IL-6 inhibitors, anti-SARS-CoV-2 monoclonal antibodies) were collected. We considered an asymptomatic infection the observation of an anti-N seroconversion between two consecutive serological tests.
2.5 Statistical analysis
A descriptive analysis of all included variables in the study was performed. Continuous variables were expressed as median and IQR and categorical variables were described as absolute values and percentages. Univariate logistic regression models were carried out to estimate the association between baseline and sixth-month clinical factors, and between the early, persistent, and post-third vaccine dose cellular and humoral immunization of the mRNA-1273 vaccine. Odds ratios (ORs) with 95% CIs were reported. No data imputation was performed and the data analyses were carried out using R statistical software version 3.6.2. For original data, please contact the corresponding author.
3 RESULTS
3.1 Patient characteristics
A total of 270 patients were included in this study. Baseline and pre-third vaccine dose demographic and clinical characteristics from patients are summarized in Table S1.
During the time, post-D2 and pre-D3, 26 patients (9.6%) changed their hematologic disease status (11 patients had a progression, 10 patients in stable disease achieved a complete response, and 5 treatment naïve patients initiated treatment and attained a partial response). Also, 37 patients changed their treatment status (13.7%): 19 patients under active therapy changed their initial treatment to another therapy, 12 patients completed their therapy prior to the third dose and 6 patients off therapy/treatment naïve initiated active therapy during the observation study period.
3.2 Persistence of humoral and cellular immunogenicity 6 months after two vaccine doses
Two vaccine doses elicited an early humoral response rate of 68.5% (185/270) with a median anti-S of 1577 BAU/mL (IQR 96 to >2080) at 4 weeks after vaccination, including the seropositive patients with prior COVID-19 infection (n = 12) and the change in the threshold of humoral responders (anti-S IgG titer ≥260 BAU/mL). Patients older than 65 years and patients diagnosed with CLL presented a seroconversion rate of 66.9% and 65.4%, respectively. Nevertheless, their median anti-S title was significantly lower (1352 BAU/mL and 650 BAU/mL, respectively, p < .05) post-D2.
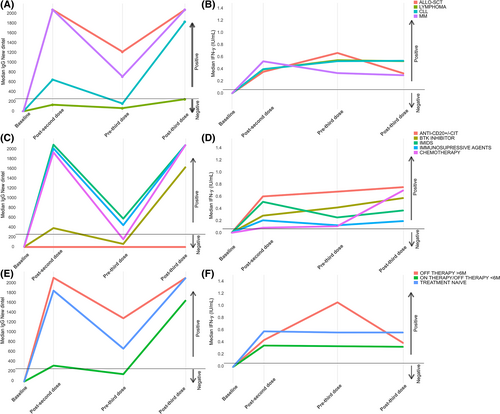
After almost 6 months (median of 23 weeks) post-D2, a decline in the seroconversion rate from 68.5% to 59.3% (147/248) and a reduction in the median anti-S antibodies titers from 1577 BAU/mL to 456 BAU/mL (IQR 33–1433) were observed. Of note, 31 out of 185 (16.8%) initially seropositive patients became seronegative. The factors associated with losing the humoral response were: >65 years of age (reduction from 66.9% to 50.9% of the seropositivity rate; p = .04), CLL as an underlying disease (reduction from 65.4% to 44.7% of the seropositivity rate; p = .04), active therapy (reduction from 53.5% to 41.4% of the seropositivity rate, p = .03) and treatment with BTK inhibitors (reduction from 56.3% to 25.9% of the seropositivity rate, p < .001). From the quantitative standpoint, the anti-S waning was observed in the whole cohort despite type of malignancy, status or type of treatment (Figure 2A, C, E).
Other factors associated with a negative humoral immunogenicity at 6 months post-D2 are described in Figure 3A. By contrast, only four patients initially negative became seropositive at this time point: two of them due to an asymptomatic SARS-CoV-2 infection with corroborated anti-N positivization, one probably caused by traces of anti-S antibodies present in transfused plasma or platelets units, and the last one due to a probable delayed humoral response to vaccination since the initial evaluation was early at Day 12 post-D2.
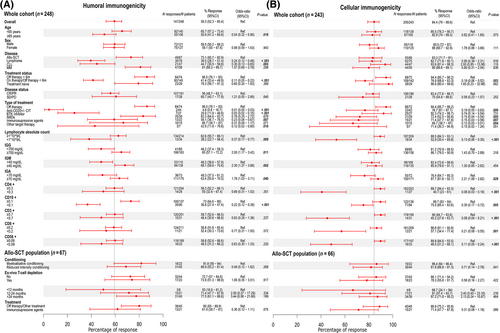
Regarding the cellular response, including the patients with prior COVID-19 infection and positive interferon-γ producing SARS-CoV-2-reactive T cells (n = 25), a total of 227 out of 270 (84.1%) patients had an early cellular immunity 4 weeks after the second dose of the mRNA-1273 vaccine with a median Ag1 of 0.4 IU/mL (IQR 0.09–1.28). Cellular response was more stable than the humoral immunogenicity maintaining a positivity rate of 84.4% (205/243) 6 months post-D2 with a median Ag1 of 0.52 IU/mL (IQR 0.1–1.77) (Figure 2B, D, F). During this time, only 17 patients (7.49%) lost their initial positive cellular response. Factors associated with a negative cellular response at this time point were: active therapy; treatment with immunomodulatory drugs (IMIDs), other target therapies and immunosuppressive agents; low IgA; and low lymphocyte, CD4+, CD8+, CD19+, and CD56+ cell counts (Figure 3B).
Moreover, 16 patients (7.8%) initially categorized as negative responders became positive at this time point without clinical or serological evidence of SARS-CoV-2 infection, all of them with negative anti-N antibodies. This subgroup of late cellular responders was composed predominantly by patients older than 65 years of age (63%) and with lymphoproliferative malignancies (5 CLL and 5 lymphoma patients).
3.3 Humoral and cellular immunogenicity after a third vaccine dose
A third vaccine dose was successful to improve the seroconversion rate from 59.3% to 75.2% (176/234) and increase the median anti-S titer from 456 BAU/mL to >2080 BAU/mL (IQR 289 to >2080). Overall, the third vaccine dose achieved a seroconversion of 42.7% (41/96) of patients without humoral response pre-D3. Regarding the subgroup of patients that did not have early humoral response post-D2, the third dose attained a seroconversion in 27.1% (19/70) of them. These patients were diagnosed mainly with CLL (42% [8/19], most of them (6/8) under BTK treatment) or lymphoma (42% [8/19] of whom 4 had finished their anti-CD20 therapy close to the initial vaccination scheme but it was ≥6 months prior to the third vaccine dose). In addition, 84.6% (22/26) of patients who had lost the response at the 6th month post-D2 regained the seropositivity. The seroconversion after the third dose was mostly attributable to vaccination, of the 16 seronegative patients post-D2 who developed SARS-CoV-2 infection only 5 had detectable anti-S antibodies and 3 anti-N positive antibodies post-D3.
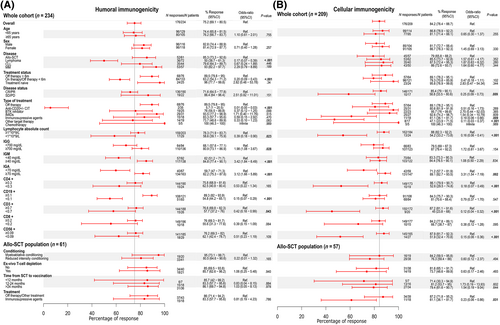
Factors associated with a lack of humoral post-D3 were: lymphoma as underlying disease, active treatment, therapy with anti-CD20 antibodies, lymphopenia, hypogammaglobulinemia and, low CD3+ and CD19+ cell count (Figure 4A), being the most important factor the anti-CD20 therapy. Thus, 33 out of 58 patients (59.9%) with absence of seroconversion were receiving active treatment with anti-CD20 antibodies, and only 2 out of 35 patients (5.7%) under active treatment with these antibodies achieved a humoral response.
The overall cellular immunogenicity rate remained stable post-D3 with an 84.2% of responders (176/209). Overall, 14 out of 168 (8.3%) patients previously positive lost their response post-D3, while 11 out of 30 (36.7%) previous nonresponders achieved cellular response. The cellular responders post-D3 were composed of 4 out of 17 patients (23.5%) primarily negative post-D2; 2 were lymphoma patients who finished their chemoimmunotherapy ≥6 months prior to this third vaccine dose and improved their lymphocyte count; however, the other 2 patients were allogeneic stem cell transplant (allo-SCT) who developed COVID-19 and also positivized anti-N antibodies. The third dose also regained T-cell response in 7 out of 13 (53.8%) patients who lost it at 6 months post-D2, 4 of them were MM patients and 3 CLL patients, most of them under active therapy.
Factors associated with a negative cellular immune response post-D3 were: absence of response to therapy with progression or stable hematologic disease, treatment with GvHD immunosuppressive therapy, treatment with targeted therapies (mainly venetoclax and daratumumab), lymphopenia and low CD3+, CD4+, and CD56+ cell count (Figure 4B).
Importantly, post-D3 only 7.2% (15/209) of the patients lacked both humoral and cellular vaccine-induced immunogenicity, most of them being lymphoma patients under active treatment (8/15), mainly with anti-CD20 antibodies (6/8).
3.4 Vaccine effectiveness against SARS-CoV-2 infection and severe COVID-19
During a median observation period of 13.8 months from March 2021 to May 2022 (since the administration of the first vaccine dose until 5 months post-D3), 67 out of 270 patients (24.8%) developed breakthrough SARS-CoV-2 infection despite vaccination, 19.4% (13/67) of them being asymptomatic. Twenty patients (29.9%) were infected post-D2 (May 2021–December 2021) and 47 patients (70.1%) post-D3 (October 2021–May 2022). Among the symptomatic cases (n = 54), 55.6% were diagnosed through rapid antigen test and 44.4% (24/54) through RT-PCR; regarding the latter, viral sequencing from 18 cases disclosed that the viral variant was delta in 5 (28%) and omicron in 13 (72%).
Age > 65 years (OR 0.56 (95% CI 0.31–0.99); p = .05) and the development of a positive serological response post-D2 (OR 0.50 (95% CI 0.28–0.89); p = .02) resulted associated with less risk to develop breakthrough SARS-CoV-2 infection.
Most of the infections were mild or moderate according to the WHO severity of illness scale (71.6%; 48 out of 67). The hospitalization rate in all infected patients was 13.4% (9/67), 9% (6/67) of patients needed supplemental oxygen and the ICU admission rate was 4.5% (3/67). The COVID-19-specific therapies administered were remdesivir (16.4%), steroids (9%), tocilizumab (3%), convalescent plasma (3%), and anti-SARS-CoV-2 monoclonal antibodies (1.5%).
Six out of 67 (9%) patients suffered a severe/critical infection, including 4 patients with lymphoma, 1 patient with CLL, and 1 allo-SCT patient; 5 of them were under active therapy (3 with anti-CD20 antibodies, 1 with BTK inhibitor, and 1 with PI3K inhibitor) and in therapeutic response. Three cases were infected by omicron variant, 1 by delta variant, and 2 could not be sequenced. It is worth mentioning that none of the severe cases had developed humoral vaccine response, while 3 out of 6 also did not develop cellular immunogenicity. Moreover, patients with a severe infection had a weaker cellular response measured by a lower median IFN-γ production (0.19 IU/mL [IQR 0.02–0.35] versus 0.35 IU/mL [IQR 0.10–1.24]) and lower median anti-S titer (<4.81 BAU/mL [IQR < 4.81–30.3]) versus 788 BAU/mL (IQR 32.11 to >2080) in comparison with asymptomatic/mild cases at the last assessment previous infection. Two out of the 6 patients who developed severe disease died due to COVID-19.
Finally, 6 out of 39 (15.4%) patients developed a prolonged virus shedding (positive RT-PCR >30 days), 5 of those patients had a negative humoral response and 4 had negative cellular immunogenicity. Three out of those 6 cases (50%) suffered a severe COVID-19.
4 DISCUSSION
In this prospective longitudinal study, the waning of anti-S titers within 6 months post-D2 of the mRNA-1273 SARS-CoV-2 vaccine was corroborated in a large cohort of patients diagnosed with hematologic malignancies. Consistent with data from other studies, patients older than 65 years of age28, 29 or with a CLL diagnosis,30 (particularly those under active therapy with BTK inhibitors who initially achieved a quantitatively weaker humoral response post-D2), were at higher risk of a significant decrease of humoral immunogenicity, with negativization of an initially positive seroconversion in 23.5% and 32.4%, of the cases respectively.
Contrary to the decline in antibodies titers, vaccine-induced cellular immunogenicity remained more robust and sustained over time in hematologic patients, similarly to previously described in immunocompetent individuals31 and convalescent patients,32, 33 and only 7.49% of cases lost the early initial cellular response 6 months post-D2 .
As previously described by Alimam et al. and Mariotti et al. regarding the influenza vaccine,34, 35 we also observed 7.8% of patients (predominantly elderly patients and those diagnosed with lymphoproliferative disorders) developing a delayed cellular immune response pre-D3 that was not achieved at a median of 26 days post-D2.
Concerning the efficacy of booster vaccination, in terms of humoral immunogenicity we found that a third dose was successful to seroconvert 42.7% (41/96) of negative patients pre-D3 and produced a meaningful increase in antibody titer. Most of these patients (26/41) corresponded to those who lost the initial humoral response at 6 months after two vaccine doses, but it should be mentioned that 27.1% (19/70) of patients without an initial humoral response post-D2 doses seroconverted too. This rate of seroconversion in patients with undetectable antibodies following two vaccine doses is similar to the one described by Herishanu et al. in CLL patients (23.8%)8 and by Terpos et al. in patients with B-cell malignancies (34.5%).36 Consistently with the findings in these studies,8, 36 the main factors related to a poor humoral response after a third vaccine dose were hypogammaglobulinemia, lymphopenia, low CD19+ cell count, and above all, active treatment with anti-CD20 monoclonal antibodies. The deleterious effect of anti-CD20 therapy in humoral immunogenicity has also been described by Aiello et al.37 and Tortorella et al.38 in patients with multiple sclerosis treated with ocrelizumab, which also failed to achieve seroconversion after booster SARS-CoV-2 vaccination.
Regarding the cellular response, a third dose was able to develop response in 36.7% (11/30) of negative patients pre-D3. Similarly to the humoral response, the booster effect was more evident in patients who lost the initial cellular immunogenicity achieving response in 53.8% (7 out of 13) of them and only in 23.5% (4 out of 17) of primarily negative patients post-D2. These rates are consistent to the ones described by Lim et al.39 with an improvement of 50% (6 out of 12) of patients who did not develop cellular response after the second dose and a sustained cellular response in 94% (16 out of 17) of initially positive patients. Shapiro et al.40 also observed in a small cohort of seronegative patients after two doses, a cellular response of 63% (20/27) that improved with the third dose to 80% (8/10). However, to the best of our knowledge, our study is the first one to provide a longitudinal analysis of the kinetics of cellular immunogenicity in a large cohort of patients with hematologic malignancies that allowed us to also detect a negativization of 8.3% (14 out of 168) of cellular responders after the third vaccine dose, an effect not seen with the humoral response post-D3. Moreover, we were able to identify factors associated with higher risk of a negative cellular response after three vaccine doses, including uncontrolled hematologic disease (stable or progressive disease), treatment for GvHD with immunosuppressive agents, lymphopenia, and low CD3+, CD4+, and CD56+ cell count.
Our longitudinal analysis of the vaccine-induced immunogenicity reveals that the humoral response rate and antibody titers significantly improved following booster vaccination against SARS-CoV-2. Meanwhile, the T-cell response rate remained stable throughout the study. These findings resemble the kinetics pattern previously reported by Farroni et al.41 and Aiello et al.37 in other immunosuppressed populations, including patients with multiple sclerosis and rheumatoid arthritis undergoing treatment with various disease-modifying therapies. Interestingly, in those studies,37, 41 the T-cell specific SARS-CoV-2 response was mainly driven by CD4+ T-cells, which could justify the impact of a pre-vaccination low CD4+ cell count in the impairment of cellular response found in our study.
Of note, the prospective character of the study allowed us to document the association between vaccine-induced immunogenicity and the clinical effectiveness and patterns of infection. We detected 24.8% (67 out of 270) of breakthrough SARS-CoV-2 infections, a higher rate than the one described by Wang et al. (13.4%),42 Piñana et al. (2.6%)43 and Salvini et al. (8%)44 probably due to our longer follow-up period that involved the first 5 months of 2022 when the Omicron variant became prevalent in European countries and the detection of asymptomatic cases by anti-N serologic positivization. Similarly, as described by Salvini and Piñana et al.,43, 44 in our cohort, developing a positive humoral vaccine-induced immunogenicity post-D2 was significantly associated with a lower risk of breakthrough SARS-CoV-2 infection while cellular immunogenicity did not have impact on the incidence of the infection. On the other hand, the lower rate of infection observed in patients older than 65 years could be related to the fact that older people, particularly immunocompromised ones are more prone to maintain isolation and self-protective measures.
Notoriously, most breakthrough SARS-CoV-2 infections were asymptomatic (19.4%) or mild/moderate (71.6%) indicating that vaccination is highly effective at reducing COVID-19 related morbidity and mortality in comparison to pre-vaccine era with rates of severe/critical COVID-19 of 63.8% and mortality rates around 31.2% in patients diagnosed with hematologic malignancies.45
Noteworthy, after a third vaccine dose a subgroup of 7.2% (15/209) of patients still remains with neither serological nor cellular vaccine-induced immunogenicity. This subgroup is composed mainly by patients diagnosed with lymphoproliferative malignancies under active treatment, mostly with immunochemotherapy including anti-CD20 antibodies. Those double negative patients are the ones who predominantly developed severe/critical COVID-19 as none out of the six severe cases had developed humoral vaccine response while three did not develop cellular immunogenicity either. Furthermore, patients with asymptomatic/mild infection had a stronger cellular response measured by a higher median IFN-γ production and a higher median anti-S titer at the last assessment previous infection. Based on our data, cellular immunogenicity plays a role in decreasing the severity of the infection as previously described by Bange et al.46 in unvaccinated hematologic patients but in some cases, it is not sufficient to avoid severe disease in the setting of a compromised humoral immunogenicity.
An impaired vaccine-induced humoral and cellular immunogenicity could also be associated with persistent virus shedding and prolonged COVID-19, a 15.4% (6/39) of infected patients in our cohort had positive RT-PCR >30 days and 5 out of the 6 cases had negative humoral response and 4 had negative cellular response. Data comparable with the 13.9% described by Lee et al. in an unvaccinated cohort of patients diagnosed with hematologic malignancies.47
In conclusion, our results highlight the importance to administer a SARS-CoV-2 booster vaccine dose to improve the seroconversion rate and overcome the natural waning of humoral response, thereby enabling the recovery of a higher antibody titers in patients with hematologic malignancies. This is particularly important for patients older than 65 years and those receiving treatment with BTK inhibitors. A booster vaccine dose might also be effective 6–12 months after having achieved a response of their hematologic disease and having suspended anti-CD20 therapy. Finally, the subgroup of patients without humoral response after a third vaccine dose would benefit from pre-exposure prophylaxis strategies with anti-SARS-CoV-2 monoclonal antibodies and an early and intensive treatment with antivirals and SARS-CoV-2 monoclonal antibodies should be encouraged in these patients with suboptimal vaccine-induced immunogenicity at the moment of infection.48
AUTHOR CONTRIBUTIONS
Concept and design were undertaken by Pau Abrisqueta, David Valcárcel, Marta Crespo, Juliana Esperalba, Moraima Jiménez, and Francesc Bosch. Data analyses were performed by Víctor Navarro and Moraima Jiménez. Collection and assembly of data were performed by Moraima Jiménez and Sandra Novoa. Laboratory analyses were performed by Candela Fernández-Naval, Juliana Esperalba, Cristina Andrés, Mónica Martinez-Gallo, and Daniel Medina. Samples collection was performed by Soraya Peralta, Gemma Pujadas, Cristina Hernández, and Carlota Pagès. All authors contributed to manuscript writing and final approval of the manuscript and are accountable for all aspects of the work.
ACKNOWLEDGMENTS
The authors sincerely appreciate the support and work of all the departments that have collaborated in the study and especially the patients and their families for their involvement. The authors also thank the Cellex Foundation for providing research facilities and equipment and the CERCA Programme/Generalitat de Catalunya for institutional support.
CONFLICT OF INTEREST STATEMENT
The following represents disclosure information provided by the authors of this manuscript. Moraima Jiménez has received honoraria from GSK and Sanofi. David Valcárcel has received honoraria from Asofarma, Astellas, BMS/Celgene, Amgen, Janssen, Jazz, MSD, Sanofi, Novartis, Takeda, and Pfizer. Pau Abrisqueta has received honoraria from Janssen, Roche, BMS, Abbie, and AstraZeneca. Marta Crespo has received research funding from Pharmacyclics, Genentech, and AstraZeneca. Francesc Bosch has received honoraria from Janssen, Gilead, Roche, BMS, Abbie, AstraZeneca, Novartis, and Lilly. All remaining authors have no conflicts to declare. Isabel Ruiz-Camps has received honoraria from Pfizer, MSD, Gilead, AstraZeneca, Janssen, and BMS.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon request.




