Quality of life comparison between esophagogastrostomy and double tract reconstruction for proximal gastrectomy assessed by Postgastrectomy Syndrome Assessment Scale (PGSAS)-45
Abstract
Aim
The current study compared the postoperative quality of life (QOL) between the esophagogastrostomy method (PGEG) and double tract method (PGDT) after proximal gastrectomy using the Postgastretomy Syndrome Assessment Scale (PGSAS)-45.
Methods
Among the 2364 patients who received the PGSAS-45 questionnaire, 300 PGEG and 172 PGDT cases responded. The main outcomes measures (MOMs) consisted of seven subscales (SS) covering symptoms, meals (amount and quality), ability to work, dissatisfaction with daily life, physical and mental component summary of the 8-Item Short Form Health Survey (SF-8), and change in body weight, and were compared between PGEG and PGDT.
Results
Overall, PGDT promoted significantly better constipation SS scores (p < 0.05), whereas PGEG tended to promote better body weight (BW) loss% (p < 0.10). A stratified analysis based on the remnant stomach size revealed that among those with a remnant stomach size of 1/2, PGDT had significantly better constipation and dumping SS scores (p < 0.05) and tended to have better working conditions (p < 0.10) compared to PGEG. Even among those with the remnant stomach size of 2/3, PGDT had significantly better diarrhea SS scores, lesser dissatisfaction with symptoms, and better dissatisfaction with daily life SS scores (p < 0.05) and tended to have better constipation SS scores and lesser dissatisfaction with work (p < 0.10) compared to PGEG.
Conclusions
After comparing the QOLs of PGEG and PGDT, the stratified analysis according to remnant stomach sizes of 1/2 and 2/3 revealed that PGDT was relatively superior to PGEG for several MOMs.
1 INTRODUCTION
Advances in diagnostic and therapeutic techniques for gastric cancer have improved the early diagnosis of gastric cancer patients and prognosis of advanced gastric cancer.1-4 As such, the long-term quality of life (QOL) after gastrectomy has also required attention. Unfortunately, both Japan and Western countries have seen an increase in the incidence of cancers in the upper third of the stomach and gastroesophageal junction.5-7 In Japan, the incidence of gastric cancer has decreased due to the decrease in the rate of H. pylori infection and the spread of H. pylori infection detection/eradication treatment in health checkups.8 In such situations there is an increase in the rate of the upper gastric cancer.9 Proximal gastrectomy has been a frequent treatment approach for early upper third gastric cancer. Various reconstruction methods have been used for proximal gastrectomy. Among them, the esophagogastrostomy method (PGEG) and double tract method (PGDT) have recently been the most commonly utilized reconstruction methods in Japan. However, it remains unclear which reconstruction method is better for postoperative QOL.
A large retrospective study using the Postgastrectomy Syndrome Assessment Scale (PGSAS-45) reported that proximal gastrectomy promoted lower postgastrectomy burden than total gastrectomy with Roux-en-Y reconstruction.10 Hence, proximal gastrectomy has become a potential option as a function-preserving surgery for early upper third gastric cancer.11 Various reconstruction methods after proximal gastrectomy have been proposed for preventing esophageal reflux and achieving optimal food storage and outflow from the remnant stomach, which are considered crucial for maintaining postoperative QOL after proximal gastrectomy. A nationwide multi-institutional surveillance study (“PGSAS NEXT” study) was conducted to investigate the optimal gastrectomy procedures for improving postoperative QOL in patients with the cancer located in the upper third of the stomach or around the esophagogastric junction. Based on collected data, we compared the postoperative QOLs after proximal gastrectomy for patient with gastric cancer located in the upper third of the stomach according to the reconstruction procedures, esophagogastrostomy method (PGEG), and double tract method (PGDT).
2 METHODS
2.1 Patients
The patient inclusion criteria were as follows: (1) females or males aged 20 years or older; (2) cancer located at the upper third of the stomach or around the esophagogastric junction regardless of stage or histologic type; (3) achieved R0 resection; (4) no recurrence or metastasis; (5) more than 6 months after gastrectomy; (6) more than 6 months after the termination of former chemotherapy; (7) underwent gastrectomy only once; (8) performance status 0 or 1; (9) capable of understanding the questionnaire; (10) no other disease or previous surgery that may influence the results of the questionnaire aside from gastrectomy; (11) no organ failure or mental disease; (12) spontaneous agreement of the said person.
The patient exclusion criteria were as follows: (1) active dual malignancy; (2) synchronous surgery with exception of the resection or extraction of the perigastric organs to accomplish gastrectomy or lymph node dissection, as well as that equivalent to cholecystectomy.
2.2 Data of case report forms
Patient and surgical information [age, sex, height, BW, postoperative period, approach (laparotomy/laparoscope), celiac branch of vagal nerve preservation (yes/no), chemotherapy history, clinical stage, extent of lymph node dissection, information on simultaneous organ resection, details of surgical procedures, etc.] was collected from case report forms.
2.3 QOL assessment using the PGSAS-45 questionnaire
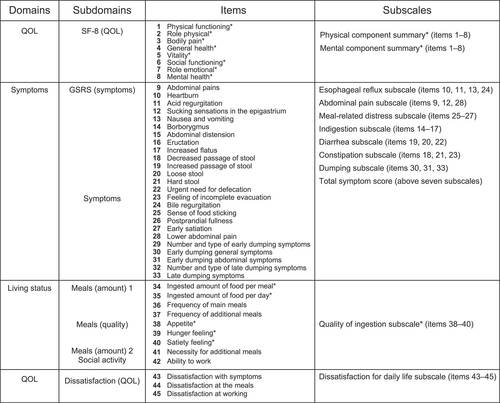
The PGSAS-45 is composed of questions pertaining to 45 items (Figure 1), which include eight items from the existing the 8-Item Short Form Health Survey (SF-8),12 15 items from the Gastrointestinal Symptom Rating Scale (GSRS),13, 14 and 22 items determined to be clinically important and newly selected by surgeons affiliated with the Japanese Postgastrectomy Syndrome Working Party. Factor analysis was performed in our previous Postgastrectomy Syndrome Assessment Study (PGSAS study), and 23 symptom items were clustered into the following seven subscales (SSs): esophageal reflux, abdominal pain, meal-related distress, indigestion, diarrhea, constipation, and dumping.15 Moreover, the total symptom score; quantity of each meal; necessity for additional meals; quality of ingestion SS; working conditions; dissatisfaction with symptoms, meals, and work; dissatisfaction with daily life SS; the physical component summary (PCS) and mental component summary (MCS) of the SF-8; and change in BW were determined as the main outcome measures. The SS scores represented the average scores for the component items or SSs except PCS and MCS of the SF-8. For items 1–8, 34, 35, and 38–40, higher scores indicated better outcomes, whereas for items 9–28, 30, 31, 33, and 41–45, higher scores indicated worse outcomes.
2.4 Study methods
This study utilized continuous sampling from a central registration system for participant enrollment. The questionnaire was distributed to eligible patients. Patients were instructed to return completed forms to the data center. All QOL data from questionnaires were matched with individual patient data collected via case report forms.
The methods used for measuring the distance from the diaphragm to the esophago-gastrointestinal anastomosis were defined as follows. The anastomotic site was confirmed by the staple line on the axial cross-sectional computed tomography (CT) image. The midpoint between the top slice where the esophageal hiatus of the diaphragm can be depicted and the bottom slice where it cannot be confirmed on the CT image was defined as the diaphragm level (D, 0 mm). The distance between the diaphragm and esophago-gastrointestinal anastomosis was assigned a positive or negative value depending on whether the latter was located below or above the diaphragm, respectively.
This study was registered with the University Hospital Medical Information Network's Clinical Trials Registry (UMIN-CTR; registration number 000032221) and was approved by the ethics committees of all institutions.
2.5 Statistics
Patient characteristics and main outcome measures were compared between PGEG and PGDT using t-tests and Fisher's exact tests. All outcome measures were further analyzed using multiple regression analyses. Ten factors—reconstruction method, age, sex, postoperative period, operative approach, preservation of the celiac branch of the vagus, chemotherapy, clinical stage, extent of lymph node dissection, combined resection—were included in the multiple regression analysis as explanatory variables. These factors were selected according to their clinical importance and based on the results of previous Postgastrectomy Syndrome Assessment Studies. Statistical significance was set at p < 0.05. Where multiple regression analysis yielded a p-value <0.1, the standardization coefficient of regression (β) and the p-value are shown in a table. Cohen's d, β, and R2 were used to measure the effect sizes. Interpretation of effect sizes were ≥0.2 = small, ≥0.5 = medium, and ≥0.8 = large in Cohen's d; ≥0.1 = small, ≥0.3 = medium, and ≥0.5 = large in β; and ≥0.02 = small, ≥0.13 = medium, and ≥0.26 = large in R2. All statistical analyses were performed using JMP12.0.1 software (SAS Institute Inc.).
3 RESULTS
3.1 Retrieving the questionnaire
Between July 2018 and December 2019, the PGSAS-45 questionnaire was distributed to 2364 patients across the 70 institutions that participated in this study. Among those who received a questionnaire, 1950 (82.5%) responded, of which 41 were deemed ineligible. As a result, 1909 questionnaires (80.8%) were ultimately included for analyses related to the PGSAS NEXT study. Among the 1909 patients included, 518 had undergone proximal gastrectomy for upper-third gastric cancer (Figure 2).
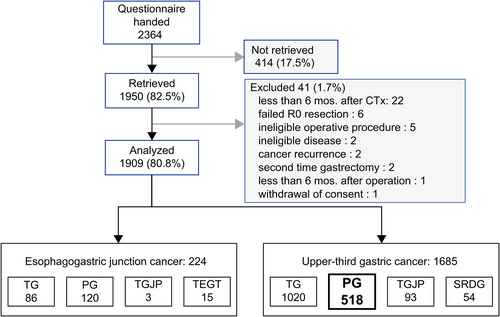
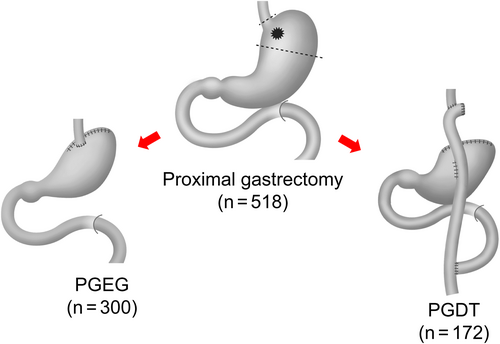
The current study analyzed 300 patients and 172 who had undergone PGEG and PGDT, respectively (Figure 3). Other reconstruction methods used for proximal gastrectomy included only 30 cases of jejunal interposition and 16 cases of jejunal pouch interposition and were considered insufficient for analysis.
The PGEG group was consisted of 276 cases who received the anti-reflux method (double flap method, 153 cases; creation of pseudofornix and/or Hisoid angle, 67 cases; fundoplication method, 44 cases; side overlap with fundoplication using the Yamashita [SOFY] method,16 11 cases; other procedures, 11 cases), 21 cases who did not receive the anti-reflux method, and three unknown cases. Reconstruction procedures were not regulated by the protocol and depended on the principle of the institution or discretion of each surgeon.
3.2 Patient characteristics
Patient characteristic of both groups are summarized (Table 1). The PGDT group was younger than the PGEG group (PGEG, 70.8 years old; PGDT, 68.6 years old; p = 0.013). The esophageal resection length was longer in the PGDT group (PGEG, 4.1 ± 6.5 mm; PGDT, 7.2 ± 8.2 mm; p < 0.001), whereas the distance between the anastomosis site and the diaphragm was shorter in the PDGT group (PGEG, 7.2 ± 16.0 mm; PGDT, 1.0 ± 14.4 mm; p < 0.001).
| PGEG (n = 300) mean (SD) | PGDT (n = 172) mean (SD) | t-test p -value | |
|---|---|---|---|
| Age (year) | 70.8 (9.5) | 68.6 (9.3) | 0.013 |
| Preoperative BMI (kg/m2) | 22.8 (3.0) | 23.0 (3.2) | 0.446 |
| Postoperative BMI (kg/m2) | 20.8 (2.8) | 19.9 (2.5) | 0.287 |
| Postoperative period (month) | 40.3 (33.8) | 39.3 (22.1) | 0.720 |
| Length of esophageal resection (mm) | 4.1 (6.5) | 7.2 (8.2) | <0.001 |
| Distance from diaphragm to anastomosis (mm) | 7.2 (16.0) | 1.0 (14.4) | <0.001 |
| PGEG (n = 300) | PGDT (n = 172) | Fisher's exact test p -value | PGEG (n = 300) | PGDT (n = 172) | Fisher's exact test p -value | ||
|---|---|---|---|---|---|---|---|
| Gender | 0.232 | cStage (JGCA 14th) | 0.745 | ||||
| Male | 237 | 128 | I | 282 | 163 | ||
| Female | 63 | 44 | IIA/IIB | 9 | 7 | ||
| Abdominal approach | 0.001 | III | 7 | 2 | |||
| Open | 96 | 31 | IVA/IVB | 1 | 0 | ||
| Laparoscopy | 204 | 141 | Chemotherapy | 0.529 | |||
| Celiac branch of vagus | <0.001 | Postoperative | 20 | 9 | |||
| Preserved | 77 | 15 | Pre and postoperative | 2 | 0 | ||
| Divided | 216 | 152 | None | 278 | 163 | ||
| Tumor location (JGCA 14th) | 0.026 | Extent of lymphnode dissection | 0.259 | ||||
| UE (Siewert type III) | 6 | 4 | D0 | 3 | 0 | ||
| U | 284 | 152 | D1 | 25 | 14 | ||
| UM | 8 | 9 | D1+ | 265 | 149 | ||
| MU | 2 | 7 | D2 | 6 | 8 | ||
| Extent of esophageal resection | 0.001 | Combine resection | 0.548 | ||||
| Lower thoracic | 5 | 1 | None | 278 | 157 | ||
| Abdominal | 144 | 112 | Gallbladder | 19 | 15 | ||
| None | 149 | 59 | Spleen | 2 | 0 | ||
| Level of esophago-GI anastomosis | 0.002 | Pancreas | 1 | 0 | |||
| Ti | 39 | 23 | Others | 0 | 0 | ||
| D | 106 | 89 | Size of remnant stomach | <0.001 | |||
| A | 148 | 59 | Greater than or equal to 3/4 | 57 | 1 | ||
| Around 2/3 | 165 | 60 | |||||
| Around 1/2 | 73 | 97 | |||||
| Around 1/3 | 0 | 13 | |||||
- Abbreviations: BMI, body mass index; PGEG, Proximal gastrectomy with esophagogastrostomy reconstruction; PGDT, Proximal gastrectomy with double tract reconstruction; SD, standard deviation.
Laparoscopic surgery was common in both groups, whereas the rate of laparoscopic surgery was higher in the PGDT group than in the PGEG group (PGEG, 68%; PGDT, 82.0%; p = 0.001). The preservation rate of the celiac branch of vagus nerve was lower in the PGDT group than in the PGEG group (PGEG, 27.2%; PGDT, 9.0%; p < 0.001). Proximal gastrectomy was mainly localized in the U region and cStage I. D1 + or less lymph node dissection was selected in almost all cases. Combined resection was performed in 22 cases (7.3%) in the PGEG group and 15 cases (8.7%) in the PGDT group and mainly involved the gall bladder.
The size of the remnant stomach was significantly associated with the selection of reconstruction method (i.e., PGEG or PGDT). The size of the remnant stomach was significantly smaller in the PGDT group. Cases with a remnant stomach of ≥3/4 accounted for 57 cases (98.3%) in the PGEG group and one case (1.7%) in the PGDT group. Cases with a remnant stomach of 2/3 accounted for 165 cases (73.3%) in the PGEG group and 60 cases (26.7%) in the PGDT group. Cases with a remnant stomach of 1/2 accounted for 73 cases (42.9%) in the PGEG group and 97 cases (57.1%) in the PGDT group. In contrast, only 13 cases had a remnant stomach of 1/3, all of whom were in the PGDT group.
3.3 Comparison of postoperative QOL between PGEG and PGDT
The main outcome measures of PGSAS-45 were compared between the PGEG and PGDT groups (Table 2). Notably, the PGDT group tended to have had greater BW loss than the PGEG group (PGEG, 11.4% vs. PGDT, 13.0%; p = 0.052; Cohen's d = 0.19). However, the constipation SS was significantly lower in the PGDT group than in the PGEG group (PGEG, 2.5 vs. PGDT, 2.1; p = 0.002; Cohen's d = 0.30), which indicated better status in the PGDT group. No significant differences in the other main outcome measures were observed. Multiple regression analysis was also performed to adjust the effects of multiple clinical factors including approaches, but the effect of the reconstruction method on the postoperative QOL for proximal gastrectomy was almost the same as the result of univariate analysis (Table 3).
| Domain | Main outcome measures | PGEG (n = 300) | PGDT (n = 172) | t-test | Cohen's d | ||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p-value | |||
| Symptoms | Esophageal reflux SS | 1.9 | 1.0 | 2.1 | 1.0 | 0.156 | |
| Abdominal pain SS | 1.7 | 0.8 | 1.8 | 0.8 | 0.658 | ||
| Meal-related distress SS | 2.6 | 1.1 | 2.7 | 1.0 | 0.357 | ||
| Indigestion SS | 2.1 | 0.9 | 2.2 | 1.0 | 0.338 | ||
| Diarrhea SS | 2.3 | 1.3 | 2.2 | 1.2 | 0.572 | ||
| Constipation SS | 2.5 | 1.3 | 2.1 | 1.0 | 0.002 | 0.30 | |
| Dumping SS | 2.2 | 1.3 | 2.1 | 1.0 | 0.407 | ||
| Total symptom score | 2.2 | 0.9 | 2.2 | 0.7 | 0.844 | ||
| Living status | Change in BW* | −11.4% | 8.4% | −13.0% | 7.6% | 0.052 | 0.19 |
| Ingested amount of food per meal* | 6.2 | 1.8 | 6.3 | 1.9 | 0.767 | ||
| Necessity for additional meals | 2.2 | 0.9 | 2.3 | 0.9 | 0.598 | ||
| Quality of ingestion SS* | 3.6 | 1.0 | 3.7 | 1.0 | 0.725 | ||
| Ability for working | 2.1 | 1.0 | 2.1 | 1.0 | 0.929 | ||
| QOL | Dissatisfaction with symptoms | 2.0 | 1.0 | 2.0 | 1.0 | 0.709 | |
| Dissatisfaction at the meal | 2.6 | 1.2 | 2.7 | 1.1 | 0.400 | ||
| Dissatisfaction at working | 1.9 | 1.0 | 2.0 | 1.0 | 0.424 | ||
| Dissatisfaction for daily life SS | 2.2 | 0.9 | 2.2 | 0.9 | 0.613 | ||
| PCS of SF-8* | 48.9 | 6.4 | 49.4 | 5.8 | 0.380 | ||
| MCS of SF-8* | 49.6 | 6.0 | 49.5 | 5.8 | 0.820 | ||
- Note: Outcome measures with “*”: higher score indicating better condition. Outcome measures without “*”: higher score indicating worse condition. The interpretation of effect size in Cohen's d: ≥0.2 as small, ≥0.5 as medium, ≥0.8 as large.
- Abbreviations: BW, body weight; MCS, mental component summary; PCS, physical component summary; SS, subscale; SD, standard deviation; PGEG, Proximal gastrectomy with esophagogastrostomy reconstruction; PGDT, Proximal gastrectomy with double tract reconstruction.
| UGca | Reconstruction method [PGDT] | Age (Yrs) | Gender [male] | Postoperative period (Mons) | Approach [laparoscopic] | Celiac branch of vagus [preserved] | CTx [Y] | cStage [III/IV] | LN dissection [D1+] | LN dissection [D2/D2+] | Combined resection [Y] | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Domain | Main outcome measures | β | p-value | β | p-value | β | p-value | β | p-value | β | p-value | β | p-value | β | p-value | β | p-value | β | p-value | β | p-value | β | p-value | R 2 | p-value |
| Symptoms | Esophageal reflux SS | 0.024 | 0.445 | ||||||||||||||||||||||
| Abdominal pain SS | −0.135 | 0.005 | −0.085 | 0.090 | −0.125 | 0.010 | 0.048 | 0.026 | |||||||||||||||||
| Meal-related distress SS | −0.154 | 0.001 | −0.083 | 0.088 | −0.082 | 0.086 | −0.085 | 0.078 | 0.058 | 0.005 | |||||||||||||||
| Indigestion SS | −0.083 | 0.086 | −0.097 | 0.052 | 0.039 | 0.089 | |||||||||||||||||||
| Diarrhea SS | −0.168 | 0.000 | −0.087 | 0.065 | −0.087 | 0.069 | 0.099 | 0.031 | 0.075 | 0.000 | |||||||||||||||
| Constipation SS | −0.153 | 0.002 | 0.105 | 0.029 | 0.046 | 0.034 | |||||||||||||||||||
| Dumping SS | −0.147 | 0.003 | 0.106 | 0.052 | −0.132 | 0.008 | 0.071 | 0.001 | |||||||||||||||||
| Total symptom score | −0.104 | 0.036 | −0.104 | 0.038 | 0.042 | 0.088 | |||||||||||||||||||
| Living status | Change in BW* | −0.090 | 0.073 | −0.114 | 0.018 | 0.025 | 0.434 | ||||||||||||||||||
| Ingested amount of food per meal* | 0.132 | 0.008 | 0.024 | 0.459 | |||||||||||||||||||||
| Necessity for additional meals | −0.136 | 0.006 | 0.044 | 0.045 | |||||||||||||||||||||
| Quality of ingestion SS* | 0.081 | 0.098 | 0.032 | 0.219 | |||||||||||||||||||||
| Ability for working | 0.270 | <0.0001 | 0.083 | <0.0001 | |||||||||||||||||||||
| QOL | Dissatisfaction with symptoms | −0.091 | 0.058 | −0.108 | 0.021 | 0.104 | 0.031 | −0.099 | 0.040 | 0.055 | 0.009 | ||||||||||||||
| Dissatisfaction at the meal | −0.103 | 0.031 | 0.143 | 0.007 | 0.057 | 0.006 | |||||||||||||||||||
| Dissatisfaction at working | 0.094 | 0.084 | 0.023 | 0.488 | |||||||||||||||||||||
| Dissatisfaction for daily life SS | 0.126 | 0.019 | −0.080 | 0.096 | 0.046 | 0.033 | |||||||||||||||||||
| PCS of SF-8* | −0.193 | <0.0001 | −0.086 | 0.070 | 0.139 | 0.004 | 0.070 | 0.001 | |||||||||||||||||
| MCS of SF-8* | 0.092 | 0.070 | 0.020 | 0.638 | |||||||||||||||||||||
- Note: Outcome measures with “*”: higher score indicating better condition. Outcome measures without “*”: higher score indicating worse condition. If β is positive, the score of the outcome measure of the patients belonging to the category in [brackets] is higher in cases when the factor is a nominal scale, and the score of outcome measure of the patients with larger values is higher in cases when the factor is a numeral scale.
-
Abbreviations: CTx, chemotherapy, LN, lymphnode, [Y], yes.
The interpretation of effect size β R 2 Small ≧0.1 ≧0.02 Medium ≧0.3 ≧0.13 Large ≧0.5 ≧0.26
Subsequently, a stratified analysis based on the remnant stomach size was performed for those with a remnant stomach size of approximately 1/2 (Table 4) and 2/3 (Table 5), which were the main targets of proximal gastrectomy.
| Domain | Main outcome measures | PGEG (n = 73) | PGDT (n = 97) | t-test | Cohen's d | ||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p-value | |||
| Symptoms | Esophageal reflux SS | 2.1 | 1.1 | 2.0 | 0.9 | 0.843 | |
| Abdominal pain SS | 1.9 | 0.9 | 1.7 | 0.8 | 0.106 | ||
| Meal-related distress SS | 2.8 | 1.2 | 2.7 | 1.1 | 0.466 | ||
| Indigestion SS | 2.3 | 1.0 | 2.2 | 1.0 | 0.342 | ||
| Diarrhea SS | 2.4 | 1.5 | 2.3 | 1.3 | 0.650 | ||
| Constipation SS | 2.5 | 1.3 | 2.1 | 1.0 | 0.007 | 0.42 | |
| Dumping SS | 2.3 | 1.1 | 2.0 | 0.9 | 0.025 | 0.37 | |
| Total symptom score | 2.4 | 0.9 | 2.2 | 0.7 | 0.126 | ||
| Living status | Change in BW* | −12.4% | 8.2% | −14.0% | 8.1% | 0.217 | |
| Ingested amount of food per meal* | 6.0 | 1.8 | 6.2 | 1.9 | 0.478 | ||
| Necessity for additional meals | 2.4 | 1.0 | 2.3 | 0.9 | 0.460 | ||
| Quality of ingestion SS* | 3.5 | 1.1 | 3.7 | 0.9 | 0.163 | ||
| Ability for working | 2.3 | 1.1 | 2.0 | 1.0 | 0.098 | 0.26 | |
| QOL | Dissatisfaction with symptoms | 2.2 | 1.1 | 2.0 | 1.0 | 0.243 | |
| Dissatisfaction at the meal | 2.7 | 1.1 | 2.7 | 1.2 | 0.924 | ||
| Dissatisfaction at working | 2.0 | 1.1 | 2.0 | 1.0 | 0.899 | ||
| Dissatisfaction for daily life SS | 2.3 | 0.9 | 2.2 | 0.9 | 0.642 | ||
| PCS of SF-8* | 48.0 | 6.1 | 49.5 | 5.9 | 0.111 | ||
| MCS of SF-8* | 48.6 | 6.6 | 49.5 | 5.9 | 0.386 | ||
- Note: Outcome measures with “*”: higher score indicating better condition. Outcome measures without “*”: higher score indicating worse condition. The interpretation of effect size in Cohen's d: ≥0.2 as small, ≥0.5 as medium, ≥0.8 as large.
- Abbreviations: BW, body weight; MCS, mental component summary; PCS, physical component summary; SS, subscale; SD, standard deviation; PGEG, Proximal gastrectomy with esophagogastrostomy reconstruction; PGDT, Proximal gastrectomy with double tract reconstruction.
| Domain | Main outcome measures | PGEG (n = 165) | PGDT (n = 60) | t-test | Cohen's d | ||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p-value | |||
| Symptoms | Esophageal reflux SS | 1.9 | 1.0 | 2.0 | 1.0 | 0.754 | |
| Abdominal pain SS | 1.7 | 0.8 | 1.8 | 0.8 | 0.425 | ||
| Meal-related distress SS | 2.6 | 1.1 | 2.6 | 1.0 | 0.668 | ||
| Indigestion SS | 2.1 | 0.9 | 2.2 | 1.0 | 0.623 | ||
| Diarrhea SS | 2.3 | 1.2 | 2.0 | 0.8 | 0.033 | 0.33 | |
| Constipation SS | 2.5 | 1.2 | 2.2 | 1.1 | 0.075 | 0.27 | |
| Dumping SS | 2.2 | 1.3 | 2.1 | 1.0 | 0.489 | ||
| Total symptom score | 2.2 | 0.8 | 2.1 | 0.7 | 0.437 | ||
| Living status | Change in BW* | −11.2% | 8.1% | −11.2% | 6.4% | 0.982 | |
| Ingested amount of food per meal* | 6.2 | 1.8 | 6.6 | 1.8 | 0.105 | ||
| Necessity for additional meals | 2.2 | 0.8 | 2.2 | 0.8 | 0.745 | ||
| Quality of ingestion SS* | 3.6 | 0.9 | 3.5 | 1.0 | 0.571 | ||
| Ability for working | 2.0 | 0.9 | 2.1 | 0.9 | 0.530 | ||
| QOL | Dissatisfaction with symptoms | 2.1 | 1.0 | 1.8 | 0.9 | 0.050 | 0.30 |
| Dissatisfaction at the meal | 2.7 | 1.2 | 2.4 | 1.0 | 0.111 | ||
| Dissatisfaction at working | 1.9 | 1.0 | 1.7 | 0.8 | 0.087 | 0.26 | |
| Dissatisfaction for daily life SS | 2.2 | 0.9 | 2.0 | 0.7 | 0.040 | 0.32 | |
| PCS of SF-8* | 49.5 | 5.8 | 50.1 | 5.6 | 0.478 | ||
| MCS of SF-8* | 49.8 | 5.3 | 49.7 | 5.5 | 0.914 | ||
- Note: Outcome measures with “*”: higher score indicating better condition. Outcome measures without “*”: higher score indicating worse condition. The interpretation of effect size in Cohen's d: ≥0.2 as small, ≥0.5 as medium, ≥0.8 as large.
- Abbreviations: BW, body weight; MCS, mental component summary; PCS, physical component summary; PGEG, Proximal gastrectomy with esophagogastrostomy reconstruction; PGDT, Proximal gastrectomy with double tract reconstruction; SS, subscale; SD, standard deviation.
Among those with a remnant stomach size of around 1/2, the constipation SS (PGEG, 2.5 vs. PGDT, 2.1; p = 0.007; Cohen's d = 0.42) and dumping SS (PGEG 2.3 vs. PGDT 2.0; p = 0.025; Cohen's d = 0.37) were significantly lower in the PGDT group than that in the PGEG group, which indicated a better status in the PGDT group. Working ability tended to be lower in the PGDT group than in the PGEG group (PGEG, 2.4 vs. PGDT, 2.0; p = 0.098; Cohen's d = 0.26). No differences in the other main outcome measures, including body weight (BW) loss (%), were found between the two groups.
Among those with a remnant stomach size of around 2/3, the diarrhea SS (PGEG, 2.5 vs. PGDT, 2.0; p = 0.033; Cohen's d = 0.33), dissatisfaction with symptoms (PGEG, 2.1 vs. PGDT, 1.8; p = 0.050; Cohen's d = 0.30), and dissatisfaction with daily life SS (PGEG, 2.2 vs. PGDT, 2.0; p = 0.040; Cohen's d = 0.32) were significantly lower in the PGDT group than that in the PGEG group, which indicated a better status in the PGDT group. Additionally, the constipation SS (PGEG, 2.5 vs. PGDT, 2.2; p = 0.075; Cohen's d = 0.27) and dissatisfaction with work (PGEG, 1.9 vs. PGDT, 1.7; p = 0.087; Cohen's d = 0.26) tended to be lower in the PGDT group than in the PGEG group. No differences in the other main outcome measures, including BW loss (%), were found between the two groups.
4 DISCUSSION
The Japan Postgastrectomy Syndrome Working Party has created a questionnaire (i.e., PGSAS-45) to evaluate postgastrectomy disorders, utilizing the Japanese version for clinical applications.15 According to the Japanese gastric cancer treatment guidelines, standard gastrectomy is defined as the resection of at least two-thirds of the stomach, including D2 lymph node dissection.11 The PGSAS study showed that proximal gastrectomy promoted better QOL compared to total gastrectomy with Roux-en-Y reconstruction, leading to the widespread introduction of proximal gastrectomy. However, the optimal proximal gastrectomy reconstruction method has yet to be established.10, 17
Following the previous PGSAS study, “the PGSAS NEXT study,” a study on the optimal gastrectomy procedures to improve postoperative QOL for the cancer located at the upper third of the stomach or around the esophagogastric junction using the PGSAS-45 questionnaire, was planned.
Various reconstruction methods have been utilized for proximal gastrectomy procedures. Japanese gastric cancer treatment guidelines have proposed three types of reconstruction methods for proximal gastrectomy: esophagogastrostomy, jejunal interposition, and double tract method.11 Analysis of the proximal gastrectomy cases collected in this study revealed that PGEG and PGDT were most popular reconstruction methods utilized in Japan.
Inada et al. reported that scores for esophageal reflux and dissatisfaction with meals were higher in patients who had not undergone an anti-reflux procedure in the PGSAS study, which indicated that an appropriate anti-reflux method is strongly recommended for PGEG.18 In the current study, 276 cases (93%) underwent anti-reflex procedures for PGEG, whereas only 21 cases (7%) did not. The types of anti-reflux methods for PGEG include the double flap method,19, 20 creation of pseudofornix and/or Hisoid angle, fundoplication,21, 22 and SOFY,16 all of which have been reported to have excellent anti-reflux effects. The double flap method, which had been performed in 154 cases, had the best anti-reflux effects for esophagogastric anastomosis with fundoplication based on valvuloplasty by creating the seromuscular double flaps, although this method is technically difficult and time consuming.23 PGDT requires 8–15 cm of interposed jejunum between the esophagus and the remnant stomach and an escape route using gastrojejunostomy.24, 25 The double tract method requires plural anastomosis (i.e., esophagojejunostomy, jejunogastrostomy, and jejunojejunostomy), although each anastomosis procedure is technically easy and familiar to many surgeons. Since the risk of an internal hernia is a concern, it is better to do suture closure of the mesentery gap caused by reconstruction.
From the background of both groups, the laparoscopic approach for proximal gastrectomy has been the mainstream approach for gastric cancer. In the previous PGSAS study, the laparoscopic surgery rate for proximal gastrectomy was only 17.2%,10 whereas that for proximal gastrectomy in the current PGSAS NEXT study, which was conducted nearly 9 years after the PGSAS study, has since increased. Currently in Japan, laparoscopic proximal gastrectomy is growing more popular than open proximal gastrectomy for early upper third gastric cancer. The JCOG1401 trial published in 2019 confirmed the safety of laparoscopic total/proximal gastrectomy for clinical stage I gastric cancer.26 An increasing number of facilities are introducing robotic surgery for stomach cancer.27 In particular, for upper gastric cancer and esophageal gastric junction cancer, the merit of performing robotic surgery is considered to be great because surgical operation can be performed easily and precisely in a narrow field of view, and its spread is expected.
Surgical options, such as the size of the remnant stomach, anastomosis method, adding procedures to create an anti-reflux function, length of the resected abdominal esophagus, and preservation of the celiac branch of the vagus nerve, can influence the QOL after proximal gastrectomy. The PGDT group had a greater esophageal resection length and shorter distance from the diaphragm to the anastomosis site, indicating that PGDT had been employed for cases requiring longer esophagus resection lengths and allows anastomosis at a higher diaphragm level. The PGEG group had a significantly larger remnant stomach size and higher preservation rate of the celiac branch of the vagus nerve than the PGDT. Thus, the remnant stomach size affected the choice of the reconstruction method and the rate of function preserving procedure after proximal gastrectomy. Nonetheless, introducing an effective anti-reflux method in cases with a small remnant stomach remains difficult; therefore, PGDT may be preferred in such cases.
In all cases, BW loss (%) tended to be smaller in the PGEG group than the PGDT group. The possible explanations for this include a larger remnant stomach size acting as a reservoir and the total passage of the ingested meal through the physiological duodenal route in the PGEG group. In contrast, the PGDT group had a rather small remnant stomach size, possibly inducing and postpancreaticocibal asynchrony if a significant proportion of the ingested meal directly passes through the escape jejunal route, which may result in nutrient indigestion. In PGDT, if food does not flow into the residual stomach, nutritionally it will be the same as total gastrectomy using Roux-en-Y reconstruction, so it is important to devise a jejunogastrostomy procedure so that more food flows into the residual stomach. Several devices have been reported, one of which is to increase length of jejunogastrostomy (6 cm or more). In the part of the jejunum used for jejunogastrostomy, the luminal structures necessary for peristalsis to transport food is lacking, so it is expected that food will flow into the residual stomach by lengthening this portion. In the report by Kamiya et al., the weight loss was less in the longer jejunogastrostomy group, namely, −12.5% in the longer group (6 cm or more), compared to −14.9% in the shorter group (5 cm or less). Although there was no significant difference.28
The current study showed that the QOL of the PGDT groups was slightly superior to that of the PGEG group across several main outcome measures of the PGSAS-45 during stratified analysis according to remnant stomach sizes of around 1/2 and 2/3. Among those with a remnant stomach size of 1/2, the PGDT group was superior in three main outcome measures, namely constipation SS, dumping SS, and working ability, compared to the PGEG group. Similarly, among those with a remnant stomach size of 2/3, the PGDT group was superior in five main outcome measures, namely diarrhea SS, dissatisfaction with symptoms, dissatisfaction with daily life SS, constipation SS, and dissatisfaction with work. No difference in the other main outcome measures, including body weight loss, was observed between the groups.
The use of a fiberscope can be expected to improve the early diagnosis of upper third gastric cancer. The Japanese gastric cancer treatment guidelines recommended proximal gastrectomy for cN0, cT1 tumors where more than half of the distal stomach can be preserved.11 Though the present study showed that PGDT can promote slightly better QOL than PGEG, the reconstruction method for proximal gastrectomy can be selected according to each surgeon's institutional principles or discretion at this point. Given that the procedures employed for PGEG and PGDT are still incomplete, further techniques for improving both methods and studies revaluating PGEG and PGDT in the future are required. One limitation of the present study was its retrospective nature and the unbalanced number of patients in each group. A well-designed prospective study should therefore be conducted in the future.
5 CONCLUSION
Our results indicated that the QOL of the PGDT group was slightly superior to that of the PGEG group for several main outcome measures of the PGSAS-45 during stratified analysis according to remnant stomach sizes of around 1/2 and 2/3, with a small effect size. The revaluation of both reconstruction methods after compensating the shortcomings of each procedure (i.e., preventing esophageal reflux for PGEG and maintaining physiological food passage for PGDT) will be a substantial issue in the future.
ACKNOWLEDGMENT
This study was supported by a grant from the Jikei University and the Japanese Gastric Cancer Association. The authors thank all physicians who participated in this study and the patients whose cooperation made this study possible.
FUNDING INFORMATION
This study was supported by a grant from the Jikei University and the Japanese Gastric Cancer Association.
CONFLICT OF INTEREST
Dr. H.M. is an editorial member at the Annals of Gastroenterological Surgery. The other authors declare that they have no conflicts of interest.
ETHICS STATEMENTS
This study was approved by the local ethics committees of each participating institution and was in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1964 and later versions. Written informed consent was obtained from all enrolled patients. Trial registration: The University Hospital Medical Information Network Clinical Trials Registry (registration number 000032221).


