Targeted Long-Read Sequencing as a Single Assay Improves the Diagnosis of Spastic-Ataxia Disorders
Ira W. Deveson and Kishore Raj Kumar joint-senior authors.
Funding: This work was supported by the Medical Research Futures Fund (Grants 2023126 and 2025138). The project receives partial in-kind support from Oxford Nanopore Technologies under an ongoing collaboration agreement. I.W.D. is supported by the National Health and Medical Research Council (NHMRC) (Grant 2035037). H.G. is supported by the Australian Research Council (ARC) DECRA Fellowship (Grant DE230100178).
ABSTRACT
Objective
The hereditary spastic-ataxia spectrum disorders are a group of disabling neurological diseases. The traditional genetic testing pathway is complex, multistep and leaves many cases unsolved. We aim to streamline and improve this process using long-read sequencing.
Methods
We developed a targeted long-read sequencing strategy with the capacity to characterise the genetic variation of all types and sizes within 469 disease-associated genes, in a single assay. We applied this to a cohort of 34 individuals with unsolved spastic-ataxia. An additional five individuals with a known genetic diagnosis were included as positive controls.
Results
We identified causative pathogenic variants that would be sufficient for genetic diagnosis in 14/34 (41%) unsolved participants. The success rate was 5/11 (45%) in those who were naïve to genetic testing and 9/23 (39%) in those who were undiagnosed after prior genetic testing, completed on a clinical basis. Short tandem repeat expansions in FGF14 were the most common (7/34, 21%). Two individuals (2/34, 6%) had biallelic pathogenic expansions in RFC1 and one individual had a monoallelic pathogenic expansion in ATXN8OS/ATXN8. Causative pathogenic sequence variants other than short tandem repeat expansions were found in four individuals, including in VCP, STUB1, ANO10 and SPG7. Furthermore, all five positive controls were identified.
Interpretation
Our results demonstrate the utility of targeted long-read sequencing in the genetic evaluation of patients with spastic-ataxia spectrum disorders, highlighting both the capacity to increase overall diagnostic yield and to streamline the testing pathway by capturing all known genetic causes in a single assay.
1 Introduction
The hereditary spastic-ataxia spectrum disorders are a group of rare, disabling neurologic conditions [1]. Hereditary cerebellar ataxia (HCA) and hereditary spastic paraplegia (HSP) can be considered as part of a spastic-ataxia spectrum disorder [2]. Despite advances in genetic testing methods and broader access to next-generation sequencing (NGS), up to 71% of individuals with HCA and 45%–50% of individuals with a HSP phenotype currently do not receive a genetic diagnosis [1, 3-8].
There are several potential contributors to this ‘diagnostic gap’. For example, only the most common short tandem repeat (STR) expansions are evaluated as part of a ‘spinocerebellar ataxia (SCA) panel’, the content of which may vary between sites, while other less common or recently described STR expansions may be overlooked. For instance, presently in Australia, clinically accredited testing for STR expansions in FGF14 or RFC1, which causes late-onset ataxia [9], is not available locally.
Long-read sequencing (LRS) is an emerging group of genomic technologies, which overcome several limitations posed by earlier methods, including NGS [10]. LRS provides improved detection of STR expansions, structural variants (SVs) and copy number variants (CNVs); the ability to resolve repetitive regions and homologous gene families or gene-pseudogene pairs; variant phasing without parental sequencing data and DNA methylation profiling at no additional cost [10]. LRS is particularly advantageous for genotyping STR expansions, where it can clearly identify expansion size, sequence, methylation state and zygosity, even for large, complex or GC/AT-rich repeats.
The adaptive sampling or ‘ReadUntil’ functionality on LRS instruments from Oxford Nanopore Technologies (ONT) enables selective sequencing of specific genes using genetic coordinates provided programmatically and without the need for additional laboratory processes for target enrichment [11]. Targeted LRS can be used to evaluate a large number of STRs in a single test and has demonstrated success in concurrent testing of a set of 37 genes associated with neurologic disease [12] as well as concurrent evaluation of 10 STR loci associated with ataxia [13]. Although these studies establish the analytical validity of this approach for detection of STR expansions, they did not examine non-STR causative variants for ataxia/neurologic disease, nor assess the capacity of LRS to solve otherwise undiagnosed patients.
Given the capacity to evaluate a broad variety of genes and genetic variant types in a single streamlined assay, we reasoned that ONT-targeted LRS could be used to improve the diagnosis of spastic-ataxia spectrum disorders. To test this, we designed a targeted assay for the evaluation of STR expansions, single-nucleotide variants (SNVs), small insertions and deletions (indels), SVs and CNVs across all diagnostically relevant genes (n = 469). We applied this to a cohort of genetically undiagnosed patients with a spastic-ataxia spectrum phenotype and assessed the potential to improve the rate of diagnosis, relative to traditional testing. Here we report the clinical and genetic findings from this cohort, demonstrating a strong improvement in diagnostic rate and highlighting the strengths of LRS for genetic evaluation of these disorders.
2 Methods
2.1 Patient Recruitment, Clinical Evaluation and Study Approval
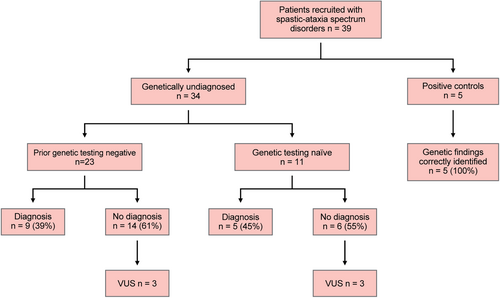
A cohort of 34 patients with clinically diagnosed spastic-ataxia spectrum disorders, without a genetic diagnosis, were recruited from the Neuromuscular Clinic at Concord Repatriation General Hospital in Sydney, Australia, between April 2023 and March 2024. The cohort consisted of 11 individuals who were naïve to genetic testing and 23 individuals who had previously undertaken genetic testing, as per standard clinical practice in Australia. Prior genetic testing had been arranged by the patients' treating clinicians, and included various combinations of testing for STR expansions in selected SCA genes (SCA 1, 2, 3, 6, 7, 12, 17), FXN for Friedreich ataxia, ATN1 for dentatorubral-pallidoluysian atrophy, FMR1 for Fragile X Tremor/Ataxia Syndrome (FXTAS); NGS [targeted gene panel, whole-exome sequencing (WES) or whole-genome sequencing (WGS)]; and auxiliary testing [single gene testing, multiplex ligation-dependent probe amplification (MLPA), microarray, mitochondrial genome sequencing], as appropriate. An additional five individuals with spastic-ataxia spectrum disorders and a known genetic diagnosis were included as positive controls. Demographic, clinical, neuroimaging, neurophysiological and genetic testing data were collected at the time of clinical evaluations and from patient healthcare records. The protocol for the study has received prior approval by the appropriate Institutional Review Board, and informed consent was obtained from each subject (approval number 2019/ETH12538).
2.2 Targeted Long-Read Sequencing Workflow
Peripheral blood samples were obtained from all 39 individuals (34 study participants and five controls). Samples were processed at The Garvan Institute of Medical Research in Sydney, Australia. High-molecular weight (HMW) genomic DNA was extracted from peripheral blood samples using the Nanobind CBB kit (PacBio, Cat# 102-301-900). HMW genomic DNA was sheared to ~30-kb fragment size using the Diagenode Megaruptor 3 DNA shearing system and visualised on an Agilent Femto Pulse using the Genomic DNA 165 kb Kit. ONT libraries were prepared from ~3 μg of sheared HMW genomic DNA using a ligation prep (SQK-NBD114.24). Three samples were barcoded and pooled into one library and loaded on an ONT PromethION R10.4.1 flow cell (FLO-PRO114M) and sequenced on either a PromethION 2 Solo or PromethION 48 instrument, with live target selection/rejection executed by the Readfish software package (v0.0.10dev2) [11] targeting a custom panel of 469 genes associated with spastic-ataxia spectrum disorders, plus the mitochondrial genome (Table S1). Gene targets were encoded relative the T2T-chm13 (v2) reference genome and ReadFish was executed with the following configurations: config_name = ‘dna_r10.4.1_e8.2_400bps_5khz_fast_prom’; min_chunks = 0; max_chunks = 16; single_on = ‘stop_receiving’; multi_on = ‘stop_receiving’; single_off = ‘unblock’; multi_off = ‘unblock’; no_seq = ‘proceed’; no_map = ‘proceed’. Genes included in the targeted panel were selected via detailed literature review [9] and review of gene panels for ataxia, spastic-ataxia and HSP through PanelApp [14] and other genetic testing laboratories including Invitae, Blueprint Genetics, PathWest and Victorian Clinical Genetics Services (VCGS). Libraries were run for 72 h, with nuclease flushes and library reloading performed at approximately 24- and 48-h time points.
2.3 Analysis of Genetic Variation
After completing a targeted sequencing run, raw ONT sequencing data were converted to BLOW5 format [15] and base-called with Dorado, using the ‘super-accuracy’ model (dna_r10.4.1_e8.2_400bps_5khz_sup_prom.cfg) and the Buttery-eel wrapper to enable BLOW5 input [16]. The resulting ‘pass’ FASTQ files were aligned to both the hg38 and T2T-CHM13v2.0 reference genomes using minimap2 (v2.14-r883) [17]. SNVs and indels were called using Clair3 (v1.0.4) [18] and phased using WhatsHap (v2.1) [19]. SVs (including CNVs) were called with Sniffles2 [20] (v2.07), which takes haplotagged BAM alignment files to generate phased SV calls. SNVs and indels were functionally annotated using variant effect predictor (VEP) [21] (v110) and SVs were annotated using AnnotSV [22] (v3.3.4).
Variants were prioritised for further consideration based on the following criteria: (i) those annotated in ClinVar with a clinical significance of ‘pathogenic’, ‘likely pathogenic’ or ‘uncertain’; (ii) variants predicted to cause a frameshift; (iii) missense variants with a REVEL score greater than 0.5 and classified as ‘pathogenic’, ‘likely pathogenic’, ‘uncertain’ or without an assigned clinical significance and (iv) variants with high or moderate impact that are similarly classified as ‘pathogenic’, ‘likely pathogenic’, ‘uncertain’ or lacking an assigned clinical significance in ClinVar. All pre-selected variants were within MANE Select transcripts and either were not found in gnomADv4 or had allele frequency of less than 0.1, resulting in a list of rare and potentially causative variants. Additionally, SVs intersecting protein-coding genes were prioritised for further consideration.
2.4 Analysis of STR Expansions
We analysed STRs in 21 genes associated with spastic-ataxia spectrum disorders (Table S2). During this project, STR expansions in THAP11 and ZFHX3, were identified to cause HCA [23-25], and these genes were subsequently added to our target panel. All individuals underwent sequencing of THAP11, however, 24 samples had already undergone sequencing prior to the addition of ZFHX3, and therefore, these individuals were not evaluated for SCA4. Furthermore, TBP was omitted from the initial panel, and therefore also was only evaluated in 15/39 samples.
STRs were genotyped using a method demonstrated previously [12]. Each STR region was first inspected in integrated genome viewer (IGV; T2T-CHM13v2 reference) [26]. For sites showing evidence of expansions (i.e., large insertions within alignments spanning an STR site), reads were retrieved within a 50 kb window centred on the target STR and assembled de novo with Flye (v2.8.1-b1676) [27] to create a pseudo-haploid contig encompassing the STR region. The starting reads were then realigned to this contig and phased into separate haplotypes using Longshot (v0.4.1) [28]. The initial assembled contig was re-polished with reads from each haplotype using Racon (v1.4.0), generating two distinct haploid contigs encompassing the STR site. The precise position of the STR site was identified by mapping 150-bp unique flanking sequences extracted from T2T-CHM13v2.0 using minimap2 (v2.22). The reads from each haplotype were mapped to their respective polished contigs and re-inspected in IGV to identify potential discrepancies in phasing of reads, which could be manually corrected before re-polishing [26]. The intervening distance between unique mapped flanking sequences was used to determine the size of the STR on each haplotype, and sequences were extracted to determine the STR motif present, and/or presence of interruptions (where relevant). Custom sequence bar plots were created in Prism to enable visualisation of STR alleles within and between patients, with multi-read bar plots used to verify the presence of interruptions or noncanonical motifs (see Figure 1 and Figure S2B for examples).
2.5 Validation of Results
Our analysis method for genotyping STR expansions with ONT data has previously been validated in a cohort of 37 individuals including patients with neurogenetic diseases, premutation carriers and controls [12]. In the present study, five individuals with a known genetic diagnosis were included as positive controls: one individual with FXTAS due to an FMR1 STR expansion; one with SCA3 due to an ATXN3 STR expansion, one with HSP due to homozygous SPG7 variants; one with recessive ataxia due to compound heterozygous variants in ANO10 and one with recessive ataxia due to compound heterozygous variants in TDP2. Novel findings were confirmed on orthogonal testing in nine individuals.
2.6 Statistical Analysis
Quantitative variables were described as means and ranges. Categorical variables were expressed with frequencies and percentages. Comparison of groups was undertaken using independent samples 𝜒2 testing for binomial variables. Statistical significance threshold was set at p-value < 0.05.
2.7 Data Avalability Statement
Anonymised data that support the findings of this study are available from the corresponding author, upon reasonable request.
3 Results
3.1 Targeted LRS Assay for Spastic-Ataxia Spectrum Disorders
We developed an ONT ReadUntil targeted LRS assay [11] designed to capture the full suite of genetic features implicated in spastic-ataxia spectrum disorders in a single assay. This encompasses 469 genes considered diagnostically relevant plus the mitochondrial genome (Table S1). Genes included in the targeted panel were selected via detailed literature review [9], PanelApp [14] and review of gene panels from other genetic laboratories. We designed target regions covering each gene and flanking regions 50 kb upstream and downstream to ensure all exons, introns, gene promoters, untranslated regions (UTRs) and other local regulatory sequences are captured. This panel covers STR sites in 21 genes where expanded alleles are known to cause spastic-ataxia spectrum disorders (Table S2). In total, the target panel covers 91.8 Mbases or 3.0% of the human genome sequence.
Targeted LRS enables multiple patient samples to be analysed in a single experiment to reduce cost, while still obtaining sufficient coverage depth for accurate and comprehensive analysis. Our study cohort (see below) was sequenced at a rate of three patients per flow cell (ONT PromethION), which equates to an approximately threefold reduction in cost per patient, relative to whole-genome ONT sequencing. We obtained median 42-fold coverage depth across target gene regions, with a median absolute deviation (MAD) of 16.79. The coverage depth across individual target regions ranged from a minimum median coverage of 23 to a maximum median coverage of 50 (Figure S1A,B). For on-target statistics per sample, see Table S3, for average mitochondrial DNA coverage and read length see Table S4.
To validate our approach, positive controls were analysed by blinded investigators (see Materials & Methods). Our targeted ONT LRS approach identified STR expansions in FMR1 and ATXN3 in patients with known FXTAS and SCA3, respectively, with relatively similar repeat lengths identified compared to that generated by standard clinical testing (85 vs. 87 copies for FMR1; 73 vs. 76 for ATXN3; Figure S2A,B). Additionally, ONT LRS was able to identify the presence and position of two AGG interruptions within the FMR1 CGG STR expansion (Figure S2B). AAG interruptions are known to influence the propensity of the FMR1 CGG STR expansion to further expand in subsequent generations [29]. We identified SNVs and small indels in three positive control patients with recessive spastic ataxic disorders (Figure S2A,C). Our ONT LRS approach also allowed for phasing of these variants, confirming an apparently homozygous SNV in SPG7 was biallelic and confirming that patients with two heterozygous variants in ANO10 and TDP2 carried these variants in trans (Figure S2C). Interestingly, the patient with known autosomal recessive SCA due to variants in TDP2 was also found to carry a (GAA)240 STR expansion in FGF14, close to the (GAA)250 threshold considered pathogenic.
3.2 Cohort Characteristics
We evaluated the performance of our targeted LRS strategy on a cohort of 34 patients (Table 1) with genetically undiagnosed spastic-ataxia spectrum disorders, in addition to five positive controls (see above).
| Characteristic | Mean (years) (range) |
|---|---|
| Age at examination | 64.0 (24–84) |
| Age at onset | 53.0 (0.08–80) |
| Disease duration | 10.9 (1–37) |
| Sex | n (%) |
| Female | 16 (47) |
| Male | 18 (53) |
| Ancestry | |
| White/European | 20 (59) |
| Lebanese | 2 (6) |
| Chinese | 1 (3) |
| Filipino | 1 (3) |
| Indian | 1 (3) |
| Iraqi | 1 (3) |
| Mixed | 2 (6) |
| Unknown | 6 (18) |
| Family history | |
| Sporadic | 20 (59) |
| AD | 11 (32) |
| AR | 1 (3) |
| Unknown | 2 (6) |
| Predominant phenotype | |
| Ataxia | 29 (85) |
| Spastic-ataxia | 3 (9) |
| Spastic paraparesis | 2 (6) |
- Abbreviations: AD, autosomal dominant; AR, autosomal recessive.
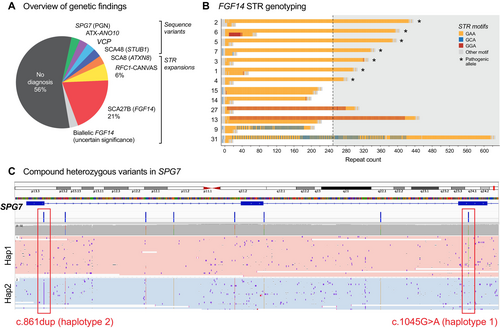
3.3 Genetic Findings
Our targeted LRS strategy identified a causative pathogenic variant sufficient for diagnosis in 14/34 (41%) of participants (Figure 1A). In the group who had previously undergone unsuccessful genetic testing, a diagnosis was obtained in 9/23 (39%) participants, while a diagnosis was obtained in 5/11 (45%) who were naïve to genetic testing. A heterozygous GAA-FGF14 STR expansion ≥ 250 repeats, consistent with a diagnosis of SCA27B, was identified in 7/34 (21%) of the study cohort and was found in 5/23 (22%) of the testing-negative group (Figure 1B). RFC1 CANVAS/spectrum disorder due to biallelic RFC1 STR expansions was identified in 2/34 (6%). A single individual was identified to have each of: a CAG·CTG STR expansion in ATXN8/ATXN8OS; a homozygous splicing variant in ANO10 (c.1163-9A>G); a heterozygous missense variant in VCP (c.475C>T, p.Arg159Cys); a heterozygous in-frame deletion in STUB1 (c.433_435del, p.Lys145del); one instance of compound heterozygous missense and nonsense variants in SPG7 (c.1045G>A, p.Gly349Ser and c.861dup, p.Asn288Ter; Figure S3A–D). For the latter case, variants were phased with LRS without parental sequencing data. The 75.7 kb phased block contained six additional heterozygous variants between the two variants of interest, supported by 23 long reads, providing robust evidence to establish compound heterozygosity (Figure 1C). Genetic and clinical features for individuals with a positive diagnosis are summarised in Table S5.
This left 20/34 (59%) of individuals without a genetic diagnosis. Two of these individuals were found to harbour pathogenic/likely pathogenic variants in GCH1 and PRRT2, respectively, although these findings were of uncertain clinical relevance to the cerebellar ataxia phenotype (File S1). A further individual was identified to have biallelic STR expansions in FGF14, within the range of 200–249 repeats (File S1), of uncertain clinical relevance. Overall, one or more variants of uncertain significance (VUS) were identified in 6/20 (30%) of individuals without an identified causative variant (Table S6).
To validate our findings, eight individuals (patients 1, 3, 4, 5, 7, 8, 12 and 13) underwent confirmatory testing through an independent clinically accredited laboratory. One further individual (patient 11) had previously undergone WGS on a research basis and data were available for reanalysis. Five individuals with FGF14 STR expansions, as well as one individual with biallelic RFC1 STR expansions, underwent confirmatory testing with standard flanking PCR and repeat-primed PCR, followed by capillary electrophoresis. Results demonstrated similar GAA repeat lengths between the two methods and in all cases, the pathogenicity of expansions was correctly classified (Table S7). The biallelic RFC1 AAGGG STR expansion identified in patient 8 was also confirmed with standard flanking PCR and repeat-primed PCR followed by capillary electrophoresis, although the specific repeat length was not reported. The homozygous ANO10 variant identified in patient 12, and the heterozygous VCP variant identified in patient 13 were confirmed on clinically accredited WES. The STUB1 in-frame deletion in patient 11 was confirmed on the retrospective review of prior research WGS data, and further confirmatory testing with a clinically accredited WES is pending.
3.4 Clinical Findings
3.4.1 FGF14-GAA (SCA27B); demographic and Clinical Features
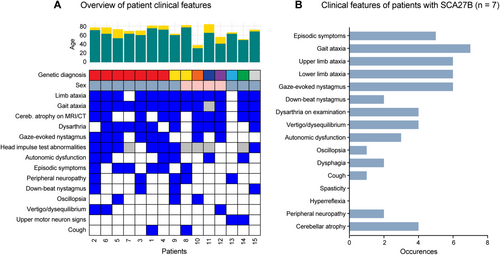
A total of 7/34 (21%) individuals were identified to have GAA ≥ 250 FGF14 STR expansions, consistent with a diagnosis of SCA27B. The mean GAA repeat length was 348 repeats (range 274–425, SD ±56) (Figure 1B, Videos S1 and S2). All seven individuals were male. Ancestry was known in 6/7 (86%) individuals, with white/European ancestry in all known cases. An autosomal dominant family history was apparent in 3/7 (43%) of individuals, with maternal inheritance in all cases. The mean age at examination was 75.4 years (range 69–81), the mean age of onset was 65.3 years (range 53–75) and the mean disease duration was 10.1 years (range 6–20). 4/7 (57%) required a mobility aid (stick 2/4 and walker 2/4), with a mean time to mobility aid requirement of 5.8 years from disease onset (range 4–10). The mean SARA score was 11.6 (range 6.5–18). Clinical features of all genetically diagnosed patients, and those with SCA27B specifically, are indicated in Figure 2A,B.
3.4.2 Non-pathogenic FGF14 STR Expansions
Four individuals were identified to have noncanonical interrupted GAA FGF14 STR expansions, three of which were ≥ 250 copies in length (Figure 1B). The longest was a mixed GAA/GCA expansion of 616 repeats, with a maximal uninterrupted GAA length of 195 repeats, while two patients had mixed GAA/GGA repeats with total repeat lengths of 440 and 330 respectively. These findings were not considered pathogenic as none of these noncanonical alleles contained an uninterrupted stretch of ≥ 250 GAA copies.
Two further participants, as well as one of the positive controls, were found to have GAA repeats in the 200–249 range (Figure 1B). Patient 13 had a STR expansion of 213 pure GAA repeats, in addition to the nonpathogenic mixed 440 GAA/GGA repeat. This individual was identified to have a pathogenic variant in VCP, with a matching phenotype, and the FGF14 GAA213 STR expansion was not felt to be contributory to his presentation. The other individual was identified to have a biallelic FGF14 GAA repeat expansion, with 213/201 repeats, which may contribute to their phenotype. This case is discussed in File S1.
3.4.3 RFC1-CANVAS; Clinical and Genetic Features
RFC1-CANVAS was identified in two individuals. Both presented with the full CANVAS syndrome including cerebellar ataxia, neuropathy and vestibular areflexia, with additional clinical features indicated in Table S5. Patient 8 was of white/European background and had biallelic (AAGGG)n expansions, with 651 and 693 repeats. The second individual (patient 9), from the Philippines, was compound heterozygous for an (AAGGG)1000 expansion and an (ACAGG)2000 expansion.
3.5 Clinically Relevant Sequencing Variants
We identified clinically relevant sequencing variants in the VCP and STUB1 genes in unsolved participants (Figures 1A and 2A).
The VCP variant carrier (patient 13), a 66-year-old man of white/European ancestry, presented with features of complex HSP (Table S5). He had a history of osteoporosis, migraines and had previously suffered a right cerebellar stroke, with complete symptomatic recovery. There were no cognitive symptoms. Family history revealed that his father had mobility issues and dementia, and his brother had frontotemporal dementia. MRI demonstrated a small right chronic cerebellar infarct. A dual-phase whole-body bone scan did not demonstrate scintigraphic evidence of Paget's disease. ONT LRS identified a pathogenic missense variant in VCP (c.475C>T, p.Arg159Cys). This was confirmed on clinically accredited WES and has been previously reported [30].
Patient 11, the STUB1 variant carrier, an 84-year-old lady of German/European ancestry presented with autosomal dominant ataxia, with similar features reported in her mother and mild features in her daughter. Clinical features are indicated in Table S5. ONT LRS identified an in-frame deletion in STUB1 (c.433_435del). Prior SCA17 testing had identified normal TBP repeat length (35/36 repeats). The patient had previously undergone WGS, and data were retrospectively reviewed, with confirmation of the STUB1 variant. Since the time WGS was originally undertaken, additional cases with the same STUB1 variant have been published, permitting classification of the variant as likely pathogenic [31, 32].
4 Discussion
Our study evaluated 34 patients with genetically undiagnosed spastic-ataxia spectrum disorders using targeted ONT LRS and identified a likely diagnosis in 14/34 (41%; summarised in Figure 3). In the group who had not previously undergone genetic testing, the diagnostic yield was 45% (5/11). In those who had previously had a genetic evaluation, with no diagnosis found, ONT LRS identified a cause in 39% (9/23). The high success rate in the latter group demonstrates the potential for LRS to greatly improve the rate of genetic diagnosis in these disorders.
There are multiple explanations for the diagnostic uplift achieved by our LRS assay. STR expansions account for most diagnoses in the cohort (10/14, 71%). The most common diagnosis was SCA27B, identified in 7/34 (21%), while two individuals (6%) were identified to have RFC1-CANVAS. These two STR expansions were both only relatively recently identified [33-35], and currently, testing in Australia is only offered on a research basis. Lack of access to clinical testing combined with the relatively high frequency of FGF14 and RFC1 STR expansions in patients with HCA underpins the high rate of detection of these two variants within our cohort. Another individual was diagnosed with SCA8, caused by an STR expansion in ATXN8/ATXN8OS [36]. While clinical testing is available, this is one of several rare STR expansions that are not universally incorporated into standard genetic testing pathways, or routine ‘SCA panels’ so may be overlooked, as was the case in our reported patient. The ability to identify these unexpected diagnoses is an advantage of our integrated approach that captures all relevant STRs on every test. The remaining 4/14 (29%) of diagnoses were accounted for by conventional sequence variants (SNVs and indels). Two of these patients had undergone previous genetic testing, without identification of the causative variant. One patient was diagnosed with SCA48 caused by a variant in STUB1, which was not recognised as pathogenic at the time of previous genetic evaluation due to the lack of recognition of dominant inheritance at the time of initial analysis [37]. The other was found to have a variant in VCP; this gene was not included for evaluation on a prior HSP panel. A further patient, who was genetic testing naïve was identified to have a compound heterozygous variant in SPG7, which could not be phased using short-read NGS to confirm pathogenicity. The capacity of LRS to phase autosomal recessive or de novo variants or identify both an STR expansion and sequence variant in a compound heterozygous state (as can occur in RFC1-CANVAS/spectrum disorder and Friedreich ataxia [38, 39]), is especially valuable for late-onset conditions, where availability of both parents for testing may be a barrier to diagnosis.
Pathogenicity of STR expansions may depend not only on repeat length, but on the repeat motif composition and the presence of interruptions. For example, RFC1-CANVAS/spectrum disorders are usually caused by biallelic (AAGGG)n STR expansions, but there are other pathogenic motifs, including (ACAGG)n, as well as several nonpathogenic motifs, including (AAAAG)n, (AAGAG)n, (AAAGGG)n and smaller (AAAGG)n expansions [31]. In such disorders, it is vital to obtain both repeat length and sequencing data, to confirm a diagnosis. Clinical testing may be undertaken with a combination of flanking PCR and repeat-primed PCR, which will identify specific common repeat motif(s), but will typically not detect the less common motifs, requiring further testing for such cases [40, 41]. In contrast, LRS can detect and characterise the various repeat motif types, as well as repeat length in a single test. For example, patient 9, with RFC1-CANVAS, was identified to have both an (AAGGG)n and an (ACAGG)n expansion. Furthermore, several individuals were found to have non-pure GAA FGF14 STR expansions. In these cases, LRS provided the capacity to quantify the maximum pure GAA repeat length, which fell below the 250-repeat threshold for pathogenicity in all cases [42]. This highlights the strong utility of LRS for the evaluation of STR expansions, being the only technique that can identify size, motif composition, zygosity, presence of interruptions, flanking variation and methylation status in a single assay [12].
The ability to capture the full variety of genomic features potentially implicated in spastic-ataxia spectrum disorders in a single assay not only improves diagnostic yields but can greatly streamline the diagnostic pathway. In clinical practice, STR expansions are commonly evaluated with repeat-primed PCR and/or Southern blot, which require separate assays and/or specific primers/probes for each different gene [12]. In addition to requiring multiple sequential tests for some patients, this also incurs delays in the availability of clinical testing for newly described STR expansions as each new gene-specific assay must be developed and validated [5]. In contrast, new genes and/or STR targets can be added to an ONT adaptive sampling assay simply by updating the set of genome targets provided programmatically during sequencing, requiring no change to either the upstream laboratory processes or downstream bioinformatics analysis pipelines [18]. Therefore, ONT LRS facilitates adaptability and rapid addition of new genes as they are discovered. This was exemplified in our study, as during the project, two additional ataxia-associated STR expansions were identified; THAP11 (‘SCA51’) [23, 43] and ZFHX3 (recently identified as the basis of SCA4) [24, 25]. Both genes were subsequently added to our gene panel. However, 24 samples had already undergone sequencing and were not evaluated for STR expansions in ZFHX3. Conversely, this also brings to light a limitation of targeted ONT LRS, which is the inability to reanalyse data for variants at additional ‘off-target’ loci, if new genes are discovered. This would be negated using whole-genome LRS, with either ONT or Pacific Biosciences (PacBio) technology but would come at an additional cost per patient.
Despite the described benefits of LRS, there remain several limitations and obstacles to the clinical uptake of this technology. On a read-by-read level, accuracy for detecting SNVs and small insertions/deletions (indels) is lower than NGS [44]. Targeted ONT LRS can identify 98.8% of SNVs, although greater inaccuracy is seen in samples with reduced depth of coverage [44]. Detection of indels suffers from a greater level of inaccuracy, on account of an increased propensity for slippage errors [11]. Software tools are less well-developed for data analysis, mapping and variant calling compared to NGS [10]. Due to the need for HMW DNA, freshly collected samples and specialised DNA extraction procedures are generally required [10]. In our study, fresh blood samples were generally provided for HMW DNA extraction and sequencing within 48 hours of collection, ensuring high-quality DNA could be obtained. Where samples are not freshly collected and/or properly stored (−80°C), or where HMW DNA extraction protocols are not used, the presence of shorter DNA fragments is likely to adversely impact both the sequencing yield and read-lengths obtained, both of which can impact detection of difficult variants like SVs, STR expansions, etc. Other important variables impacting sequencing yields per patient in our study were the initial ONT flow cell quality (MUX scan) and the ability to achieve precise balance in the molarity of different patient samples multiplexed on a single flow cell, which determines the relative sequencing yield on each sample. Finally, the cost of LRS remains higher than short-read NGS, although pricing has, and is predicted to continue to improve [10].
Beyond technology-related factors, other limitations of this study include potential selection bias. Patients were recruited from a tertiary centre, receiving referrals predominantly from primary care physicians and neurologists, with recruitment undertaken by authors L.I.R, D.Y. and K.R.K., neurologists, with subspecialty experience in neurogenetic disorders. The application of study protocols to broader neurology clinics could potentially impact the cohort characteristics and diagnostic rate. Most individuals in our cohort were of white/European background, and therefore results may differ when applied to broader population groups. Furthermore, although patients with features consistent with any spastic-ataxia spectrum disorder were eligible for the study, 29/34 (85%) had a primary HCA phenotype, while only two individuals had a primary HSP phenotype. This was likely contributed to by referral patterns, with several HCA patients referred to our research centre by local neurologists aware of this study, as well as the commencement of recruitment in late 2023 for a concurrent study, for which individuals with a primary HSP phenotype were eligible.
After undergoing genetic evaluation, many individuals with spastic-ataxia spectrum disorders remain without a genetic diagnosis, which has implications for clinical care. The provision of a genetic diagnosis may terminate a prolonged and arduous diagnostic odyssey, avoid further unnecessary and invasive investigations, permit disease-specific management and provide more accurate prognostic information. Although only few gene-specific treatments are presently available (e.g., treatment with 4-aminopyridine for SCA27B) [45], eligibility for clinical trials may also be affected by a genetic diagnosis. Furthermore, there are important implications for reproductive choices and family counselling, with the ability to offer genetic testing in at-risk family members [46]. Evidently, there is clear impetus to improve current genetic testing methods, to close this diagnostic gap. Our study begins to address this gap through the development and evaluation of a targeted LRS strategy that accurately identifies all classes of genomic variation across a panel of genes implicated in spastic-ataxia spectrum disorders. A sizeable fraction of the diagnoses we made were not STR expansions and would have been missed using previously published LRS approaches for ataxia [13]. Therefore, our approach represents a clinically meaningful advance by being more comprehensive compared to LRS methods focused primarily on the detection of repeat expansion disorders. This integrated ‘single test’ approach is now poised to replace fragmented genetic testing regimes, leading to improved diagnostic rates and streamlining the diagnostic pathway for patients.
Author Contributions
L.I.R., I.W.D., and K.R.K. contributed to conception and design of the study. L.I.R., I.S., D.Y., A.L.M.R., S.R.C., P.L.C., H.G., L.W., K.A., M.H., A.H., S.K., V.S.C.F., M.H., A.M., D.M., M.T., K.N., M.L.K., I.W.D., and K.R.K. were involved in acquisition and analysis of data. L.I.R., I.S., D.Y., A.L.M.R., S.R.C., I.W.D., and K.R.K. drafted a significant portion of the manuscript or figures.
Acknowledgements
We thank our colleague David Pellerin for aiding in interpreting potential atypical cases of SCA27B. Kishore Raj Kumar receives funding from the Medical Research Future Fund (grants 2023126, 2023357 and 2024888) and from the Ainsworth4Foundation, unrelated to the current project.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
Data is available on request.




