Evidence of blood–brain barrier dysfunction and CSF immunoglobulin synthesis in Down Syndrome Regression Disorder
Abstract
Objectives
This study sought to evaluate proteomic, metabolomic, and immune signatures in the cerebrospinal fluid of individuals with Down Syndrome Regression Disorder (DSRD).
Methods
A prospective case–control study comparing proteomic, metabolomic, and immune profiles in individuals with DSRD was performed. Samples were obtained from a biorepository of affected individuals and compared to clinically available data and previously obtained neurodiagnostic studies. Individuals with DSRD were compared to individuals with established neuroinflammatory conditions (e.g., multiple sclerosis), and neurotypical controls undergoing a lumbar puncture for headaches. Samples underwent high-throughput proteomic, metabolomic, and immune marker profiling. Data was compared across groups and clinical phenotypes. Gene set enrichment analysis and pathway analyses were utilized to analyze the data.
Results
In total, 34 individuals with DSRD, 22 neuroinflammatory controls, and 27 neurotypical controls were enrolled in the study. We observed a highly significant concordance in dysregulated proteomics signatures in DSRD and neuroinflammatory controls versus healthy controls, most prominently upregulation of many immunoglobulin sequences. In addition, individuals with DSRD displayed strong upregulation of liver-derived plasma proteins and erythrocyte proteins in the CSF, indicating poor blood–brain barrier integrity. The immune marker profile of DSRD is clearly similar to other neuroimmunological conditions, including strong elevation of MIP3-α, eotaxin, and IFN-γ.
Interpretation
Individuals with DSRD have unique CSF proteomic and metabolomic signatures consistent with neuroinflammation and increased blood–brain barrier permeability. The CSF of individuals with DSRD was more comparable to individuals with neuroinflammatory disorders than neurotypical controls, indicating the potential for an immune etiology of disease.
Introduction
Down syndrome (DS) is a leading cause of intellectual and developmental disability worldwide and occurs in ~1 in 700 live births in the United States.1 A rare but increasingly diagnosed condition in this population is Down Syndrome Regression Disorder (DSRD) which is an acute or subacute loss of previously acquired developmental skills in the areas of language, communication, cognition, executive function, behavioral, and adaptive skills.2-6 Individuals with DSRD frequently manifest bradykinesia, catatonia, hallucinations, rapid-onset insomnia, and other neuropsychiatric manifestations.3-8 The symptoms of DSRD are profound and can significantly impact both the quality of life and autonomy of individuals with DS and their caregivers.
Clinical investigations of DSRD have identified that only a fraction of individuals have abnormalities in their cerebrospinal fluid (CSF) indicative of neuroinflammation.5, 9 These abnormalities can include pleocytosis, restricted oligoclonal banding, and elevations in IgG index. However, these features are present in ~17% of individuals, a much lower overall rate relative to the response to immunotherapy in this population, which is as high as 80%.5, 9 This diagnostic-therapeutic paradox has created a need for further investigation into the CSF of individuals with DSRD as clinically available studies may be insufficient to detect the underlying etiology. This possibility has become increasingly relevant over the past half-decade where multiple investigations into the roots of inflammation and autoimmunity in individuals with DS have identified abnormalities in interferon activation and JAK/STAT signaling cascades,10-14 abnormal autoantibody production,15, 16 and abnormal T-cell function.15, 17, 18 As such, it remains possible that commercial CSF testing may not be capturing key pathological processes in individuals with DSRD, warranting further exploration.
This study sought to explore proteomic, metabolomic, and immune signatures in the CSF of individuals with DSRD and compare them to individuals with known neuroinflammatory disorders without DS and neurotypical controls. Moreover, we sought to evaluate if specific biosignatures were associated with clinical characteristics and therapeutic outcomes.
Materials and Methods
Standard protocol approvals, registrations, and patient consents
This study was approved by the Children's Hospital Los Angeles (CHLA) Institutional Review Board (21-00026 and 24-00184). Participants in this study were enrolled prospectively with assent (if <18 years of age) and consent (if >18 years) obtained when possible. In circumstances where individuals did not have the capacity to assent, consent, or both, a parent or guardian was consented in lieu of the patient.
Patient populations
- DSRD: Individuals with DSRD of any age were eligible in this study if they met either possible or probable international consensus criteria for the condition and had a full neurodiagnostic workup performed.19 Individuals who could not obtain an MRI (e.g., cardiac pacer present) obtained a CT scan in lieu of neuroimaging and were still eligible for the study.
- Neuroinflammatory Controls: Individuals had to have a prior diagnosis of a pediatric onset neuroimmunologic or neuroinflammatory condition (e.g., multiple sclerosis (MS), myelin oligodendrocyte glycoprotein associated disease (MOGAD), autoimmune encephalitis (AIE)) per established criteria.20-23
- Neurotypical Controls: As a non-inflammatory control group, individuals with either idiopathic intracranial hypertension (IIH) or “rule out” of autoimmune encephalitis when clinical criteria were not met,21, 22 were enrolled to be controls.
Exclusion criteria were uniform between all groups and included prior neurosurgical intervention (e.g., shunt placement), prior or active receipt of radiotherapy or xchemotherapy, or use of immunotherapy of any kind within the last 6 months. A conservative 6-month immunotherapy-free period was used to minimize any potential for altered interpretation of proteomic or metabolomic profiling. Individuals could not be on any psychotropic (e.g., antidepressants and/or antipsychotics) or benzodiazepines within 4 weeks of the lumbar puncture in order to avoid perturbation of metabolomic profiling, which assesses a variety of neurotransmitters. Individuals in the control group also could not have any immunologic, rheumatologic, genetic, cardiopulmonary, gastrointestinal, or neurologic condition. Finally, individuals with missing data, or who were lost to follow-up after 6 months, were excluded from the study.
Patient identification and clinical responses
Individuals in the DSRD cohort were recruited from the two sites involved in this study (Children's Hospital Los Angeles (CHLA) and the University of Colorado). Individuals from the Neuroinflammatory Control group and Neurotypical control group were all recruited at CHLA. CSF could have been obtained at an external site and shipped directly to CHLA prior to clinical evaluation but all patients required clinical evaluations at one of the host sites.
Clinical response to immunotherapy in the DSRD cohort was defined in an identical manner to prior studies9, 24 as ≥50% response on the Bush-Francis Catatonia Rating Scale and/or the Neuropsychiatric Inventory Questionnaire. These assessments were made after CSF collection and 3 months after initiation of immunotherapy in the DSRD cohort.
CSF sample processing
CSF was flash-frozen at −80°C within 15 min of collection. All CSF utilized was drawn off the last (4th) collection tube. Samples utilized in this study had previously never been thawed. Samples with more than 5 RBC/hpf were excluded.
Data collection
Chart-based data extraction of demographic and clinical data in addition to neurodiagnostic studies and clinical LP study results was performed for all patients. Determination of abnormalities in the DSRD cohort on either EEG, MRI, or LP was performed by two clinicians (JDS and MMK) after individualized review using criteria published in prior studies (Methods S1).5, 9, 24 Definition of response to immunotherapy was determined as a greater than 50% improvement on either the Bush-Francis Catatonia Rating Scale (BFCRS) or the Neuropsychiatric Inventory Questionnaire (NPI-Q) after 3 months on monotherapy.
Sample processing
Processing of samples for proteomic,25 metabolomic,10, 12 and inflammatory biomarker10 discovery was performed in an identical manner to previously published studies by the author group. Full methodology is available on proteomics (Methods S2), metabolomics (Methods S3), and inflammatory biomarker processing (Methods S4).
Statistical analysis
Data pre-processing, statistical analysis, and plot generation for all data sets were carried out using R (R 4.0+/RStudio 2022.12.0+/Bioconductor 3.16+)26, 27 as detailed below. All figures were assembled in Adobe Illustrator v25.1.
Pre-processing of CSF proteomics and metabolomics data
Peak intensity data from LC–MS of proteomics and metabolomics was processed and analyzed using R. Analytes with more than 70% missingness in all three sample groups were excluded from the analysis. For the remaining analytes, zero values were replaced with random values sampled from 0 to 0.5x the minimum non-zero value for that analyte. Data was normalized by applying a scaling factor, which was calculated by dividing the global median intensity value across all analytes by each sample median intensity.
Pre-processing of CSF MSD data
Concentration values (pg/mL) for each of the cytokines and related immune factors measured across multiple MSD assay plates were imported to R and combined, and analytes with >10% of values outside of detection or fit curve range were flagged. For each analyte, missing values were replaced with either the minimum (if below-fit curve range) or maximum (if above-fit curve range) calculated concentration per plate/batch and the means of duplicate wells used for subsequent analysis.
Differential abundance analysis
Extreme outliers were determined on a per-group and per-analyte basis as values greater than three times the interquartile range below the first quartile or above the third quartile and omitted from further analysis. Differential abundance of analytes was evaluated using linear regression with log2-transformed relative abundance as the dependent variable, and group (i.e., DSRD, neuroinflammatory controls, and neurotypical controls or DSRD clinical subgroups) as the independent variable, adjusting for age, sex, and where applicable, experimental batches. Multiple hypothesis correction was performed with the Benjamini-Hochberg method using a false discovery rate (FDR) threshold of 10% (q < 0.1). Prior to visualization, data were adjusted for age, sex, and batch using the removeBatchEffect() function from the limma package (v3.44.3).28
Data visualization
Comparisons of data distributions between different groups were shown by sina plots using ggplot2 and the geom_sina() function from the ggforce R package.29, 30 For sina plots, all points were jittered by local density with overlayed boxes representing medians and interquartile ranges. For comparison of fold change data, XY scatterplots were generated using ggplot2,30 and Spearman correlation statistics were generated by rcorr() function from the Hmisc R package. Heatmaps were generated using the ComplexHeatmap R package.
Gene set enrichment analysis (GSEA)
GSEA31 of proteomics data was carried out in R using the fgsea package (v 1.14.0),32 using Hallmark gene sets33 and log2(fold change) multiplied by −log10(p-value) as the ranking metric.
Ingenuity pathway analysis (IPA)
IPA of proteomics and metabolomics data was carried out in QIAGEN IPA (version 101138820 QIAGEN Inc., https://digitalinsights.qiagen.com/IPA) for all proteins with FDR <0.1 and log2(fold change) >0 and for all metabolites with FDR <0.1.34 The analysis was limited to the analytes detected in the study by specifying reference set as ‘User Dataset’ and only direct relationships were considered. Results were exported and visualized using R and RStudio.
Metascape analysis
Metascape analysis of proteomics data was performed using Metascape v3.5 for all proteins with FDR <0.1 and log2(fold change) >0.35 The list of all proteins included in proteomics analysis was provided as the background gene list. Results were exported and visualized using R and RStudio.
Results
Clinical and demographic data
In total, 34 individuals with DSRD, 22 neuroinflammatory controls, and 27 neurotypical controls were enrolled in the study. Of the DSRD samples, 31/34 (91%) were processed by the CHLA site with 7/31 (23%) being obtained outside of the institution and shipped in prior to clinical evaluation. All neuroinflammatory control and neurotypical control samples came from CHLA. Clinical and demographic data on these cohorts is presented in Table 1. There were no statistically significant differences between the demographics of groups. Among individuals with DSRD, abnormalities in EEG, MRI, and CSF were observed in 29% (10/34), 47% (16/34), and 47% (16/34) of patients respectively.
| DSRD (n = 34) | Neurotypical Controls (n = 27) | Inflammatory Controls (n = 22) | p-value | 95% CI | |
|---|---|---|---|---|---|
| Age at symptom onset (median, IQR) | 15.7 (13.4–18.0) | 16.1 (14.6–17.3) | 14.9 (12.1–17.9) | 0.62 | 0.57–4.29 |
| Age at collection (median, IQR) | 18.9 (15.8–20.9) | 16.9 (14.9–17.2) | 15.2 (12.3–17.3) | 0.49 | 0.39–2.36 |
| Sex | |||||
| Male | 14 (42%) | 17 (63%) | 12 (54%) | 0.11 | 0.20–1.17 |
| Female | 20 (58%) | 10 (37%) | 10 (46%) | ||
| Race | |||||
| White | 32 (94%) | 23 (85%) | 18 (82%) | 0.17 | 0.62–15.73 |
| Black | 1 (3%) | 1 (4%) | 2 (9%) | ||
| Asian | 1 (3%) | 3 (11%) | 2 (9%) | ||
| Ethnicity | 0.14 | 0.21–1.25 | |||
| Hispanic | 15 (41%) | 17 (63%) | 12 (54%) | ||
| Not hispanic | 20 (54%) | 10 (37%) | 10 (46%) | ||
| History of personal autoimmune disease | 16 (47%) | 0 (0%) | 6 (27%) | 0.14 | 0.75–7.52 |
| EEG abnormal | 10/34 (29%) | ||||
| MRI abnormal | 16/34 (47%) | ||||
| CSF abnormal | 16/34 (47%) | ||||
| Multiple CSF abnormalities | 8/16 (50%) | ||||
| Any neurodiagnostic abnormal | 26/34 (76%) | ||||
| Multiple neurodiagnostic studies abnormal | 16 (47%) | ||||
| EEG + MRI | 5 (31%) | ||||
| EEG + CSF | 8 (50%) | ||||
| MRI + CSF | 3 (19%) | ||||
| Catatonia | 25/34 (73%) | ||||
| BFCRS >20 at diagnosis | 13/25 (52%) | ||||
| Response to immunotherapya | 25/30 (83%) |
- BFCRS, Bush-Francisc Catatonia Rating Scale; CSF, cerebrospinal fluid; EEG, electroencephalogram; IQR, interquartile range; MRI, magnetic resonance imaging.
- a Response to immunotherapy defined as >50% improvement between baseline BFCRS or Neuropsychiatric Inventory Questionnaire (NPI-Q).
Analysis of proteomics signatures dysregulated in DSRD
To characterize the pathophysiology of DSRD, we evaluated proteomic changes using mass-spectrometry data relative to the neurotypical controls and those in the neuroinflammatory control group. We completed linear modeling adjusting for age and sex to identify differentially abundant proteins across groups (Fig. S1A, Table S1). Dozens of proteins with lower or higher abundance in DSRD and/or the neuroinflammatory controls were identified. A comparison of fold changes revealed a highly significant concordance in overall proteomics changes in DSRD and neuroinflammatory controls versus neurotypical controls (Fig. 1A). For example, when visualizing the top 10 significantly upregulated and downregulated proteins in each group comparison, we observed consistent changes in DSRD and the neuroinflammatory group, such as upregulation of the immunoglobulin sequences IGKV1D-33 and IGHV3-11 and downregulation of FTO (Fat Mass and Obesity Associated, Alpha-Ketoglutarate Dependent Dioxygenase), an RNA demethylase with emerging roles in neuropsychiatric disorders,36 and CCN2 (Cellular Communication Factor 2, IGFBP8), a mitogen secreted by vascular endothelial cells involved in oligodendrocyte differentiation with ties to multiple sclerosis.37 To investigate which biosignatures were dysregulated across groups, we completed pathway analysis using Metascape, Ingenuity Pathways Analysis (IPA), and Gene Set Enrichment Analysis (GSEA) (Fig. 1D). These analyses revealed enrichment of proteins involved in the adaptive immune response in both DSRD and inflammatory controls, as documented by strong signatures of Adaptive Immune Response and Immunoglobulin Production (by Metascape) and related IPA signatures (e.g., FCGR-dependent phagocytosis and signaling by the B cell receptor). These signatures are driven largely by the upregulation of dozens of immunoglobulin sequences (Fig. 1E, Fig. S1C,D, Table S1). To define which immunoglobulins would be generally elevated in the plasma of individuals with DS, we analyzed a mass-proteomics dataset from a cohort of participants with DS without a DSRD diagnosis versus euploid non-inflammatory controls. This exercise revealed that whereas some immunoglobulins are generally elevated in the plasma of people with DS (e.g., IGHV4-34), many are not (e.g., IGHV3-11), potentially indicative of intrathecal antibody production by a humoral response in the CNS38 (Fig. 1B, Fig. S1C).
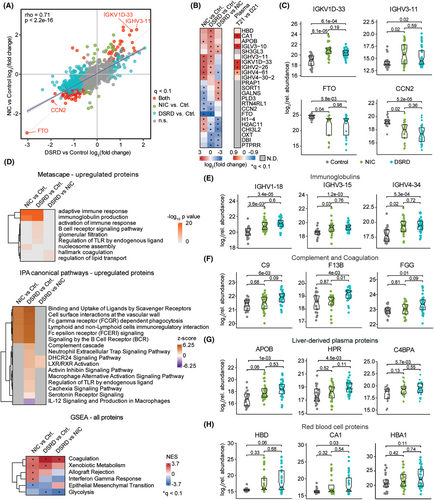
Pathway analyses also revealed upregulation of factors involved in the interconnected complement and coagulation cascades, with higher fold changes in the DSRD group (Fig. 1D). Prominent examples include C9, F13B, FGG, MST1, C8G, and CPB2 (Fig. 1F, Fig. S1E, Table S1). We also observed upregulation of signatures associated with liver-derived abundant plasma proteins (e.g., LXR/RXR activation, Xenobiotic Metabolism, Fig. 1D), once again with stronger signals in the DSRD group. Key examples are APOB, HPR, C4BPA, APOL1, APOM, and PZP (Fig. 1G, Fig. S1D, Table S1). Notably, the three most elevated proteins in DSRD vs. healthy controls were the red blood cell (RBCs) proteins HBD, CA1, and HBA1 (Fig. 1H).
Altogether, these findings reveal signs of neuroinflammation and disruption of the blood–brain barrier in DSRD.
Metabolomics assays reveal profound metabolic dysregulation in the CNS of DSRD cases
As with the proteomics analysis, we completed pairwise comparisons using linear modeling with adjustment for age and sex to identify analytes differentially abundant in DSRD cases versus healthy controls and/or the neuroinflammatory control group (Fig. S2A, Table S2). Once again, we observed a significant concordance in the global pattern of changes between DSRD and neuroinflammatory controls relative to healthy controls (Fig. 2A). Nevertheless, analysis of the top dysregulated analytes in each group comparison revealed important differences. For example, dopamine was elevated in the neuroinflammatory group but not in DSRD, however, this was driven by a small subset of participants (Fig. S2B). Kynurenine, a tryptophan catabolite produced during immune activation39 was more strongly elevated in the neuroinflammatory group (Fig. S2B, Table S2). In contrast, the DSRD group showed many significant changes not observed in the neuroinflammatory controls, such as elevation of thymidine, butanoyl-l-carnitine, and nicotinamide (Fig. 2B, Fig. S2A). Notably, many fatty acids were significantly elevated in DSRD but not in neuroinflammatory controls (e.g., tetradecenoic acid, heptanoic acid, octanoic acid) (Fig. 2B).
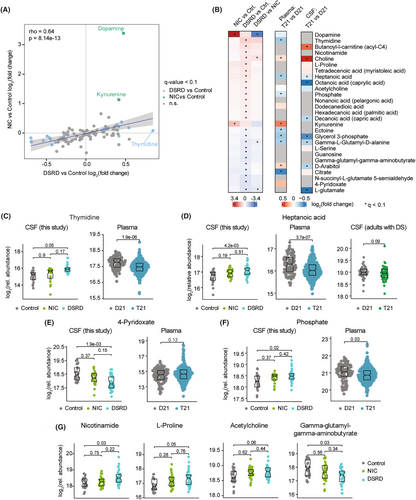
In order to define to what extent the metabolomics changes observed in the CSF of DSRD cases could simply be an effect of trisomy 21 status we analyzed datasets previously reported by our team using similar metabolomics platforms for analysis of plasma samples10 and CSF samples from adults with DS without DSRD.12 Although the overlap in measured analytes is not complete, this comparative exercise revealed that where a few changes could be broadly associated with DS, most were specific to the DSRD diagnosis. For example, elevation of choline is also observed in plasma samples of a large cohort of individuals with DS and in the CSF of older adults without DSRD (Fig. 2B, Fig. S2C, Table S2). Likewise, glycerol-3-phosphate is depleted in the CSF of DSRD cases, the plasma of participants with DS, and the CSF of adults with DS (Fig. S2D, Table S2). Depletion of glutamate is also conserved between DSRD CSF samples and CSF of adults with DS (Fig. S2E, Table S2). In contrast, many changes were only observed in the CSF of DSRD cases. For example, thymidine levels were significantly elevated in the CSF of DSRD cases, but not elevated in the CSF of neuroinflammatory controls or plasma samples of DS (Fig. 2B,C). Notably, thymidine levels were found to be elevated in the CSF of patients with Disorders of Consciousness (DoC).40 Heptanoic acid (enanthic acid, a seven-carbon saturated fatty acid), is highly elevated in DSRS CSF but not so in plasma of DS or CSF of adults with DS (Fig. 2D). Conversely, levels of 4-pyridoxate, a catabolic product of vitamin B6, was strongly depleted in DSRD CSF but not in DS plasma samples (Fig. 2E). Other notable changes in DSRD samples that could not be correlated in the other studies include elevation of nicotinamide, L-proline, and acetylcholine, and decrease in gamma-glutamyl-gamma-aminobutyrate (Fig. 2G).
Altogether, these results illuminate profound metabolic dysregulation in the central nervous system of DSRD cases.
Association between proteomic and metabolomic signatures and clinical phenotypes
Next, we tested for the presence of biosignatures associated with specific symptoms of DSRD. Toward this end we created clinical subgroups based on the presence or absence of neurodiagnostic abnormalities (Fig. S3A, Table S3), as these features have been previously shown to be predictive of response to immunotherapies in DSRD.5, 8, 9, 24 When completing linear modeling with adjustment of age and sex to find proteomic differences among those with or without a given clinical feature, only catatonia showed multiple statistically significant differences (Fig. 3A,B, Fig. S3B,C, Table S3). Consistently, DSRD cases without catatonia show unique differences in protein biomarkers relative to healthy controls not observed in those with catatonia, such as depletion of FTO and differential elevation of PNOC, LEFTY2, SIGLEC14, PAMR1, HLA-A, FCGR2A, and GRB2.
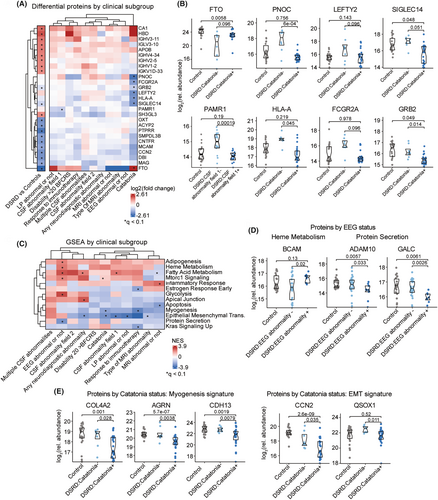
We then completed GSEA for the proteomic biosignatures associated with each clinical subgroup, which revealed multiple pathways that were significantly enriched across a subset of DSRD cases. For example, those with abnormal EEGs show an elevation of protein signatures associated with Heme Metabolism (BCAM) and Protein Secretion (ADAM10, GALC). In contrast, those with catatonia show depletion of proteins associated with the related Myogenesis (e.g., COL4A2, AGRN, CDH13) and Epidermal-to-Mesenchymal Transition (EMT, e.g., CCN2, QSOX1) signatures. When performing an analogous exercise for metabolomics, the only significant difference was a greater depletion of guanosine among those with multiple CSF abnormalities (Fig. S3C–E, Table S3).
Altogether, these findings suggest that the clinical heterogeneity of DSRD could be accompanied by variations in underlying molecular signatures.
DSRD is accompanied by a unique neuroinflammatory profile
Given the biosignatures of immune dysregulation observed in the proteomics dataset, we completed a quantitative targeted analysis of 81 inflammatory and immune markers using Meso Scale Discovery assays on a subset of the cohort. This effort identifies many changes in the levels of immune markers with an overall significant concordance in neuroinflammatory controls versus DSRD cases, with some notable exceptions (Fig. 4A,B, Table S4). For example, MIP3-α, IL13, VEGF-α, and eotaxin were significantly elevated in both groups relative to healthy controls (Fig. 4C). However, some markers were differentially dysregulated in neuroinflammatory controls versus DSRD, such as TNF-α and IL-10 (elevated in neuroinflammatory controls) or IFN-γ (elevated in DSRD). When assessing which of these changes are characteristic of the peripheral inflammatory milieu of DS, we identified both changes that are clearly conserved in the plasma proteome of DS, such as elevation of MIP3-α, VEGF-α, and eotaxin, versus changes unique to the CSF compartment such as elevation of IL13 and IFN-γ, which are not significantly elevated in plasma. Most strikingly, some cytokines clearly elevated in the plasma of people with DS are not increased in the CSF samples, most prominently IL-22 and TSLP, indicative of differential inflammatory milieus in the CSF versus periphery. The smaller sample size for this analysis did not allow for the identification of changes unique to clinical subgroups in DSRD (e.g., with versus without catatonia).
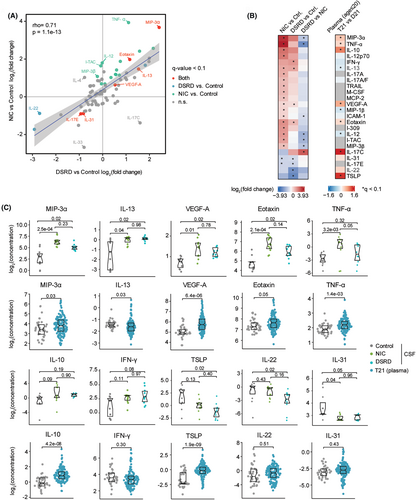
Altogether, these results indicate that DSRD is characterized by a neuroinflammatory profile that is reminiscent of other neuroimmunological conditions, including changes that are not usually observed in the peripheral immune compartment of people with DS, pointing to a potential immune etiology for the regression phenotypes, which would have to be confirmed through a direct comparison of individuals with DS with versus without DSRD.
Discussion
These results reveal unique molecular signatures consistent with compromised integrity of the blood–brain barrier and elevation of immunoreactive proteins in the CSF of a cohort of individuals with DSRD. Prior studies have reported that a small minority of individuals with DSRD have abnormal clinical biomarkers in the CSF,5 making our findings more notable in that they match the high rates of response to immunotherapy observed in this condition.5, 8, 9, 24 These findings further indicate that individuals with DSRD share proteomic and metabolomic profiles more consistent with individuals with established neuroinflammatory disorders than neurotypical controls.
Multiple proteins identified in the CSF of individuals with DSRD were serum-based proteins that would not have been anticipated to be present in the CSF. Liver-derived proteins such as APOB (apolipoprotein B) and HPR (haptoglobin-related protein) in addition to proteins specific to red blood cells such as HBD (hemoglobin D) and CA1 (carbonic anhydrase 1) were also abundant in the CSF, where they would not necessarily have a clear function. This was even more surprising in that no sample obtained in this study had RBC contamination on obtainment. The ingress of serum proteins into the CNS via a potentially compromised BBB could be a driving factor in the pathogenesis of DSRD. The specific cause for this finding is unknown but multiple studies have identified an increase in BBB permeability in neuroinflammatory disorders41-45 and neurodegenerative diseases such as Alzheimer's and dementia.46, 47 A critical question remains as to whether this observed increased permeability is reflective of a physiologic anomaly intrinsic to individuals with DS or is secondary to an active neuroinflammatory process unique to DSRD.
In both individuals with DSRD and those with established neuroinflammatory diseases, we observed strong upregulation of immunoglobulins and components of the interconnected complement and coagulation cascades. Although the overall proteomic signatures showed strong concordance across the two groups, there were also notable differences. As the CNS is considered to have minimal immune activation in healthy individuals, these proteins would not be anticipated to be present in the absence of changes in BBB integrity or active CNS-specific neuroinflammatory disease. More strikingly, while some of these elevations have been previously observed in the plasma of individuals with DS, multiple immunoglobulin sequences were unique to DSRD cases, potentially indicative of intrathecal production of (auto)antibodies in those with this condition. However, this finding will need to be reassessed through a direct comparison of individuals with DS with versus without regression, as individuals with DS are known to display excessive autoantibody production.15 Yet as we have learned over the last decade, immune privilege is not as binary as previously presumed, with several studies demonstrating a variety of immune surveillance mechanisms in the CNS.48, 49 As such, a comparison of our DSRD cohort to both individuals with neuroinflammatory disorders and those without is highly relevant and demonstrates that DSRD should be studied and managed with approaches employed in the field of neuroimmunology.
Metabolomic analysis revealed widespread metabolic dysregulation in the CNS of individuals with DSRD, including changes potentially associated with dysregulated energy metabolism (e.g., elevated nicotinamide, decreased glycerol-3-phosphate), neurotransmitter synthesis and turnover (e.g., elevated acetylcholine, decreased gamma-glutamyl-gamma-aminobutyrate), and fatty acid metabolism (e.g., elevated tetradecenoic acid, heptanoic acid, and octanoic acid). The clinical significance of these findings is obscure at this point and warrants further investigation. Importantly, the metabolic differences in DSRD depart from the overall concordance in proteomic and inflammatory signatures relative to the NIC group, which could be explained in part by the fact that DS causes global metabolic reprogramming not conserved in other neuroimmunological conditions.10, 12
In the context of DSRD as an emerging condition, significant resources have been employed to identify biomarkers of disease activity. While neurodiagnostic biomarkers have been previously identified as being predictive of immunotherapy responsiveness5, 24 and risk of relapse,9 these tests have been neither sensitive nor specific for the diagnosis of DSRD nor linking to the severity of symptoms.5, 7 We did not find diagnostic abnormalities strongly associated with unique proteomic or metabolomic signatures, with the exception of catatonia, but the results shared here justify a larger study to identify symptom-specific signatures, which could illuminate new therapeutic approaches.
In prior studies, clinical phenotypes have generally been poorly predictive of subsequent therapeutic responses5 whereas neurodiagnostic abnormalities have been much more demonstrative of responsiveness to immunotherapy.5, 9, 24 Although most biomarkers of disease severity were not associated with differences in proteomic profiling, catatonia was associated with unique proteomic findings such as depletion of FTO, elevation of PNOC, HLA-A, SIGLEC14, FCGR2A, and LEFTY2. In prior cohorts, nearly 75% of individuals with DSRD had catatonia present5 although it has been established that those without catatonia are more treatment-refractory, particularly to immunotherapeutic interventions. A question arising from this data set is if individuals with DSRD without catatonia may have different pathophysiologic mechanisms that are less immunologic although the low number of patients analyzed in this cohort makes generalization difficult. The hypothesis that individuals with DSRD who do not have catatonia may have different mechanisms of disease has been previously reported by thist study group,24 wherein individuals without catatonia are much less likely to have susceptibility-weighted imaging (SWI) abnormalities in the basal ganglia than those with catatonia. As these abnormalities are often observed in interferon-mediated diseases,50, 51 it is possible that this is also a biomarker of less inflammatory mechanisms of disease in this subpopulation. Embedded within this biomarker difference is if primary psychiatric disease with clinical symptomatic overlap allowing for meeting DSRD criteria may be present as well. Further exploration of these potential mechanistic differences is critical in that they may influence therapeutic selection, outcomes, and prognostication.
This study is not without limitations. Samples used in this study were collected at multiple sites but were processed uniformly. Samples were transferred to the analysis site in two unique batches. The authors corrected for batch effect in sample analysis. It is likely that ascertainment and severity bias are present in the DSRD cohort given the rarity of this condition and that symptoms were severe enough to warrant comprehensive neurodiagnostic workup. This is confirmed in the higher rates of neurodiagnostic (EEG, MRI, and CSF) abnormalities observed in the DSRD cohort compared to previously published studies.5-7, 9 Individuals in the inflammatory cohort would have also had ascertainment and severity bias in sample collection given the infrequency of pediatric-onset inflammatory disorders of the brain. This same cohort was heterogenous regarding primary inflammatory diagnosis and underlying pathophysiology which could have dampened some of the comparisons against the DSRD cohort. A heterogeneous group was chosen for comparison as the exact etiology of DSRD remains unknown. Ultimately, the ability to compare pre-treatment individuals with neuroinflammatory disorders directly to pre-treatment individuals with DSRD is a strength of this study although a cohort of individuals with DS without regression would have optimized the comparison. We attempted to alleviate this by evaluating plasma biomarker differences between individuals with DS and euploid controls although this is acknowledged to be imperfect as a comparator. Direct comparison of individuals with DSRD versus DS without regression will be highly necessary as individuals with DS are under a significant amount of inflammatory stress10, 12, 13, 17 which may alter the production of cytokines and potentially disrupt the BBB, a phenomenon that would not be anticipated in the other control groups. Given the exciting nature of the findings in this study, there is a clear need to prospectively evaluate serum and CSF proteomic and metabolomic profiles, as performed in this study, in a well-powered comparison of individuals with DS with versus without DSRD to define biosignatures that are unequivocal to regression symptoms. Finally, while exciting, broad proteomic, metabolomic and cytokine profiling takes an exploratory approach to understanding the potential mechanisms for DSRD. As such this study is best suited to use as a springboard for further mechanistic discovery as opposed to drawing conclusions regarding shared mechanisms of inflammatory disease.
Conclusions
In a diverse cohort of individuals with DSRD, unique CSF proteomic and metabolomic signatures emerged consistent with increased BBB permeability and ingress of immune reactive proteins to the CNS. The CSF of individuals with DSRD was more comparable to individuals with neuroinflammatory disorders than neurotypical controls, further indicating the potential for immune regulated mechanisms of disease. Further exploration, particularly the CSF of individuals with DS without regression is needed.
Acknowledgments
The investigators wish to thank the patients and caregivers who engaged in this research. The investigators also wish to thank the Byrne family and the Global Down Syndrome Foundation for their support of research efforts in DSRD.
Funding Information
The study was funded via the NICHD (R61HD109748) and NHLBI (K23HL155898).
Conflict of Interest
The authors report no relevant conflicts of interest-related data presented.
Author Contributions
JDS was responsible for the conception and design of the study, acquisition of data and analysis of data, drafting significant portions of the manuscript, and editing intellectual content. NPE and MDG were responsible for data analysis, tablet generation, figure generation, and editing for intellectual content. MMK, SJ, NKB, BNV, LR, LK, EB, and HRL were responsible for data acquisition, data analysis and interpretation, drafting significant portions of the manuscript, and editing intellectual content. ALR and JME were responsible for the conception and design of the study, analysis and interpretation of the data, and editing for intellectual content.
Open Research
Data Availability Statement
Anonymized data are available in Tables S1–S4. Additional anonymized clinical and demographic information is available to qualified investigators upon request contingent on IRB approval.




