Expert perspective: Highlights on myeloma cell therapy from the American Society of Hematology Annual Meeting 2019
Abstract
Autologous stem cell transplantation has been part of standard of treatment in multiple myeloma for 2 decades. The data showed improving progression-free survival and overall survival. Despite the improvement of new drugs, myeloma patients continue to relapse New cell therapy, chimeric antigen receptor T-cell therapy have been explored in heavily pretreated patients. In this commentary, we highlight studies presented at the American Society of Hematology (ASH) Annual Meeting held in Orlando, FL in December 2019.
1 INTRODUCTION
The use of high dose melphalan with stem cell rescue (ASCT) has been part of the standard of care for myeloma (MM) patients since the 1990s. Recent data from the European Group for Blood and Marrow Transplantation (EBMT) were presented, which included 103 032 patients from 54 countries from 1993 to 2017. This showed an increasing number of patients receiving ASCT and longer progression-free survival (PFS) and overall survival (OS) overtime along with increased use of novel agents and triple-therapy initially.1 Fortunately, nine agents have been approved for the treatment of MM by regulatory agencies from 2003 to 2019 with resulting improvement in clinical outcomes. Despite this improvement, the majority of MM patients continue to relapse. New cell therapy options, including chimeric antigen receptor (CAR)-T-cell therapies, are being explored in heavily pretreated and high-risk/early relapse patients.2 In this commentary, we highlight studies presented at the American Society of Hematology (ASH) Annual Meeting held in Orlando, FL in December 2019.
2 HIGH DOSE MELPHALAN AND STEM CELL RESCUE IN MYELOMA
2.1 Does ASCT remain relevant in the treatment of multiple myeloma?
Upfront ASCT has been associated in a phase III study with deep response, MRD negativity and longer PFS when compared to delayed ASCT. Despite this, OS has been similar in upfront vs delayed transplant using novel agents as initial therapy.3 The FORTE study in newly diagnosed myeloma (NDMM) compared four cycles of carfilzomib/lenalidomide/dexamethasone (KRd) followed by ASCT versus 12 cycles of KRd without ASCT both showing similar rates of MRD negativity. Despite similar MRD negativity, patients that received ASCT relapsed less compared to patients without ASCT, particularly in patients with Revised International Staging System (R-ISS) II/III.4 Another study of 124 NDMM patients (93 had upfront and 31 delayed ASCT) showed similarly that upfront ASCT provided significantly better PFS1 (from diagnosis to 1st relapse, PFS1 = 6.45 vs 1.25 years, P < .001) and PFS2 (from diagnosis to 2nd relapse, PFS2 = 9.19 vs 3.69 years, P < .001).5
2.2 What are the factors that may predict early relapse after ASCT?
Disease relapse is an obstacle to long-term survival in MM patients. The analysis of 474 NDMM patients from the FORTE study identified three factors that can predict early disease relapse at 18 months. These factors include: R-ISS II/III (particularly those patients with high LDH level), presence of circulating plasma cells and MRD status prior to ASCT.6 The Center for International Blood and Marrow Transplant Research (CIBMTR) has generated a model to predict relapsed disease in patients treated with upfront ASCT.7 The model included cytogenetic risk, initial therapy, bone marrow plasma cell infiltration prior to ASCT and number of lines during initial therapy and is divided into a low-risk (score 0-3) and high-risk score (score 4-6). The 3-year PFS was 27%-28% versus 51%-60% in the high- and low-risk sore groups, respectively. These factors may signal the need for more intensive maintenance therapy in patients who have a high-risk relapse score.
2.3 High dose melphalan: Are there any alternatives to improve patient outcomes?
An augmented conditioning regimen with busulfan plus melphalan (Bu-Mel) has been developed to improve the outcomes obtained with single agent high-dose melphalan. Qazilbash et al reported results of a phase 3 study comparing Bu-Mel versus melphalan alone in NDMM patients.8 The Bu-Mel group had significantly longer PFS than melphalan alone even in patients with high-risk cytogenetics (median PFS 64.7 vs 43.5 months, P = .022 and not reached (NR) versus 25 months, P = .0087 in all patients and high-risk cytogenetics patients, respectively). Importantly, intravenous busulfan did not increase the risk of sinusoidal obstructive syndrome (SOS). Long-term results of another study (MCRN-001) that used Bu-Mel augmentation in 78 NDMM resulted in high response rates (≥very good partial response 96%), however, high-risk patients still had significantly poorer outcomes with an estimated 5-year PFS of 28% vs 67% (P = .0169) and 5-year OS 61% vs 86% (P = .0170) in high-and standard-risk patients, respectively.9 In addition, 10 out of 78 patients developed second primary malignancies. A phase I/II study was presented including long-term data (7-year follow-up) of 43 NDMM patients treated with Bu-Mel plus bortezomib. When compared with historical controls that had been treated with high-dose melphalan alone (162 patients) the results indicated the similar results to those reported by Qazilbash et al: no increase in SOS and longer PFS for the Bu-Mel group.10 Bu-Mel may be an option for upfront treatment, especially in high-risk myeloma.
2.4 What is the role of ASCT in the treatment of high-risk multiple myeloma?
Patients with high-risk features such as high risk cytogenetics, presence of extramedullary disease at diagnosis, high LDH, or R-ISS stage II/III respond to treatment initially, however, their clinical course if characterized by early relapse. Total therapy-7 incorporated daratumumab into their induction (carfilzomib, thalidomide, dexamethasone, cisplatin, doxorubicin, cyclophosphamide, etoposide-KTD-PACE), tandem ASCT (fractionated melphalan 50 mg/m2 × 4 with peritransplant datumumab, carfilzomib, dexamethasone -DaraKD), and maintenance (alternating daratumumab, carfilzomib, dexamethasone and daratumumab, lenalidomide, dexamethasone) therapy for treatment of patients with high-risk myeloma features.11 With 11-months of median follow-up, 68% of patients had achieved a CR. PFS and OS were 87.2%, and 91.6%, respectively. Early results are encouraging, however, longer follow-up is needed to determine whether this regimen can improve outcomes in high-risk patients.
Long-term follow-up of the phase 3 HOVON-65/GMMG-HD4 study demonstrated similar OS between bortezomib/adriamycin/dexamethasone (PAD) initial therapy follow by ASCT and bortezomib maintenance compared to vincristine/Adriamycin/dexamethasone (VAD) prior to ASCT with thalidomide maintenance (12 year OS 36% vs 32% in the PAD and VAD arms, P = .11).12 However, patients with renal insufficiency (creatinine > 2) or deletion 17p who were treated with PAD can significantly reduce risk of death. For these patients, OS was 39% in the PAD arm vs 5% in the VAD Arm (HR 0.34, CI 0.20-0.59, P < .0001). Use of bortezomib as initial therapy and maintenance after ASCT provided a significant OS benefit. This may indicate the need for prolonged treatment with proteasome inhibitors in high-risk myeloma.
2.5 Are there any updates on continuous therapy/maintenance after a second ASCT?
A second ASCT has shown benefit in patients who achieve a long-term remission after a first ASCT. The additional benefit of maintenance/continuous therapy after second ASCT had not been evaluated. At ASH 2019, Modi et al retrospectively compared the outcome of maintenance either with lenalidomide or bortezomib (n = 39) to observation (n = 78) after second ASCT.13 The maintenance cohort showed significantly longer median PFS (24 vs 14 months, P = .04) and OS (NR vs 41 months, P = .02). The Nordic group also conducted the phase 2 CARFI trial in first relapse MM patients. Patient were treated with four cycles of carfilzomib, cyclophosphamide, dexamethasone; conditioning with carfilzomib/melphalan and randomized after second ASCT to observation or carfilzomib/dexamethasone.14 The median time to progression for the maintenance arm was 28.8 months vs 18.5 months in the observation arm (HR 0.42; 95% CI 0.26-0.68, P = .0003). These studies underscore the benefit of maintenance after second ASCT.
3 CELL THERAPIES INCLUDING CAR-T-CELL THERAPY AND BISPECIFIC T-CELL ENGAGER AGENTS (BITE)
3.1 How many different CAR-T-cell products are currently under investigation?
CAR-T-cell therapy in MM has been reviewed extensively elsewhere.15 Currently, a multitude of CAR-T-cell targets are being investigated for use in relapsed myeloma. One of the most common targets is the B-cell maturation antigen (BCMA) as a single target. Additionally, bispecific targeting combining BCMA with another antigen is being actively pursued. The signaling lymphocytic activation molecule family member 7 (SLAMF7), CD138, and G protein-coupled receptor class C group 5 member D (GPRC5D) are also potentially relevant. Updated data on CAR-T-cell therapy from ASH 2019 is available in Table 1.
| CAR-T name/target |
CAR-T structure 1) Extracellular domain 2) Co-stimulation 3) Safety switch |
Lymphodepletion | Dose |
Patient characteristics 1) MM status 2) Medina lines of prior therapy 3) Prior PI/IMiDs/CD38 Antibody (%) 4) prior ASCT (%) 5) Prior anti-BCMA therapy (%) 6) High risk cytogenetics (%) |
ORR/CR/MRD negative (%) | PFS/OS (median F/U) (months) |
Complications (%) 1) Cytokine release syndrome 2) Neurotoxicity |
|
|---|---|---|---|---|---|---|---|---|
| Anti BCMA CAR-T | ||||||||
|
CRB-402 J G Berdeja, ASH 2019 (n = 22) ongoing |
bb21217/BCMA |
1) Murine scFv 2) 4-1BB (CD28) 3) None *Co-culture with PI3K inhibitor |
Cyclophosphamide 300 mg/m2 × 3 days Fludarabine 30 mg/m2 × 3 days |
150, 300, 450 800 × 106 |
1) RRMM 2) 7 (4-17) 3) 92/100/75 4) 83 5) 0 6) 58% |
83/25/18 (8% at 10−5 8% at 10−6 of sensitivity) |
Ongoing |
1) 67/8% grade 3 2) 25%/8% grade 4 |
|
Legend-2 B Y Wang and L Chen ASH2019 (n = 74) |
LCAR-B38M |
1) dual epitope-binding to BCMA 2) 4-1BB 3) None |
Cyclophosphamide 300 mg/m2 × 3 days or Cyclophosphamide plus fludarabine |
0.5-0.7 × 106 cell/kg divided infused 3 times |
1) RRMM 2) 3 (1-9) 3) 68/86/NA 4) 18 5) 0 6) NA |
88/74-82 /68-82 (sensitivity at 10−4) |
All patients: 12-20/36-NR (25) |
1) 90%-100%/7%-35% grade 3 and 5% grade 5 2) 2% |
|
CARTITUDE-1 D. Madduri ASH2019 (n = 25) |
JNJ-68284528 (identical to LCAR-B38M) |
1) dual epitope-binding to BCMA 2) 4-1BB 3) None |
Cyclophosphamide 300 mg/m2 × 3 days Fludarabine 30 mg/m2 × 3 days |
0.73 × 106 cell/kg one infusion |
1) RRMM 2) 5 (3-16) 3) 100/100/100 4) NA 5) NA 6) NA |
21 patients: 91/24/40 (sensitivity at 10−5) |
Ongoing |
1) 88%/4% grade 3 and 4% grade 5 2) 9% |
|
C Li, ASH2019 (n = 16) |
CT103A/BCMA |
1) fully human scFv 2) CD8a hinger, and 4-1BB 3) None |
Cyclophosphamide and fludarabine | Dose escalation 1, 3, 6, 8 × 106/kg |
1) RRMM 2) ≥3 lines of therapy 3) NA 4) NA 5) 31 (murine BCMA CAR-T) 6) NA |
100/38/100 (sensitivity at 10−4) | Ongoing |
1) 100%/37% grade 3/4 2) 0% |
|
PRIME (phase 2) CL Costello, ASH2019 (n=) |
P-Bcma-101/BCMA |
1) Centyrins: Fully human scFv 2) 4-1BB 3) safety switch *piggyBac DNA modification |
Cyclophosphamide 300 mg/m2 × 3 days Fludarabine 30 mg/m2 × 3 days |
6-15 × 106 cell/kg | Ongoing | Ongoing | Ongoing | Ongoing |
|
W Fu, ASH 2019 (n = 46) |
CAR-T (EGFRt)/BCMA |
1) NA 2) 4-1BB 3) EGFRt |
Cyclophosphamide 300 mg/m2 × 3 days Fludarabine 25 mg/m2 × 3 days |
9 × 106 cells/kg |
1) RRMM 2) ≥2 lines of therapy 3) NA 4) NA 5) NA 6) NA |
44 patients: 80/41/36 (unknown sensitivity level) |
15/not reach (NA) |
1) 30%/7% grade 3 2) NA |
|
A J Cowan, ASH2019 (n = 10) |
Fully human BCMA + gamma secretase inhibitor/BCMA |
1) fully human scFv 2) 4-1BB 3) EGFRt |
Cyclophosphamide 300 mg/m2 × 3 days Fludarabine 25 mg/m2 × 3 days |
5 pt: 50 × 106 3 pt: 150 × 106 2 pt: 300 × 106 |
1) RRMM 2) 10 (6-23) 3) NA 4) 90% 5) 20% 6) 50% |
100%/30%/NA | Ongoing |
1) 100/40% grade 3-4 2) 60% |
| Bispecific CAR-T | ||||||||
|
N S Raje, ASH2019 (n = 23) On going |
PF-06863135/BCMA and CD3 |
1) Humanized IgG to BCMA and CD3 2) 3) None |
NA | Dose escalation 7 levels once weekly dose |
1) RRMM 2) 10 3) All patients prior receive PI, IMiDs, anti-CD38 4) NA 5) 22% 6) 26% |
Overall (22 pt): 1 CR, 2MR, 9SD Highest DL (5 pt): 1CR, 1MR, 2SD |
Ongoing |
1) 48 (all of them are grade 1-2, dose dependent) 2) NA |
|
ChiCTR1800018143 C. Li, ASH2019 (n = 16) |
BCMA and CD38 |
1) NA 2) 4-1BB 3) None |
Cyclophosphamide 300 mg/m2 × 3 days Fludarabine 25 mg/m2 × 3 days |
0.5, 1, 2, 3, 4 × 106 cell/kg |
1) RRMM 2) ≥2 lines of therapy 3) NA 4) NA 5) NA 6) NA |
88/50/88 (unknown sensitivity level) |
Not reach (9) |
1) 63%/25% grade 3 |
|
H. Zhang, ASH2019 (n = 5) |
BCMA and CD19 |
1) BCMA-CD19 scFv linked by CD8 hinge 2) NA 3) None |
Cyclophosphamide plus Fludarabine | Dose escalation |
1) RRMM 2) 4 (1-6) 3) NA 4) NA 5) NA 6) NA |
100/20/NA | Ongoing |
1) 75% all grade 1 2) 0% |
| Bispecific T-cell engager | ||||||||
|
L J Costa ASH2019 (n = 30) |
CC-93269/BCMA and CD3 |
Humanized IgG1 Ab to 1) BCMA: bivalent 2 + 1 2) CD3: monovalent |
— |
2 stages dose escalation 1) Fixed dose 2) dose escalation |
1) RRMM 2) 35 (3-13) 3) 100/100/97 4) 77% 5) 0 6) 30 |
All patients (n = 30): 43/17/17 10 mg cohort (n = 9): 89/44/44 (sensitivity at 10−5) |
Ongoing |
1) 77/3% grade 5 2) — |
- Abbreviations: BCMA, B-cell maturation antigen; CD, cluster of differentiation; CR, complete remission; EGFRt, epidermal growth factor receptor; MRD negative, minimal residual disease negative; NA, not available; NR, not reach; ORR, overall response rate; OS, overall survival; PFS, progression free survival; pt, patients; scFv, single chain variable fraction.
3.1.1 Anti-BCMA CAR-T-cell
BCMA is an important protein for survival and proliferation of myeloma cells. Most CAR-T-cell studies focus on this target. Various CAR-T-cell constructs have been developed including: (a) murine derived, (b) humanized, (c) human, and (d) adding a safety switch to prevent toxicity of engineered CAR-T-cells.
bb21217
bb21217 contains the same molecule as bb2121, however, in bb21217, the engineered cells are co-cultured ex vivo with Phosphatidylinositol-3-kinase inhibitor (bb007) to enrich for memory-like CD8 T-cells. This may lead the CAR construct to be more persistent and potent. Updated data of the phase 1 dose escalation of bb21217 in 22 relapsed/refractory MM (RRMM) patients showed high response rate (83% ORR, 25% CR with 18% MRD negativity).16 Cytokine release syndrome (CRS) and neurotoxicity occurred in 67% and 25% of patients, respectively, with grade 3-4 in 8% of patients.
L-CAR B38M
L-CAR B38M, a CAR-T with dual epitope-binding to BCMA, showed an ORR of 88% with 74%-82% CR and 63%-82% MRD negativity at a sensitivity of 10−4.17, 18 Seventy-four patients were treated with a median of three prior lines of therapy. The median PFS (12-20 months) and OS (36 months - NR) were encouraging.19, 20 CRS occurred in all patients. About 7%-35% of patients experienced grade 3 CRS and one patient died on study.
Another study in 25 RRMM patients with JNJ-68284528 (identical to L-CAR B38M) in the United States (CARTITUDE-1) showed high ORR of 91% (CR in 40%) and confirmed the recommended phase 2 dose of this CAR-T-cell product at 0.75 × 106/kg.21 Translational work of JNJ-68284528 showed expansion of CD8 + CAR+T-cell after infusion (peaking at day 10-14).22 The phase 2 study of L-CAR B38M in China (CARTIFAN-1, NCT03758417) is under investigation.
P-BCMA-101
P-BCMA-101 is a CAR-T-cell product that uses the piggyBac DNA modification system instead of a virus vector. In addition, the extracellular part of this construct consists of centyrins (fully human scFv) that are thought to be smaller, more potent, and less immunogenic than conventional scFv. This CAR-T-cell is under investigation in a phase 2 study with plans for a phase 3 study in the future.23
Anti-BCMA CAR-T-cell therapy with a safety switch (tEGFR)
Fu et al conducted a BCMA CAR-T-cell therapy study with tEGFR as safety switch in 46 RRMM patients.24 The ORR was 80% with CR in 41%. All grade CRS was reported in only 30% of patients (7% grade 3).
Anti-BCMA CAR-T-cell therapy with a gamma secretase inhibitor
Anti-BCMA CAR-T-cell therapy provides excellent responses, however, most patients relapse within a year of engineered cells administration.2 A possible reason for this is an immune escape mechanism by which myeloma cells express less BCMA after BCMA-directed therapy. This can occur as the enzyme γ-secretase cuts BCMA from the myeloma cell surface and releases it into the blood as soluble BCMA. This lead to decreased BCMA expression in the myeloma cell and a possible resistance mechanism.
The γ-secretase inhibitor (GSI) JSMD194 was tested in preclinical studies. The studies showed that this inhibitor blocks BCMA cleavage from myeloma cell lines and patients tumor cells. This led to an increase of surface BCMA density in myeloma cells and decreased soluble BCMA levels.25 This led to a new application of the combined use of JSMD19 with anti-BCAM CAR-T-cell therapy (Figure 1).
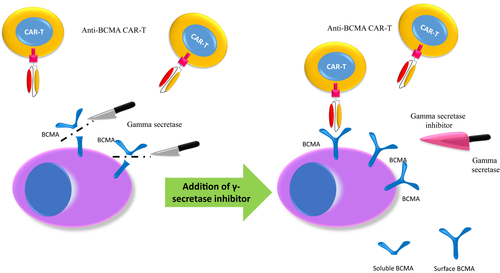
Cowan et al treated seven patients with a median of 10 prior lines of therapy (range, 4-23). Patients were treated with three oral doses (run in) of JSMD194 and prebone and postbone marrow assessments showed increase in BCMA expression in plasma cells from 75% to 99% with a two-fold decrease of soluble BCMA.26 Responses (including one patient who did not respond to prior BCMA CAR-T-cells and another patient that progressed on a BCMA bispecific antibody) were reported: 100% ORR (5 VGPR, 1 PR with 5/6 patients MRD negative by flow. CRS toxicity occurred in 100% of patients (grade 3-4 in 40%) and neurotoxicity in 60% of patients. One patient passed away at day 33 posttreatment because of CRS and concurrent fungal infection.
A meta-analysis of 258 RRMM patients from 15 studies who were treated with anti-BCMA CAR-T-cell therapy demonstrated high ORR of 82% (36% CR; 77% MRD negative).27 The median PFS was 10 months. CRS was seen in 69% of patients––all grades––and 15%––grade 3 and 4––while neurotoxicity was seen in 18% of patients, overall. Despite initial response, most patients relapsed within 18 months of therapy. These results are promising; however, larger studies with longer follow-up are needed.
3.1.2 Bispecific monoclonal antibodies and dual-target CAR-T-cell therapy
BCMA and CD3 monoclonal antibodies
Raje et al evaluated the bispecific BCMA and CD3 monoclonal antibody (PF-06863135) in a phase 1 study of 23 heavily pretreated MM patients (median 10 lines of prior therapy with 22% previously exposed to anti-BCMA therapy).28 They reported an 80% overall clinical benefit rate (response at least stable disease).
CC-93269 is a humanized IgG1 that binds to BCMA in the plasma cell and CD3 on T-cell. The preliminary results of a phase 1 dose escalation study demonstrated a high response rate (ORR 89%, CR and MRD negative 44%) in the highest dose cohorts (10 mg and 6 mg ->10 mg).29 Seventy-seven percent of patients had CRS and one patient died from severe CRS at the 10 mg dose.
BCMA and CD38 CAR-T-cells
ChiCTR1800018143 is a dual-target BCMA and CD38 with 4-1BB signaling and CD3 zeta domains.30 Sixteen RRMM patients were treated with this CAR-T-cell construct with ORR of 88%. A 4.0 × 106 cells/kg was the dose selected for an expansion cohort, which is currently ongoing.
BCMA-CD19 CAR-T-cells
CD19 is an antigen that may be expressed by myeloma progenitor cells. Therefore, there is therapeutic interest in a dual-targeting CD19 and BCMA CAR-T-cell product. A phase 1 dose escalation study currently ongoing showed 100% ORR and mild (grade 1-2) CS without neurotoxicity in the first five patients dosed.31
3.1.3 Allogeneic CAR-T-cell therapy
Apart from surface BCMA downregulation on plasma cells, T-cell exhaustion may be another mechanism of resistance to BCMA-CAR-T-cell therapy. In a preclinical study, healthy donor anti-BCMA CAR-T-cells eliminated primary myeloma cells even when those cells expressed low levels of BCMA.32 Interestingly, once exposed to CD138+ cells, the healthy donor CAR-T-cell were functionally more active than patient derived anti-BCMA CAR-T-cells. Patient derived cells can express checkpoint inhibitory molecules that may be involved in disease relapse. These interesting preclinical results prompted the start of the first phase 1 in human study of allogeneic anti-BCMA CAR-T therapy, which is currently ongoing.
3.1.4 Future and ongoing car cell therapy trials
Ongoing clinical trials on the CAR-T therapy space in multiple myeloma include the use of upfront CAR-T in high-risk patients. For example, studies using BCMA CAR-T with or without CD19 CAR-T are currently underway (NCT03549442 and NCT03455972). Moreover, a phase 3 study using clarithromycin and lenalidomide in combination with BCMA CAR-T therapy is accruing in China (NCT04287660). Additionally, investigations using sequential CAR-T and natural killer (NK) CAR therapy are under investigation. All these studies may alter the standard of care in myeloma in the future.
4 CONCLUDING REMARKS
ASCT remains an important treatment modality for NDMM and RRMM. The addition of busulfan to melphalan has been shown to increase PFS when compared to melphalan alone. Multiply relapsed myeloma patients have dismal outcomes with poor overall survival. Cell therapies with engineered CAR-T-cells and the new bispecific antibodies offer hope to patients and physicians with high response rates and acceptable durability of response. Despite this, most patients continue to relapse. Additionally, some toxicities including deaths may be prohibitive of certain therapies. Head to head comparisons of engineered T-cell therapy to ASCT are likely in the future. This may drastically change how we treat multiple myeloma.
All cited results are in line with data presented at the American Society of Hematology Annual Meeting 2019 and should not impact publication of cited abstracts as full manuscripts.
ETHICS STATEMENT
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to. No ethical approval was required as this is a perspective article with no original research data.
ACKNOWLEDGMENTS
This work was supported in part by The MD Anderson Cancer Center Support Grant (P30 CA016672), the Leukemia and Lymphoma Society Specialized Center of Research (LLS SCOR), the Dr Miriam and Sheldon G. Adelson Medical Research Foundation, the Multiple Myeloma Research Foundation, the Perelman Family Foundation and the University of Texas MD Anderson Moon Shot Program. We would like to thank participating patients and their families.
CONFLICT OF INTERESTS
C. Kunacheewa reports no conflicts of interest. E. Manasanch has received research support from Sanofi, Quest Diagnostics, Novartis, JW Pharma, Merck; consultant fees from GSK, Takeda, Celgene, Sanofi, and Adaptive Biotechnologies.