The Long Term Effects of a 12-Session Community Exercise Program on Health Measures in Cancer Patients
Funding: The authors received no specific funding for this work.
ABSTRACT
Purpose
To assess the long-term effects of a community cancer exercise program on quality of life, fatigue, weight, waist circumference, physical activity levels, lower extremity strength, body mass index (BMI), heart rate, and blood pressure, across non-metastatic and metastatic patients.
Methods
A total of 918 participants (F/M: 1.77; mean age = 61 years, SD = 13.233) diagnosed with cancer within the last five years completed a 12-session guided physical activity program. Sessions included functional, aerobic, and resistance training aligned with ACSM guidelines for cancer patients. Blood pressure, quality of life, fatigue, BMI, lower extremity strength, body weight, and physical activity levels were measured at baseline, 12 sessions, and at 6 months, and 12 months during follow-up. The Wilcoxon signed-rank test was used to assess changes over time.
Results
Significant improvements were observed in physical activity levels, health-related quality of life, and overall quality of life, sustained at 6- and 12-month follow-ups. Waist circumference, fatigue, and blood pressure significantly decreased across all time points. Lower extremity strength improved up to 6 months but was not significant at 12 months. No significant changes were observed in body weight or BMI. Non-metastatic patients experienced significant improvements in blood pressure, waist circumference, fatigue, and functional ability, while metastatic patients maintained their baseline health measures, suggesting a stabilizing effect.
Conclusions
This study demonstrates that a community-based exercise program benefits non-metastatic cancer patients by improving quality of life, physical activity levels, and functional health, while helping metastatic patients maintain health outcomes. These findings highlight the importance of structured exercise programs in cancer care and support their implementation in real-world settings.
1 Introduction
In England, there were 329,665 new cancer diagnoses in 2021, reflecting a 1% increase in cancer diagnoses for men and a 3% rise for women since 2019 [1]. Due to COVID-19, many patients have been awaiting cancer treatment on the NHS [2]. In December 2021, more than 43,000 people were missing a cancer diagnosis [3]. In March 2022, the number of patients starting treatment was still 37,000 lower than expected [4].
A decrease in mortality rates of 17% in men and 16% in women was projected from 2003 to 2023 [5], while an average survival at 10 years has risen to 46.2% in comparison with 23.6%, which would have been found more than 30 years ago [6]. These statistics highlight the increased number of cancer survivors in the United Kingdom living with side effects of treatment [7]. Modifying lifestyle behaviors has been shown to effectively reduce the risk of developing cancer, with unhealthy lifestyle choices contributing to 35%–50% of all cancer diagnoses and morbidity [8]. Physical inactivity is linked to an increased risk of obesity [9], promoting elevated levels of insulin, glucose, and insulin-like growth factors. These factors accelerate cell growth and inhibit cell death, creating a tumor-promoting environment that increases cancer risk and recurrence [10]. This underscores the importance of exercise for cancer patients to counteract lifestyle-driven dysregulation [8]. Physical activity can improve health outcomes in cancer patients [11]. However, cancer patients are at an increased risk of cancer recurrence and the development of comorbidities due to treatment [12]. There is strong evidence that exercise at the right dose and intensity can improve anxiety, depressive symptoms, fatigue, quality of life, lymphedema, and physical function in certain cancer types [13-15].
The American College of Sports Medicine guidelines for cancer survivors recommend at least 150 minutes of moderate-intensity exercise or 75 minutes of vigorous-intensity exercise of aerobic training and two resistance sessions a week [16]. However, many studies are not conducted in community settings, and research mainly focuses on breast, prostate, lung and colorectal cancer [17].
In addition, only a few studies assess the long-term effect of exercise on cancer patients within the community setting [18, 19]. Therefore, the primary objective of this study was to assess the impact of a community exercise program on several measures, including quality of life, fatigue levels, physical activity levels, lower extremity strength, BMI, weight, waist circumference, heart rate, and blood pressure over 12 months.
The purpose of this study was to evaluate the long-term effects of a 12-session community exercise program across various cancer types and tumor stages.
1.1 Aim
The primary aim of this study was to assess the long-term effectiveness of a community-based cancer exercise program and determine whether its effects were evident at 6 and 12 months post-intervention. Additionally, the study aimed to compare the impact of exercise between nonmetastatic and metastatic participants.
This study examined the long-term effects of the program on quality of life, fatigue levels, physical activity levels, lower extremity strength, BMI, weight, waist circumference, heart rate, and blood pressure. It also investigated the differences in these effects between nonmetastatic and metastatic participants.
2 Method
2.1 Participants and Recruitment
A total of 918 participants (64% female, 36% male; mean age, 61 years) with a cancer diagnosis within the last five years participated in a 12-session guided physical activity program. Participants had to be over 18 years old and had a cancer diagnosis in the last five years. The participant also needed to live or have access to healthcare in Barnet, Camden, Enfield, Haringey, or Islington. All participants were referred by a healthcare professional who would complete the referral form for the service user and send it directly by email or post to the health and well-being manager. The health and well-being manager would then cascade referral forms to the Level 4 cancer rehabilitation personal trainer to begin pre-rehabilitation or rehabilitation.
2.2 Exercise Program
The program was a 12-session guided physical activity program for adults diagnosed with cancer in the last five years. The aim of the program was to help optimize a patient's health outcome before, during, and after cancer treatment and is tailored to help increase physical output. Level 4 cancer rehabilitation trainers were present at all 12 sessions, each for at most an hour.
Each session consisted of functional, aerobic, and resistance training with conventional warm-ups and cool-downs, and all programs were written with the aim of getting participants to the ACSM guidelines of physical activity for cancer patients (3 sessions of 50 minutes of moderate-intensity or 3 sessions of 25 minutes of vigorous-intensity exercise a week, with two of these sessions including resistance exercise a week targeting major muscle groups). Outcome measures were used at baseline, 12 sessions, 6 months, and 12 months after completing the 12 sessions.
This study used a quasi-experimental pre-exercise and post-exercise design to assess the program. All service users from 2015 to 2021 who entered the program were automatically added to the service evaluation. Data collected by Level 4 cancer rehabilitation trainers at the four time points at baseline, after completing 12 sessions, and at 6 months, and 12 months follow-ups, (the 6 and 12 month measures were taken after 6 months and 12 months after completion of the 12 sessions) and analyzed. These included blood pressure, waist circumference, body mass index (BMI), fatigue, quality of life, health-related quality of life, sit-to-stand scores, physical activity scores, heart rate, and weight.
No incentives were provided to participate in the evaluation. Ethical approval for the use of anonymized data captured from the program was granted by the Staffordshire University Research Ethics Committee.
2.2.1 Measures
Several measures were taken as follows:
2.2.2 Demographic and Health Characteristics
All self-reported data related to ethnicity, age, educational status, housing status, current treatment status, cancer diagnosis, and cancer status were collected using a questionnaire. BMI, sit-to-stand test scores, waist circumference, blood pressure, and heart rate were collected by the Level 4 cancer rehabilitation personal trainers.
2.2.3 Physical Activity Scoring
The Scottish Physical Activity Screening Questionnaire was used to assess physical activity levels. The questionnaire is divided into two main categories, a seven-day recall of all activities and a stage of change in exercise behavior. The Scottish Physical Activity Screening Questionnaire has been used as an outcome measure in various cancer rehabilitation journals [20, 21]. The physical activity questionnaire has also been found to be a valid and reliable tool for use in exercise interventions [22].
2.2.4 Health-Related Quality of Life
FACT-G is a 27-item health-related quality-of-life questionnaire that consists of four subscales. Physical, functional, social, and emotional well-being. Yost's study [23] found that FACT-G is a reliable and valid tool (reliability > 0.7 and validity, r = 0.43). FACT-G has also been established as an effective tool in predicting treatment response and survival outcomes in metastatic gastrointestinal cancers [24], which highlights its effectiveness as a cancer-specific tool regardless of cancer stage.
2.2.5 Quality of Life
Quality of life was assessed using the 5-item EuroQol 5-dimension VAS analogue scale. The VAS is a visual scale from 0 (being the worst imaginable health) to 100 (being the best imaginable health). The EurolQol 5- 5-dimension VAS analog scale is a valid and reliable tool to assess quality of life (Cronbach alpha = 0.76) [25].
2.2.6 Fatigue
Fatigue was assessed using the FACIT-F questionnaire. The questionnaire is a 13-item questionnaire with a 5-point Likert scale that focuses on psychosocial and physical well-being [26]. The scoring system ranges from 0 to 52. The higher the score, the better the fatigue levels. This questionnaire has been found to be a valid and effective tool in various studies [27]. It has also been reviewed as a valid assessment of quality of life [28].
2.2.7 Lower Extremity Strength
The 30-second sit-to-stand test is used to assess lower extremity strength. The test requires participants to complete as many sit-to-stands as possible in 30 seconds. The total number of sit-to-stands is then recorded and compared with normative data for the participant's age [29].
2.3 Classification of Metastatic and Nonmetastatic Cancer Status
Cancer status was determined using self-reported data exclusively from the Cancer Physical Activity Standard Evaluation Framework Questionnaire [30]. Based on their responses to cancer-related questions, participants were categorized into assumed metastatic or nonmetastatic groups.
Participants who reported “My cancer is stable” or “Remission or cancer-free” were classified as assumed nonmetastatic, unless they also indicated having advanced, secondary, or metastatic cancer. These terms suggest that the cancer is controlled or localized to a specific area. Conversely, participants who reported “advanced, secondary, or metastatic cancer” were classified as assumed metastatic, as these terms are associated with stage IV disease.
2.4 Handling of Ambiguous or Missing Cancer Status Responses
Participants who selected “My cancer has recurred or relapsed,” “My cancer status is not known/undergoing diagnosis,” or “Other” were not classified into either the metastatic or nonmetastatic groups due to insufficient information.
2.5 Statistical Analyses
All data were analyzed using IBM Statistics v29.1, and normality was checked using the Kolmogorov–Smirnov test (p < 0.05), from which the data were found to be not normally distributed. The Wilcoxon rank test was used to assess significant (p < 0.05) differences between baseline and each of the three-time points (at 12 sessions, at 6 months follow-up, and at 12 months follow-up). These were performed to assess whether exercise had a significant difference to the outcome measures (quality of life, health-related quality of life, blood pressure, heart rate, lower extremity strength, fatigue, BMI, and body weight) after 12 sessions and if there is a significant effect of exercise on these outcome measures after 6 months and 12 months after completion of the twelve exercise sessions.
2.6 Attrition Rates
Between 2014 and 2019, the exercise program was attended by 918 participants. Of these, 424 (46%) completed all 12 exercise sessions, while 572 (62%) finished the 12-session assessment. Some participants did not complete all 12 sessions due to work commitments, treatment complexities, or because fewer sessions were needed. 274 cancer participants (30%) completed the 6-month follow-up assessment, while 144 participants (16%) completed the 12-month follow-up evaluation.
3 Results
3.1 Age and Gender
The study looked at 918 cancer participants ranging from 18 to 96 years of age. Most participants were aged between 46 and 71 (595), while 11.7% (n = 107) were younger adults (18–45 years) and 23.5% of participants were older adults (72+ years) (Table 1). The gender demographic showed a higher proportion of females (64.2%, n = 589) than male counterparts (35.8%, n = 329). This gender disparity reflects the higher proportion of breast cancer participants referred to the program (45.2%, see Table 2).
| Age and gender | Total | Percentage |
|---|---|---|
| Age range | ||
| 18–45 | 107 | 11.7 |
| 46–71 | 595 | 64.8 |
| 72 and above | 216 | 23.5 |
| Gender | ||
| Female | 589 | 64.2 |
| Male | 329 | 35.8 |
| Variables | Frequency (n) | Percentage |
|---|---|---|
| Cancer type | ||
| Breast | 400 | 45.8 |
| Prostate | 150 | 17.2 |
| Colon | 89 | 10.2 |
| Lymphoma | 80 | 9.2 |
| Other | 154 | 17.6 |
| Cancer status | ||
| Advanced/secondary/metastatic | 29 | 4.38 |
| My cancer is stable (the cancer is neither decreasing nor increasing) (nonmetastatic) | 205 | 30.96 |
| Not known/undergoing diagnosis | 70 | 10.57 |
| Other | 34 | 5.13 |
| Partial remission | 1 | 0.15 |
| Recurrence/relapse | 12 | 1.81 |
| Remission or cancer-free (cured) (nonmetastatic) | 311 | 46.97 |
| Total | 662 | 100 |
| Treatment response | ||
| Don't Know | 23 | 3.47 |
| I'm Currently in treatment | 230 | 34.79 |
| I am not in active treatment, but I am on ‘wait and watch’ | 62 | 9.37 |
| I have finished the course of treatment, but my cancer is still present | 22 | 3.32 |
| My cancer is being treated again because it has not fully responded fully to treatment | 9 | 1.36 |
| The treatment has been effective, and I have no signs or symptoms of cancer | 300 | 45.38 |
| Treatment has not yet started | 15 | 2.26 |
| Total | 661 | 100 |
Table 2 summarizes that the most common cancer type was breast cancer (N = 389, 45%) followed by prostate (N = 147, 17%) and colon (N = 88, 10%). Table 1 shows that participants were in remission or cancer-free (N = 311, 47%) followed by “the cancer is stable” (N = 205, 31%) and not known/undergoing diagnosis (N = 70, 11%). Table 2 illustrates that predominately most participants found the treatment to be effective and had no signs or symptoms of cancer (N = 300, 45%); the second most common treatment response was “I'm currently in treatment” (N = 230, 35%) followed by “I'm not in active treatment, but I am on wait and watch” (N = 62, 9%). Table 2 also shows that the majority of participants were nonmetastatic (N = 516, 94.67%), while only a relatively small proportion were metastatic (N = 29, 5.33%) as identified with self-reported data.
3.2 Weight and BMI, Diastolic Blood Pressure, and Systolic Blood Pressure
Figure 1 shows that, exercise did not have a significant effect on body weight (baseline–12 sessions, p = .682, baseline–6 months after completion, p = .251, baseline–12 months after completion, p = .662) and BMI (baseline–12 sessions = .624, baseline–6 months after completion, p = .192, baseline–12 months after completion = .842). Significance was found with diastolic blood pressure at baseline–12 sessions (p ≤ .001), baseline–6 months after completion (p = .016), and baseline–12 months after completion (p = .012). The exercise was found to significantly improve systolic blood pressure at baseline–12 sessions (p ≤ .001), baseline–6 months after completion (p = .059), baseline–12 months after completion (p = .011) (please see Table 4 for more details on p values and Z scores).
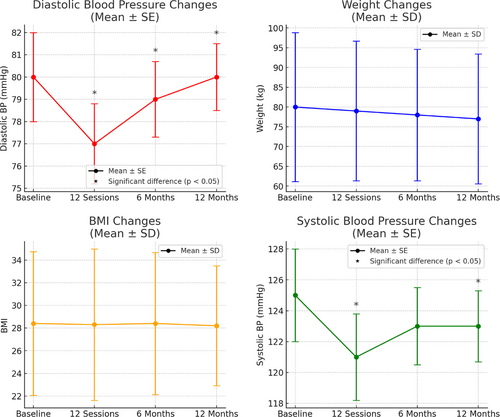
The error bars shown in these graphs indicate the standard deviation (or standard error), capturing the variability at each time point. The consistency in error bar size across time points suggests that variability in participant results remained steady throughout the study period rather than indicating an absence of significant differences. Significant changes were identified through statistical analysis and are highlighted in the text and graph annotations.
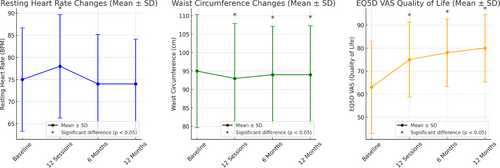
3.3 Heart Rate, Waist Circumference, and Quality of Life (EQ5D VAS)
Figure 2 shows that a significant difference was found in heart rate at baseline–12 sessions after completion (p = .003), no significance was found at baseline–6 months (p = .194) and baseline–12 months for heart rate (p = .296). Significance was found in waist circumference at the baseline–12 sessions (p ≤ .001), baseline–6 months after completion (p ≤ .001), and baseline–12 months after completion (p = .030). Significance was found in EQ5D VAS baseline–12 sessions (p ≤ .001), baseline–6 months after completion (p = .001), and baseline–12 months after completion (p ≤ .001) (please see Table 4 for more details on p values and Z scores).
3.4 FACT-G and FACIT-Physical Activity Levels and Sit-to-Stand Scores
Figure 3 shows that FACT-G showed significance at baseline–12 sessions (p ≤ .001), baseline–6 months after completion (p = .001), and baseline–12 months after completion (p ≤ .001).
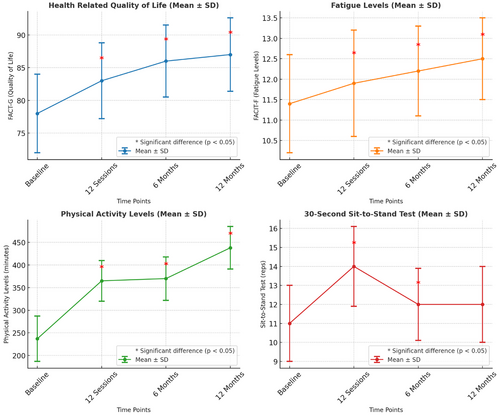
FACIT-F showed significance at baseline– 12 sessions (p ≤ .001), baseline–6 months after completion (p ≤ .001), and baseline–12 months after completion (p ≤ .001). Physical activity levels showed significance at baseline–12 sessions (p≤.001), baseline–6 months after completion (p ≤ .001), and baseline–12 months after completion (p = 0.027). Sit-to-stand scores showed significance at baseline–12 sessions (p ≤ .001), baseline–6 months after completion, (p ≤ .001), however not at baseline–12 months after completion (p = .106).
Figure 4 compares the mean EQ5D VAS scores (quality of life) between participants aged 18–45 years and those 72 years and above. The results demonstrate a statistically significant difference between age groups immediately following the 12-session exercise intervention (p = .039), with older adults reporting higher quality of life scores compared with younger adults. While this age-related difference was notable post-intervention, it was not maintained at either the 6-month (p = .075) or at the 12-month follow-up assessments (p = .681), suggesting a temporal effect of the exercise intervention on age-related quality-of-life outcomes.
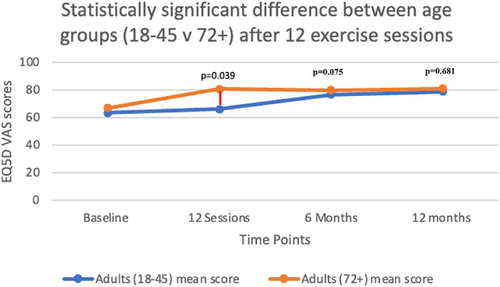
Only age and gender were assessed to determine their impact on the health outcome measures in this study. The influence of specific cancer types on these health outcomes will be explored in a separate publication.
Table 3 shows the effect sizes across the four time points for each outcome measure. Using Cohen's (1988) criteria for effect sizes, waist circumference from baseline to 12 sessions, physical activity level from baseline–12 sessions, quality of life from baseline to 12 sessions, quality of life baseline to 6 months post-completion, quality of life baseline to 12 months post-completion, health-related quality of life baseline to 12 sessions, health-related quality of life baseline to 6 months post-completion, health-related quality of life baseline to 12 months post-completion, and fatigue baseline to fatigue 12 sessions, all showed a small effect size. However, sit-to-stand baseline to 12 sessions showed a medium effect size.
| Outcome measure | Effect size | P |
|---|---|---|
| Systolic baseline—Systolic 12 sessions | 0.09 | <.001 |
| Systolic baseline—Systolic 6 months post-completion | 0.03 | .059 |
| Systolic baseline—Systolic 12 months post-completion | 0.04 | .011 |
| Diastolic baseline—Diastolic12 sessions | 0.09 | <.001 |
| Diastolic baseline—Diastolic 6 months post-completion | 0.03 | .016 |
| Diastolic baseline—Diastolic 12 months post-completion | 0.04 | .012 |
| Waist circumference baseline—Waist circumference 12 sessions | 0.09 | <.001 |
| Waist circumference baseline—Waist circumference 6 months post-completion | 0.07 | <.001 |
| Waist circumference baseline- Waist circumference 12 months post-completion | 0.1 | .030 |
| Sit-to-stand baseline—Sit-to-stand 12 sessions | 0.3 | <.001 |
| Sit-to-stand baseline—Sit-to-stand 6 months post-completion | 0.09 | <.001 |
| Physical activity level baseline— Physical activity levels 12 sessions | 0.1 | <.001 |
| Physical activity level baseline—Physical activity levels 6 months post-completion | 0.08 | <.001 |
| Physical activity level baseline—physical activity levels 12 months post-completion | 0.04 | .027 |
| Quality of life baseline—Quality of life 12 sessions | 0.2 | <.001 |
| Quality of life baseline—Quality of life 6 months post-completion | 0.1 | <.001 |
| Quality of life baseline—Quality of life 12 months post-completion | 0.1 | <.001 |
| Health-related quality of life baseline—Health-related quality of life 12 sessions | 0.1 | <.001 |
| Health-related quality of life baseline—Health-related quality of life 6 months post-completion | 0.1 | <.001 |
| Health-related quality of life baseline—Health-related quality of life 12 months post-completion | 0.1 | <.001 |
| Fatigue baseline—Fatigue 12 sessions | −0.1 | <.001 |
| Fatigue baseline—Fatigue 6 months post-completion | 0.09 | <.001 |
| Fatigue baseline—Fatigue 12 months post-completion | 0.07 | <.001 |
4 Discussion
Unhealthy lifestyle choices create an oncogenic environment [8]. Exercise has been shown to be an effective tool for regulating immune responses [31] and influencing tumor metabolism [32]. This study specifically aims to evaluate the unique psychological and physiological impacts of cancer treatment on key health outcome measures. Cancer treatment often compromises the immune system [31], leading to cancer-related fatigue in up to 90% of patients [33], reduced quality of life [34], and a heightened risk of treatment-induced comorbidities [35]. These factors underscore the importance of assessing how cancer patients, with their distinct needs, respond to exercise interventions.
The growing number of randomized control trials in the area of exercise oncology has strengthened the importance of exercise. However, most experimental designs do not reflect the general population, with studies omitting comorbidities and allowing for the homogeneity of samples. However, our study explored the effectiveness of a generalized community exercise program for participants with varying cancer diagnoses, helping to show the effectiveness of the many randomized control trials and evidenced-based research in a “real-world setting” that has been minimally assessed [36, 37].
In this research, a data set was analyzed to assess the long-term effects of a community cancer exercise program on quality of life, fatigue levels, physical activity levels, lower extremity strength, BMI, waist circumference, weight, heart rate, and blood pressure.
This research addresses a critical gap between exercise science and oncology, specifically examining the implementation of exercise interventions for cancer patients in community settings. While randomized controlled trials have established the efficacy of exercise in cancer care [13-15], there remains a significant knowledge gap regarding translating these findings into community-based programs. As Morris and co-authors [38] identified, research investigating the implementation of physical activity interventions in community settings represents a crucial area for future investigation. The present study aims to bridge this translational gap by evaluating the effectiveness of a community-based exercise program for cancer patients, thereby contributing to the evidence base for the real-world application of exercise oncology.
A factor that makes this study different from the literature already written on cancer and exercise rehabilitation is the fact that this community exercise program for cancer patients used ACSM guidelines within the “real-world setting,” while also being in North Central London, a cosmopolitan area with a wide range of ethnicities, ages (Table 1), cancer types (Table 1), educational and socioeconomic statuses that can be reflective of the major cities in the world. Our study also showed the long-term effect of exercise on cancer patients, as this study looks at 6 and 12 months after the 12 sessions are completed to assess adherence to exercise.
The proposed exercise program assessed in our study was found to show significant improvements in blood pressure, heart rate, waist circumference measurements, quality of life, health-related quality of life, fatigue, physical activity levels, and lower extremity strength. These improvements were found throughout the cancer continuum, from stage I to stage IV, and at different treatment stages.
4.1 Age and Gender
Age appeared to influence the effect of exercise on quality of life after 12 sessions, with participants over 72 showing significantly higher scores than younger adults (18–45 years) (p = .039) (Table 1). This difference may stem from several factors. Older adults often demonstrate higher adherence to exercise programs, which can enhance physical functioning and overall quality of life [39, 40]. Additionally, older adults typically have more leisure time to commit to exercise programs [41]. These findings underscore the importance of age-tailored exercise prescriptions and the incorporation of behavior-change interventions to help younger adults prioritize exercise, particularly following a cancer diagnosis. However, it should be noted that while a significant age-related difference in quality of life was observed immediately following the intervention (p = .039), this difference was not sustained at the 6-month (p = .075) or 12-month (p = .681) follow-ups. No significant differences were found between age groups or genders for any other outcome measure. This suggests that while older adults may experience initial benefits in quality of life than younger counterparts, these differences appear to converge over time, potentially due to adaptations in exercise adherence and lifestyle across age groups.
4.2 Physical Activity Levels
The program managed to significantly increase the amount of physical activity (minutes) achieved by cancer participants for 12 months after the intervention (Figure 3). These results showed that the effects of the program lasted 12 months after the initial assessment (completion of the 12 sessions); these findings are supported by the literature [42, 43]. The study shows that supervised exercise can help bring cancer patients to the recommended public health guidelines of 150 minutes of moderate activity a week. Participants in the program averaged 438 minutes (stdev = 376.909) of physical activity after 12 months of participating in the community exercise program; these results showed that the program promoted long-term exercise behavior change (Table 4). Witlox's (2018) study analyzed the beneficial effects of an 18-week supervised study on fatigue and physical activity levels in patients with breast and colon cancer. A four-year follow-up was used to assess the long-term effects of the program. Participants in the study exercised twice a week for an hour each session and were told to be active for 30 minutes on 3 other days of the week. The control group was told to maintain their regular exercise patterns. The results found that participants assigned to the exercise intervention group reported higher levels of moderate physical activity levels.
| Baseline | 12 Sessions | 6 Months | 12 Months | Average | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Check points | N | Mean | Stdv | Median | Range | N | Mean | Stdv | Median | Range | N | Mean | Stdv | Median | Range | N | Mean | Stdv | Median | Range | Average N | Average mean | Average Stdv | Average median | Average range |
| Weight (kg) | 856 | 80 | 18.853 | 77 | 136 | 548 | 79 | 17.679 | 76 | 124 | 255 | 78 | 16.631 | 75 | 120 | 131 | 77 | 16.434 | 74 | 113 | 447.5 | 78.5 | 17.39964 | 75.5 | 123.25 |
| Diastolic (mmHg) | 849 | 80 | 11.083 | 80 | 78 | 534 | 77 | 10.306 | 78 | 85 | 211 | 79 | 9.926 | 80 | 60 | 96 | 80 | 8.889 | 80 | 44 | 422.5 | 79 | 10.051 | 79.5 | 66.75 |
| BMI | 875 | 28.4 | 6.33 | 27.7 | 76.5 | 560 | 23.3 | 6.675 | 27.5 | 85.6 | 253 | 28.4 | 6.278 | 27.5 | 65.3 | 130 | 28.2 | 5.293 | 27.6 | 30.6 | 454.5 | 27.075 | 6.1444 | 27.575 | 64.5 |
| HR (BPM) | 848 | 75 | 11.7 | 75 | 84 | 538 | 76 | 11.718 | 77 | 72 | 214 | 74 | 11.169 | 74 | 74 | 97 | 74 | 10.148 | 74 | 50 | 424.25 | 74.75 | 11.18375 | 75 | 70 |
| WC (cm) | 858 | 95 | 15.355 | 95 | 134 | 556 | 93 | 14.898 | 93 | 129 | 233 | 94 | 13.178 | 94 | 81 | 104 | 94 | 13.331 | 93 | 76 | 437.75 | 94 | 14.1905 | 93.75 | 105 |
| EQ5D | 886 | 63 | 20.042 | 65 | 100 | 571 | 75 | 16.263 | 80 | 90 | 273 | 78 | 14.647 | 80 | 70 | 145 | 80 | 14.62 | 80 | 65 | 468.75 | 74 | 16.393 | 65.0105 | 81.25 |
| FACT-G | 886 | 78 | 17.581 | 80 | 182 | 570 | 83 | 16.703 | 85 | 125 | 238 | 86 | 16.865 | 90 | 112 | 107 | 87 | 17.575 | 91 | 105 | 450.25 | 83.5 | 17.18118 | 86.5 | 131 |
| FACIT-F | 895 | 11.3993 | 5.036 | 11.3 | 52 | 571 | 11.924 | 2.508 | 12.3 | 18 | 241 | 12.2 | 2.879 | 12.8 | 16.5 | 110 | 12.517 | 3.097 | 13.1 | 22.5 | 454.25 | 12.01 | 3.380755 | 12.375 | 27.25 |
| PAL (minutes) | 877 | 237.11 | 361.534 | 140 | 4230 | 246 | 365 | 400.577 | 270 | 2640 | 132 | 370 | 277.779 | 315 | 1330 | 49 | 438 | 376.909 | 480 | 1380 | 422.5 | 355 | 332.5093 | 301.25 | 2395 |
| STS (sec) | 610 | 11 | 3.839 | 11 | 24 | 408 | 14 | 3.845 | 13 | 36 | 53 | 12 | 3.47 | 12 | 20 | 15 | 12 | 4.086 | 11 | 13 | 271.5 | 12.25 | 3.81 | 11.75 | 23.25 |
| Systolic (mmHg) | 847 | 125 | 17.963 | 125 | 103 | 536 | 121 | 17.487 | 121 | 155 | 214 | 123 | 15.91 | 123 | 115 | 96 | 123 | 13.087 | 125 | 61 | 430 | 123 | 16.11175 | 123.5 | 108.5 |
In comparison, this study solely assessed the amount of physical activity completed by participants rather than intensity levels, which is a potential flaw in this study. Before entering the community exercise program, participants averaged 247 minutes (stdev = 274.772) of physical activity, increasing to 438 minutes (stdev = 376.909) 12 months after the program (Table 4), highlighting that the community exercise program had a positive effect on participant's behavior toward exercise. Similar findings were found in the study by Mutrie and Campbell's (2007) that focused solely on the effect of a 12-week supervised exercise program on breast cancer participants. The study found that physical activity significantly increased six months after the intervention.
4.3 Health-Related Quality of Life
The significant effects of the program lasted up to 12 months after the initial assessment in terms of health-related quality of life (Table 5 and Figure 3). This suggests that 12 sessions of aerobic and resistance training with supervised guidance can have a significant effect on participants suffering from cancer. These findings were supported by the study by Irwin [44], who found that community exercise was effective in improving health-related quality of life of participants over 12 weeks, visiting the gym twice a week. This study found similar findings; however, participants only attended supervised exercise once a week or depending on the treatment schedule and still saw a significant improvement in health-related quality of life 12 months after joining the program.
| Outcome measure |
Initial assessment–12 sessions p-value Z score |
Initial assessment–6 months p-value Z score |
Initial assessment–12 months p-value Z score |
12 sessions–6 months p-value Z score |
12 sessions–12 months p-value Z score |
6 months–12 months p-value Z score |
|---|---|---|---|---|---|---|
| Body weight |
.682 −410 |
.251 −1.147 |
.662 −.437 |
.633 −.478 |
.323 −.989 |
.632 −.491 |
| Diastolic blood pressure |
<.001 −5.438 |
.016 −1.891 |
.012 −2.531 |
.008 −3.078 |
.037 −1.240 |
.549 −.742 |
| Systolic blood pressure |
<.001 −5.438 |
.059 −1.891 |
.011 −2.531 |
.002 −3.078 |
.215 −1.240 |
.458 −.742 |
| Body mass index |
.624 −.490 |
.192 −1.303 |
.842 −.199 |
.597 −.529 |
.436 −.779 |
.803 −.250 |
| Heart rate |
.003 −3.010 |
.194 −1.298 |
.296 −1.046 |
.019 −2.344 |
.244 −1.165 |
.501 −.674 |
| Waist circumference |
<.001 −5.805 |
<.001 −4.177 |
.030 −2.175 |
.480 −.707 |
.564 −.577 |
.409 −.826 |
| EQ5D-5L-quality of life |
<.001 −13.234 |
<.001 −9.533 |
<.001 −7.671 |
.044 −2.011 |
.222 −1.221 |
.892 −.136 |
| FACT-G |
<.001 −9.286 |
<.001 −7.901 |
<.001 −6.737 |
<.001 −3.265 |
<.001 −3.959 |
.026 −2.221 |
| FACIT-F |
<.001 −8.333 |
<.001 −6.170 |
<.001 −4.664 |
.006 −2.747 |
<.001 −3.588 |
.002 −3.048 |
| Physical activity levels |
<.001 −6.447 |
<.001 −4.713 |
.027 −2.211 |
<.001 −3.376 |
<.001 −3.381 |
.065 −1.843 |
| Sit-to-stand test |
<.001 −14.434 |
<.001 −4.500 |
.106 −1.616 |
.902 −.124 |
.854 −.184 |
.399 −.843 |
4.4 Fatigue
Fatigue levels improved significantly in the program, with the effect of exercise lasting 12 months after the exercise intervention (Table 5 and Figure 3). The systematic review by Kessels [45] supported these findings, concluding that aerobic interventions had a significant effect on cancer-related fatigue; this study also found that aerobic interventions had a larger effect on cancer-related fatigue than a study with aerobic and resistance exercises, suggesting that although significant effects were found up until 12 months after the community exercise sessions, a possible larger effect could have been found with a prescribed aerobic only exercise program.
4.5 Blood Pressure
Despite significant advancements in cancer treatment leading to higher survival rates [7], many treatments continue to produce side effects that can severely impact patients' quality of life [46]. Cardiotoxic effects, particularly from chemotherapy, can adversely affect blood pressure. For instance, one study found that 24% of patients developed hypertension following chemotherapy, with 8% experiencing severe hypertension [47]. Monitoring blood pressure during cancer treatment is crucial, as hypertension may indicate the outcome and success of treatment [47]. This program found significant long-term improvements in blood pressure, suggesting that the program is effective not only in reducing side effects of treatment but also in attenuating the development of additional comorbidities due to treatment. With a mean reduction of 2 mmHg in systolic blood pressure between the initial assessment and 12 months of follow-up, this is a clinically meaningful discovery with research showing that a reduction of this stature is associated with fewer strokes and premature deaths [48]. Comparable findings were reported in Rajotte's [49] study, which evaluated the effectiveness and safety of a 12-week community exercise program for cancer survivors. The study demonstrated significant improvements in systolic and diastolic blood pressure from baseline to 12 weeks. However, as with the present study, the absence of a randomized control group may limit the generalizability of these results (Table 4 and Figure 1).
4.6 Waist Circumference
A high waist circumference has been found to be associated with an increased risk of cancer reoccurrence and all cause mortality [50]. This study found that a 12-session supervised community exercise has a significant effect in decreasing waist circumference in cancer patients. The median value of waist circumference was reduced by 2 cm from the initial assessment and 12 months after the community exercise program. These results show that a community exercise program can potentially have an effect in reducing the risk of comorbidities occurring and reducing the risk of reoccurrence of cancer (Table 4 and Figure 2). These results were similar to Brown's study [51] that evaluated the dose–response effects of aerobic exercise on the body composition of colon cancer survivors. Participants were split into a usual care group, a 150 minutes of aerobic exercise a week group and a 300 minutes of aerobic exercise a week group, over six months. The results showed that exercise reduced waist circumference in the 150-minute-a-week exercise group (1.5 cm) and the 300-minute group (4.5 cm). This study again highlights the importance of community exercise programs in helping to stabilize and reduce waist circumferences before, during, and after cancer treatment.
4.7 Metastatic and Non-metastatic Participants
This study did not find any significant improvements in health outcomes for metastatic cancer participants (all p > .05). However, there was no significant decline in key measures, suggesting that exercise may have helped maintain these outcomes (Table 6).
|
Outcome measure Metastatic participants |
Initial assessment–12 sessions p-value Z score |
Initial assessment–6 months p-value Z score |
Initial assessment–12 months p-value Z score |
|---|---|---|---|
| Body weight |
.477 −.711 |
.116 −1.572 |
Not enough valid cases |
| Diastolic blood pressure |
.858 −.179 |
.498 −.677 |
Not enough valid cases |
| Systolic blood pressure |
.197 −1.290 |
.500 −.674 |
Not enough valid cases |
| Body mass index |
.533 −.623 |
.116 −1.572 |
Not enough valid cases |
| Heart rate |
.185 −1.326 |
.080 −1.753 |
Not enough valid cases |
| Waist circumference |
.929 −.090 |
.893 −.135 |
Not enough valid cases |
| EQ5D-5L-quality of life |
.779 −.281 |
.715 −.365 |
Not enough valid cases |
| FACT-G |
.382 −.874 |
.916 −.105 |
Not enough valid cases |
| FACIT-F |
.701 −.384 |
.345 −.944 |
Not enough valid cases |
| Physical activity levels |
.273 −1.095 |
.655 −.447 |
Not enough valid cases |
| Sit-to-stand test |
.068 −1.827 |
.180 −1.342 |
Not enough valid cases |
Non-metastatic participants in the 12 session exercise program experienced significant improvements in diastolic (<.001) and systolic blood pressure (p = .003), waist circumference (<.001), health-related quality of life (<.001), physical activity levels (<.001), and lower extremity strength (<.001) (Table 7). These results suggest that the exercise intervention was effective in improving health outcomes for non-metastatic participants, aligning with McNeely's [52] study, which assessed the feasibility of cancer-specific community-based exercise programs. McNeely's [52] study, which excluded participants with metastasis, found significant improvements in upper and lower extremity fitness (<.05) following an 8-week exercise intervention. Similarly, the present study found improvements in lower extremity strength, which were sustained for six months after the intervention (<.001).
Rajotte's [49] study also supports these findings, demonstrating that 12 weeks of supervised exercise for non-metastatic cancer participants led to significant improvements in fatigue (p < .001), systolic and diastolic blood pressure (p < .0001), and health-related quality of life (p < .0001). These results are consistent with the present study, further emphasizing the benefits of exercise for cancer participants and the effectiveness of community-based cancer exercise programs for non-metastatic individuals.
|
Outcome measure Non-metastatic participants |
Initial assessment–12 sessions p-value Z score |
Initial assessment–6 months p-value Z score |
Initial assessment—12 months p-value Z score |
|---|---|---|---|
| Body weight |
.139 −1.478 |
.183 −1.333 |
.293 −1.051 |
| Diastolic blood pressure |
<.001 −4.180 |
.361 −.914 |
.325 −.983 |
| Systolic blood pressure |
.003 −2.963 |
.199 −1.284 |
.485 −.699 |
| Body mass index |
.210 −1.253 |
.310 −1.015 |
.258 −1.132 |
| Heart rate |
.060 −1.883 |
.027 −2.211 |
.903 −.122 |
| Waist circumference |
<.001 −3.467 |
.013 −2.481 |
.458 −.742 |
| EQ5D-5L-quality of life |
<.001 −9.842 |
<.001 −7.218 |
<.001 −4.848 |
| FACT-G |
<.001 −5.894 |
<.001 −4.972 |
<.001 −3.784 |
| FACIT-F |
<.001 −6.411 |
<.001 −5.441 |
<.001 −3.994 |
| Physical activity levels |
<.001 −4.008 |
.235 −1.187 |
.593 −.535 |
| Sit-to-stand test |
<.001 −11.666 |
<.001 −3.549 |
.207 −1.261 |
Body weight remained unchanged at 12 sessions (Z = −0.711, p = .477) and six months (Z = −1.572, p = .116). Similarly, diastolic (Z = −0.179, p = .858) and systolic blood pressure (Z = −1.290, p = .197) showed no significant changes, nor did BMI (Z = −0.623, p = .533) or heart rate (Z = −1.326, p = .185). Waist circumference (Z = −0.090, p = .929), quality-of-life measures (EQ5D-5L: Z = −0.281, p = .779; FACT-G: Z = −0.874, p = .382), and fatigue levels (FACIT-F: Z = −0.384, p = .701) also remained unchanged.
Physical activity levels (Z = −1.095, p = .273) and lower extremity strength (sit-to-stand test: Z = −1.827, p = .068) showed no significant improvements but indicated potential maintenance effects.
While no measurable gains were observed, these findings suggest that exercise may play a role in stabilizing health outcomes in metastatic cancer patients, reinforcing its potential as a supportive intervention in cancer care.
4.8 Effect Size
Significant differences were found between various time points and variables within this study; however, in the context of this study, the effect size assisted in delineating the real-world relevance of the significant results found. Only lower extremity strength from baseline to 12 sessions showed a medium effect size, highlighting that not only was there a statistically significant improvement in lower extremity strength between these time points, but it also carries practical implications (Table 3).
4.9 Strengths of the Study
This study possessed several notable strengths. Firstly, the inclusion of 918 cancer participants, representing a broad spectrum of cancer diagnoses and various stages of treatment, enabled an evaluation of the effectiveness of the ACSM guidelines at a community level. Moreover, given the scarcity of community-based studies conducted by charitable organizations in the United Kingdom, this research provides a valuable foundation for the development of additional community exercise programs.
The study's extended follow-up period is another significant strength, as it offers insight into both the immediate effects of exercise in mitigating cancer-related side effects and the lasting impact of the intervention, with follow-ups at 6 and 12 months. This long-term perspective underscores the potential of the program to foster sustained behavioral changes toward regular exercise. Additionally, by examining a broad range of health outcomes, the study highlights the multidimensional benefits of exercise for cancer patients.
The within-subject design further enhanced the study by facilitating the capture of longitudinal changes in patients' responses to exercise during cancer treatment. This approach ensured that each participant had the opportunity to potentially benefit from the intervention. By monitoring the same individuals within a community-based, real-world setting, the findings are more likely to be generalizable than those derived from controlled laboratory environments, which may not fully encapsulate the complexities of everyday life. Finally, the positive findings related to waist circumference, physical activity levels, and blood pressure suggest that exercise may play an essential role in reducing cancer recurrence risk and managing treatment-related comorbidities, thereby providing a valuable approach to addressing the side effects of cancer treatment.
4.10 Limitations
Because each program was individually tailored to participants' personal goals, including the target of 150 minutes of moderate-intensity exercise, it was not possible to reproduce a standardized program for further community-level research. Additionally, nearly half of the participants referred to the program were breast cancer patients, limiting the generalizability of the findings.
This study, being an analysis of the community cancer and exercise program, did not include a preplanned control group. This represents a limitation, as it complicates the ability to disentangle the effects of the exercise program from factors such as disease progression or regression. Additionally, the within-subject design may be subject to carryover effects, whereby participants' previous scores could influence subsequent measurements, potentially introducing bias into the results. Another limitation was the reliance on self-reported cancer status data from the Physical Activity Standard Framework, which may have led to misclassification of metastatic and nonmetastatic participants. The retrospective nature of the study further reduced accuracy, as medical records were unavailable. Future research should incorporate access to medical records and utilize the TNM tumor staging system to enhance classification accuracy and reliability, Additionally, a larger sample size of metastatic participants is needed to gain deeper insight into the effects of exercise on this population, especially in terms of long-term data for metastatic patients. Study design could be enhanced by incorporating propensity score matching (PSM) or historical control groups to strengthen causal inferences. It must also be highlighted that the FACT-G does not assess cognitive impairment, an area of significant concern for over 75% of patients undergoing treatment and 35% of cancer survivors [53]. Future studies should consider incorporating an additional quality-of-life measure alongside the FACT-G to specifically evaluate the impact of interventions on cognitive function [54].
5 Conclusion
The findings of this study show that the exercise intervention investigated in this study can have a significant long-term effect on blood pressure, health-related quality of life, fatigue, waist circumference, and physical activity levels, with effects lasting for 12 months after the 12 sessions. Significant improvements were also found for lower extremity strength; however, a significant difference could only be found up to 6 months after finishing the program. Exercise has a significant impact on nonmetastatic cancer participants, leading to measurable health improvements. Although limited data suggest that exercise does not significantly enhance health outcomes in metastatic cancer participants, it may help maintain these measures, preventing further decline. These findings illustrate the need for more community cancer exercise programs within London and throughout the United Kingdom to improve the quality of life of cancer patients. The exercise intervention program has helped to improve the physiological and psychological outcomes of cancer patients and has the potential to address issues associated with cancer treatment side effects, prehabilitation, survivorship, and palliative care.
Acknowledgments
We thank the Tottenham Hotspur Foundation (the charitable arm of Tottenham Hotspur Football Club) who ran the “Move4you” program for cancer patients in North Central London. Also, a special thank you to Katrina Heal, Chantelle Fernandes, Emiliano Bianchi, Belvin Lewis, and Adam Millar, who all helped to run the program. A thank you also to Macmillian, Big Lottery, and North Cancer London Cancer Alliance for all the funding to allow this program to exist.
Ethics Statement
Ethical approval for the use of anonymized data captured from the program was granted by the Staffordshire University Research Ethics Committee.
Conflicts of Interest
The author(s) have no conflicts of interest to disclose.
Open Research
Data Availability Statement
Data supporting the findings of this study are available from the corresponding author (IO) upon reasonable request.




