Aging-Driven Blood–Brain Barrier Dysfunction and Its Impact on CNS Cancer Susceptibility: A Comprehensive Narrative Review
Funding: The authors received no specific funding for this work.
ABSTRACT
Background
Aging significantly affects the structural and functional integrity of the blood–brain barrier (BBB), increasing the susceptibility of the central nervous system (CNS) to both primary and metastatic cancers. As the BBB deteriorates, it promotes tumor cell infiltration, alters drug permeability, and contributes to a proinflammatory microenvironment that supports tumor progression. These age-related changes present major obstacles in the effective treatment of CNS malignancies in elderly patients.
Methods
This review synthesizes current literature on the mechanisms of BBB aging and its impact on CNS cancer development and treatment. It examines key structural alterations, such as tight junction protein loss, endothelial dysfunction, and pericyte reduction, as well as functional changes including impaired immune regulation and transporter dysfunction. The review also evaluates therapeutic challenges and emerging strategies to overcome the BBB barrier in the aging brain.
Results
Aging induces BBB dysfunction by weakening cellular junctions, disrupting the neurovascular unit, and promoting chronic neuroinflammation. These alterations facilitate tumor cell entry and survival in the brain and reduce the effectiveness of cancer therapies due to impaired drug delivery. Promising interventions, including nanoparticle-based therapies, focused ultrasound techniques, and targeted chemoimmunotherapy, are under development to enhance therapeutic outcomes in elderly patients.
Conclusion
The age-related breakdown of the BBB contributes significantly to increased cancer risk and therapeutic resistance in the CNS. Addressing BBB dysfunction through age-specific interventions and advanced drug delivery strategies is critical to improving outcomes for older adults with CNS malignancies. Further research into the molecular pathways of BBB aging will support the development of personalized and effective treatments.
1 Introduction
The blood–brain barrier (BBB) is a highly specialized and dynamic structure that protects the central nervous system (CNS) by regulating the movement of molecules, cells, and pathogens between the blood and the brain parenchyma. Comprised primarily of endothelial cells, astrocytes, and pericytes, the BBB is crucial in maintaining CNS homeostasis and providing a tightly controlled environment for neuronal function [1]. However, as people age, the BBB's structural and functional integrity gradually deteriorates, increasing permeability and making the brain more vulnerable to a range of clinical disorders, including both primary and metastatic CNS malignancies [2, 3].
In aging populations, CNS tumors—both primary malignancies, such as glioblastoma, which originate within the brain parenchyma, and secondary metastases from systemic cancers—present significant treatment challenges [4, 5]. The decline of the BBB with age is associated with increased frequency and severity of CNS malignancies, though its role differs between tumor types [3]. In primary brain tumors like glioblastoma, BBB dysfunction can alter the tumor microenvironment by facilitating immune cell infiltration, modifying nutrient transport, and impacting drug delivery. Conversely, in metastatic brain tumors, a compromised BBB may allow circulating tumor cells to infiltrate the CNS more easily, promoting secondary tumor formation [3].
This review examines the intricate connection between aging, BBB dysfunction, and the risk of developing both primary and metastatic CNS cancers. In addition to discussing the implications for cancer progression and treatment, it will emphasize the mechanisms behind BBB aging and suggest future research directions. By revealing these links, we aim to provide insight into a crucial but underexplored area in aging and cancer biology.
2 Overview of the Blood–Brain Barrier
The CNS and the systemic circulation are separated by the BBB, a highly selective and semipermeable contact [6]. Its principal function is to keep the CNS in a state of homeostasis by controlling the flow of nutrients, ions, and waste products while blocking the entry of infections and potentially dangerous substances [7, 8]. In order to maintain its functionality, the BBB is made up of a neurovascular unit that consists of astrocyte end-feet, pericytes, endothelial cells, and a basement membrane [9].
Tight connections between endothelial cells are a defining characteristic of the BBB. These tight junctions are essential for limiting paracellular permeability as they are made up of proteins like occludins and claudins [10]. To maintain appropriate neuronal function, the interplay between astrocytes and pericytes further controls BBB integrity and permeability [11]. These elements work together to form a very dynamic system that can adjust to changes in the body's metabolism and physiology.
Additionally, the BBB is essential for shielding the CNS from systemic immunological reactions. The BBB contributes to the preservation of an immunologically favored milieu by controlling cytokine signaling and immune cell trafficking [12]. However, as we age, this dual role of immune surveillance and permeability regulation is weakened, which creates the conditions for both CNS malignancies and neurodegenerative disorders. A thorough schematic of the BBB's structural and functional elements is shown in Figure 1.
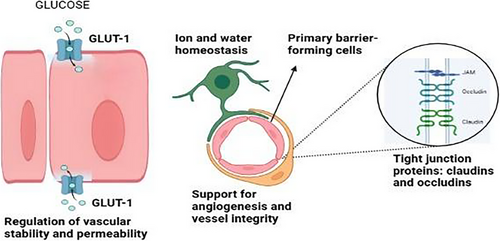
3 Aging and Structural Changes in the BBB
The BBB's structural integrity is drastically changed by aging, making the CNS more susceptible. The most noticeable alteration is the decrease in tight junction protein expression, which lowers the barrier's selective permeability and includes occludins and claudins [13, 14]. This decrease raises the possibility of dangerous drugs accessing the brain by impairing the BBB's physical barrier qualities [15]. Furthermore, the effectiveness of the barrier is further diminished by morphological changes in aged endothelial cells, such as swelling and vacuolation [16].
As people age, the basement membrane, a vital structural element of the BBB, thickens and degrades. The connection between astrocytes, pericytes, and endothelial cells is disrupted by this dual phenomenon, which hinders their ability to work together to sustain BBB function [13]. Barrier dysfunction is exacerbated when astrocyte end-feet, which envelop capillaries to offer structural and metabolic support, grow hypertrophic and lose their close association with endothelial cells [17].
The number and activity of pericytes, which are necessary for angiogenesis and vascular stability, likewise decrease with age. Increased BBB permeability and microvascular dysfunction result from the vascular network being unstable due to a decrease in pericyte density [18]. Together, these structural alterations generate an environment that encourages neurodegeneration and increases susceptibility to CNS cancers, as shown in Figure 2.
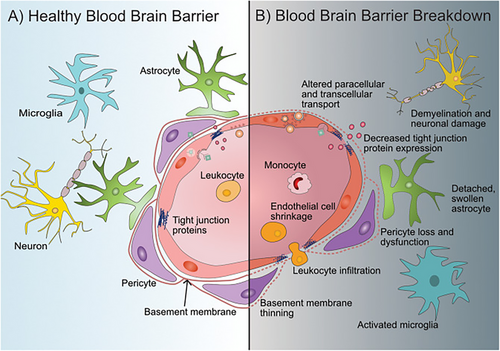
4 Neuroinflammation and BBB Dysfunction
In the BBB, neuroinflammation serves as a reaction to damage as well as a cause of dysfunction. Proinflammatory cytokines including TNF-α, IL-6, and IL-1β are produced in greater amounts as a result of persistent low-grade inflammation, or “inflammaging,” which intensifies with age [19, 20]. Increased BBB permeability results from these cytokines' disruption of endothelial cells' tight junction proteins [21]. Reactive oxygen species (ROS) and nitric oxide are released concurrently by activated microglia and astrocytes, which further harm endothelial cells and compromise the integrity of the barrier [22].
Age-related CNS diseases are made worse by the feedback loop between neuroinflammation and BBB degradation. Peripheral immune cells including monocytes and macrophages, penetrate the brain parenchyma when BBB integrity deteriorates, intensifying the inflammatory response. [23, 24] This invasion increases the risk of neurodegenerative disorders and CNS malignancies by causing neuronal injury and contributing to a self-replicating cycle of immune activation and barrier disruption [24]. Furthermore, the inflammatory environment is maintained because modified BBB transport pathways are unable to effectively remove inflammatory molecules [25].
Circulating tumor cells (CTCs) have a greater chance of invading the CNS as the BBB deteriorates with age [26]. The aging BBB's proinflammatory and oxidative stress environment improves metastatic cells' adherence and extravasation, increasing their chances of surviving within the brain parenchyma. Tumor cell migration over the barrier is further facilitated by the basement membrane's deterioration and the loss of tight junction integrity [27]. Furthermore, compromised BBB transport systems lead to the buildup of inflammatory and carcinogenic substances in the CNS, which promotes the development of tumors [28].
Additionally, the formation of primary and secondary tumors is significantly influenced by the vascular aging process. Cerebral microvessel stability is compromised by decreased pericyte covering and endothelial dysfunction, which results in aberrant neovascularization that promotes tumor growth [29]. Aging-related chronic inflammation promotes the release of vascular endothelial growth factor (VEGF), which leads to leaky, defective angiogenesis and the establishment of tumors' own blood supply in the CNS [23]. Therefore, by fostering a favorable milieu, the aging BBB actively promotes tumor growth in addition to failing to shield the brain from metastatic invasion [23].
Additionally, astrocytes and pericytes, which typically fight inflammation, lose some of their regulatory abilities as we age [13]. Depletion of pericytes impairs the barrier's ability to respond to inflammatory stimuli, whereas astrocytes lose their ability to release anti-inflammatory chemicals [30]. All of these alterations work together to produce an atmosphere in which persistent inflammation propels progressive BBB failure, jeopardizing CNS homeostasis and heightening vulnerability to pathological processes. Key routes connecting neuroinflammation and BBB breakdown in aging are listed in Table 1.
| Pathway | Mechanism | Impact on BBB |
|---|---|---|
| Proinflammatory cytokines | Increased TNF-α, IL-6, IL-1β production disrupts tight junction proteins [31]. | Elevated permeability, weakened selective barrier function [31]. |
| Reactive oxygen species (ROS) | Microglia and astrocytes release ROS, inducing oxidative damage in endothelial cells [32]. | Tight junction degradation, increased endothelial permeability [32]. |
| Peripheral immune infiltration | Monocytes and macrophages infiltrate through compromised BBB [33]. | Amplification of neuroinflammation, barrier destabilization [33]. |
| Reduced anti-inflammatory signaling | Astrocytes and pericytes fail to regulate inflammation effectively [34]. | Chronic inflammatory state exacerbating BBB dysfunction [34]. |
| Dysfunctional transporters | Dysfunction transporters fail to clear inflammatory molecules [13]. | Prolonged exposure to inflammatory mediators, sustained barrier compromise [13]. |
5 Cancer Metastases and the Aging BBB
The CNS vulnerability to cancer metastasis is greatly influenced by the aging BBB. The BBB becomes more receptive to the entry of CTCs and metastasizing cancer cells as it ages due to structural and functional changes [35]. The deterioration of tight junctions and the loss of endothelial cell integrity, which are characteristics of an aging BBB, are major causes of this increased permeability [13]. This promotes the formation of secondary tumors by facilitating the transvascular migration of tumor cells from the circulation into the brain parenchyma [28].
The proliferation and survival of metastatic cells within the brain are further supported by alterations in the tumor microenvironment that are linked to aging. An environment that is more conducive to tumor invasion is produced by the matrix remodeling enzymes and proinflammatory cytokines secreted with aging [36]. Increased vascular permeability and enhanced metastatic potential are the results of these age-related changes in conjunction with the compromised BBB. Furthermore, cancer cells may be able to elude immune surveillance and expand into the brain due to the aging immune system's diminished capacity to eradicate CTCs [37].
Crucially, brain metastases are a serious risk for several cancers, including melanoma, lung, and breast cancer [38]. The BBB's weakened integrity makes the aged brain especially susceptible because it allows tumor cells to enter while restricting the immune system's capacity to eliminate them [13]. Treatment for cancer metastases in the aging brain can be particularly difficult since older patients may respond less well to conventional BBB-targeting medicines [39]. The primary routes through which the aging BBB leads to CNS cancer metastases are highlighted in Figure 3, which illustrates the mechanisms of interaction between tumor cells and the brain endothelium, including adhesion molecule involvement and tight junction disruption.
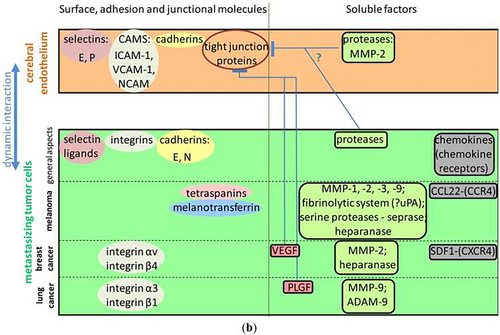
6 Primary CNS Tumors and BBB Aging
As individuals age, the BBB becomes increasingly compromised, which can have significant implications for primary CNS tumors, such as gliomas, meningiomas, and medulloblastomas [41]. Aging is associated with structural and functional changes in the BBB that facilitate tumorigenesis within the brain, including decreased integrity of endothelial cells, increased permeability, and disruption of tight junctions [14]. These alterations not only enable the initial growth of primary tumors but also affect their progression, invasiveness, and response to treatment.
Primary CNS tumors, particularly gliomas, are known to be aggressive and highly infiltrative, often spreading through the brain parenchyma [42]. The aging BBB contributes to this aggressiveness by allowing tumor cells to penetrate the brain more easily, as the endothelial cell junctions become weakened over time. Moreover, the aging microenvironment can influence the interaction between tumor cells and the surrounding neural tissue. For example, age-related changes in neuroinflammatory signaling pathways can foster an environment conducive to tumor cell survival and growth. In addition to this, aging impairs the ability of the immune system to mount an effective response against the tumor cells, resulting in a decreased capacity for immune surveillance and clearance of malignant cells [13, 37].
In the context of treatment, the compromised BBB in the aging brain can hinder the efficacy of therapeutic agents, particularly chemotherapy and immunotherapy, which rely on effective drug delivery across the barrier [43]. This barrier dysfunction also complicates the use of targeted therapies, as drugs intended to modify tumor behavior may have reduced access to the tumor site [43]. Consequently, these aging-related changes present additional challenges for the treatment of primary CNS tumors in older patients [44]. This is due to the fact that an increased level of permeability of the BBB makes it more difficult for cancer treatments to access primary CNS tumors. The impact of BBB aging on tumor progression and treatment efficacy highlights the need for age-specific therapeutic strategies that address both the tumor biology and the altered vascular characteristics of the aging brain [45].
7 Therapeutic Implications of an Aging BBB
It becomes much more difficult to deliver cancer treatments to the CNS as people age because the BBB deteriorates [46]. Because of the BBB's tight connections and selective permeability, conventional cancer treatments like chemotherapy and monoclonal antibodies sometimes have trouble passing through it [47]. These features are further compromised in the aged brain by dysfunctional endothelial cells, changed transporter activity, and compromised tight junction integrity, all of which raise the permeability of the barrier [48]. Because of this, therapeutic medicines might not be able to reach their intended target tissues in high enough concentrations to be efficacious, especially when treating primary CNS tumors or brain malignancies that have spread [49]. This issue is compounded in older patients, where systemic health factors, such as reduced liver and kidney function, may alter drug metabolism, further complicating the delivery and efficacy of treatments. Consequently, finding ways to circumvent or restore BBB function is essential for improving the delivery of therapies to the aging brain [50].
Several interesting treatment options are being investigated in response to the difficulties in delivering cancer medications across the aging BBB [51]. One of the most promising methods for enhancing medication delivery to the brain is the use of nanoparticles [52]. By taking advantage of receptor-mediated transport, transcytosis, or barrier disruption via methods like focused ultrasound, these nanoscale carriers can be designed to pass through the BBB [53]. Researchers can increase the solubility, stability, and bioavailability of medications and make sure they more efficiently reach their targets by encapsulating monoclonal antibodies or chemotherapeutic medicines in nanoparticles [54]. Larger therapeutic molecules, such as antibodies, can be delivered directly to brain tumors using focused ultrasound and microbubbles, a noninvasive method of temporarily breaching the BBB [54]. Additionally, methods for modulating the BBB itself, such as the use of pharmacological agents to transiently loosen tight junctions or inhibit efflux transporters, are under investigation as strategies to enhance drug delivery [55]. These innovative approaches hold the potential to significantly improve treatment outcomes for patients with brain tumors, especially those in the older population where BBB dysfunction is more pronounced.
Targeted BBB chemoimmunotherapy is gaining attention as a strategy to improve drug delivery for aging-related brain tumors. By combining chemotherapy with immune-based therapies, such as immune-checkpoint inhibitors or CAR T-cell therapy, researchers aim to enhance therapeutic efficacy while overcoming BBB restrictions [56]. Nanoparticle-based carriers and BBB-modulating agents, including small-molecule inhibitors and focused ultrasound, are being explored to facilitate the penetration of immunotherapies into the CNS [57]. Additionally, strategies that temporarily disrupt the BBB or modify its transport mechanisms may help improve the uptake of systemically administered immune-based treatments, ultimately enhancing antitumor responses in elderly patients with brain tumors.
One promising approach to enhancing the prognosis of elderly cancer patients is personalized cancer medicines, which consider individual variations in tumor biology and BBB integrity [58, 59]. While there are currently no direct clinical methods to assess “BBB failure,” emerging biomarkers and imaging techniques may help evaluate BBB permeability and dysfunction, allowing for more targeted treatment strategies [60]. Patients with less severe BBB alterations may respond better to traditional treatments that depend on modifying preexisting barrier mechanisms, whereas patients with advanced BBB breakdown may benefit from more aggressive interventions, such as those that employ focused ultrasound or targeted nanoparticles [61]. Incorporating age-specific considerations into treatment choices, such as evaluating the patient's immunological status, vascular health, and medication metabolism, may also increase the safety and effectiveness of treatments [62]. Personalized techniques that directly target the aging BBB may allow for more efficient delivery of cancer treatments and maybe lessen negative side effects, improving older patients' quality of life and prognosis [43].
8 Experimental Models of BBB Aging
Determining the processes of BBB aging and how they contribute to CNS disorders, such as cancer, depends heavily on in vitro models. Age-related cellular alterations, including broken tight junctions, increased permeability, and changed transporter expression, are mimicked by endothelial cell cultures subjected to oxidative stress or induced senescence. Coculture methods that include glial or neuronal cells offer more information about how different brain cell types interact to affect the integrity of the BBB as people age. The complexity of the brain in vivo, which includes systemic elements like vascular remodeling and immune system interactions, cannot be fully replicated by these in vitro models [13].
By providing a more thorough understanding of BBB aging and its involvement in brain function and cancer progression, in vivo models supplement in vitro investigations. Age-related alterations in BBB integrity seen in humans, such as decreased tight junction protein expression and increased vascular leakage, are replicated in aged rodent models. Using senescence-inducing transgenic methods, these mice are useful for investigating pathways such as inflammation and oxidative stress and testing therapeutic approaches to restore BBB function [45]. In vivo models offer important insights into systemic factors impacting the BBB, such as inflammation and vascular health, despite difficulties in simulating human aging and species heterogeneity. In order to further our knowledge of BBB aging and its connections to CNS cancer, both in vitro and in vivo models are essential [63].
9 Conclusion
In conclusion, the aging BBB plays a crucial role in the increased vulnerability of the CNS to cancer, contributing to both primary CNS tumors and metastasis. As the BBB undergoes structural and functional decline with age, it becomes more permissive to tumor cell infiltration and metastatic spread, exacerbated by chronic neuroinflammation and reduced immune surveillance [64]. These changes complicate the treatment of CNS cancers in older adults, highlighting the need for age-specific therapies and innovative drug delivery strategies. Understanding the intricate relationship between BBB aging, neuroinflammation, and cancer progression will be vital in developing targeted interventions to preserve BBB integrity, prevent metastasis, and improve therapeutic outcomes in older populations. Further research, particularly clinical studies in aging human populations, is essential to fully explain the molecular mechanisms at play and guide the development of effective treatments for CNS cancers in the elderly.
Acknowledgments
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
Data sharing is not applicable as no new data are generated.




