Understanding and Overcoming Immunotherapy Resistance in Skin Cancer: Mechanisms and Strategies
Funding: The authors received no specific funding for this work.
ABSTRACT
Background
Immunotherapy that includes immune checkpoint inhibitors (ICI) is a revolutionary arm of the treatment of skin cancers like melanoma, basal cell carcinoma, and squamous cell carcinoma. Despite this leap in clinical advances, a critically challenging area in this field is emerging resistance to immunotherapy that limits its efficaciousness in a profound segment of the population. This resistance can be classified as primary resistance, in which cancers fail to respond to initial regimen, or acquired resistance that develops after there is a favorable initial response. A comprehensive understanding of the basic mechanisms and figuring out novel strategies to combat resistance are necessary to improve patient outcomes.
Methods
A comprehensive review of recent studies was conducted with focus on preclinical and clinical evidence related to immunotherapy resistance in skin cancer with a wide literature search on databases such as PubMed, Cochrane, and Google Scholar with keywords, including “skin cancer,” “immunotherapy,” “malignant melanoma,” “drug resistance,” “mechanisms,” and “strategies” published in the last 15 years.
Results
This study aims to establish a review of the molecular and cellular mechanisms that contribute to development of drug resistance in skin cancer and to gauge emerging strategies to overcome these barriers. Insights into these mechanisms were classified into tumor-intrinsic factors, like genetic and epigenetic changes, and tumor-extrinsic factors, such as changes in tumor microenvironment (TME) and systemic immunosuppression. Therapeutic strategies that included combination therapies, newer checkpoint inhibitors, and modulation of the TME were evaluated. Key mechanisms leading to drug resistance identified include tumor-intrinsic factors, including mutations in signaling pathways, tumor-extrinsic factors, including immunosuppressive cells and changes in the TME, such as hypoxia that contributed to drug resistance. Upcoming strategies to counteract resistance included combination approaches, adoptive T-cell therapy, and newer immunomodulatory agents that target resistance pathways.
Conclusions
There is a complex interplay of cancer and immune microenvironmental mechanisms that leads to development of immunotherapy resistance in skin tumor patients. A multi-pronged approach with focus in fields of genomics and immunology as well as bioinformatics is required, along with combination therapies and novel immunomodulators, to tackle resistance and enhance clinical outcomes for patients suffering with skin tumors.
1 Introduction
The incidence of skin cancer is on the rise. This rise is thought to occur due to increased UV exposure, indoor tanning, advanced skin cancer awareness, screening programs, and increased skin biopsy rates. Immunotherapy has shown promise in helping patients reach their goals, particularly in melanoma, metastatic, or locally advanced non-melanoma cancer cases [1]. The mechanism through which immunotherapy helps in cancer is used as early as the 1800s with injecting streptococcal and Serratia bacteria, intralesional bacillus calmette guerin (BCG), cytokine therapies employing interferons, intralesional use of the oncolytic virus Talimogene laherparepvec (T-VEC), and so on. Now, it has progressed to the use of immune checkpoint inhibitors (ICI). This earlier form of therapy had high toxicity and low response rate. Thus, it helped us to keep our goal as to increase the patient's overall survival and reduce the side effects [2].
Acral and mucosal melanomas in sun-protected areas are more common in the Asian region and typically exhibit a low mutation burden. In contrast, cutaneous melanomas in sun-exposed locations, which often affect Caucasians, are associated with a high mutation burden. Although a high mutation burden generates more neoantigens that improve responsiveness to immunotherapy, certain mutations, such as V-Raf murine sarcoma viral oncogene homolog B (BRAF) mutations, may contribute to resistance. Conversely, acral and mucosal melanomas, despite having a lower mutation burden, are less sensitive to immunotherapy, potentially due to reduced neoantigen expression and unique biological characteristics [1]. Thereby developing resistance to commonly used BRAF inhibitors (BRAFis) such as vemurafenib and dabrafenib [3]. Thus, understanding and overcoming resistance with immunotherapy is of high concern. This review discusses the mechanism, strategies to overcome it, monitoring, and early intervention of immunotherapy resistance.
1.1 Immunotherapy Resistance Mechanisms
1.1.1 Immunotherapy Resistance in Skin Cancer
Immunotherapy resistance in skin cancer is a complex phenomenon that arises due to various mechanisms. Latest studies suggest that various types of immune cells, both belonging to the innate immune system and the adaptive immune system (specifically T cells and B cells), exert influence on tumor progression when they are present within the tumor microenvironment (TME), where immunotherapy resistance can be influenced by the behavior of these cells [4]. The process of cancer development triggers a robust immune response against tumors, providing a means for the immune system to eliminate cancerous cells. This theory, known as immunosurveillance, outlines the intricate interplay between immune and cancer cells. It unfolds in three stages: elimination, equilibrium, and evasion, often occurring simultaneously [5].
1.1.2 PD-1/PD-L1 Axis Regulation and Disrupted Interferon γ (IFN-γ) Signaling Pathways
The PD-1/PD-L1 axis acts as a regulatory mechanism in immune surveillance. PD-1, part of the CD28 family of transmembrane proteins, is seen on activated T cells, although it can also be observed in other cells like B cells and natural killer cells following activation. PD-1 interacts with two ligands, PD-L1 and PD-L2. PD-L1 is expressed in various immune cells like macrophages, activated T cells, B cells, and dendritic cells (DCs), as well as certain epithelial cells, especially in inflammatory conditions. Furthermore, tumor cells utilize PD-L1 as an adaptive mechanism to evade antitumor reactions. However, suppression of certain pathways can decrease PD-L1 levels, fostering a more potent antitumor immune response [4, 6, 7].
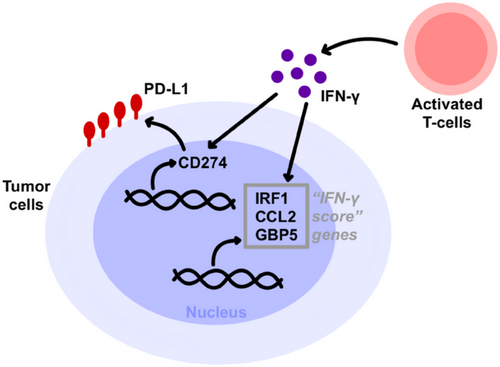
Moreover, changes in signaling pathways within tumor cells contribute to immunotherapy resistance [8]. IFN-γ, a cytokine released by effector T cells (Teffs) and antigen-presenting cells (APCs), triggers JAK2 activation upon binding with IFN-γ receptors 1/2 (IFNGR 1/2). Subsequently, IFNGR 1/2 interacts with signal transducers and activators of transcription-1 (STAT1) to regulate downstream processes, activating the transcriptional activity of IRF-1. This leads to the expression on tumor cell surfaces. Additionally, it induces the production of chemokines CXCL9 and CXCL10, facilitating the recruitment of CXCR3+ lymphocytes and other immune cells and tumor cells, thereby exerting antitumor effects. However, in patients undergoing immunotherapy, tumor cells can develop resistance by downregulating or modifying IFN-γ signaling pathways. This can involve loss-of-function mutations in genes encoding JAK 1/2 and alterations in STAT1, allowing them to evade the influence of IFN-γ [6-9]. Tumor cells harboring a mutation in the JAK1/2 genes display reduced sensitivity to the cytotoxic effects of IFN. This leads to a decrease in the expression of PD-L1, rendering the tumor cells less responsive to PD-1/PD-L1 monoclonal antibody treatment.
1.1.3 Hypoxia Signaling and Lactate Accumulation: Dual Challenges in Immunotherapy Resistance in Skin Cancer
Furthermore, in the context of oncogenes, the hypoxia signaling pathway assumes a pivotal role with profound implications for both tumor progression and the immune response. Within this pathway, the transcriptional complex formed by HIF-1α and HIF-2α takes center stage in enabling tumor cells to adapt to oxygen-deficient environments [5]. Recent research has shed light on the crucial role of HIF-1α in orchestrating the mobility and functional capacities of CD8+ T cells. An important way in which hypoxia signaling disrupts the functionality of T cells is by inducing the expression of PD-L1 on myeloid suppressor cells when these cells are exposed to low-oxygen conditions. On a molecular level, HIF-1α directly influences the expression of PD-L1 by binding to the hypoxia response element (HRE) located on the PD-L1 promoter [5, 10, 11].
Moreover, in conditions marked by low-oxygen levels, cancer cells exhibit heightened glycolytic activity, resulting in the accumulation of lactate and subsequent acidification of the TME. This leads to a substantial 40-fold increase in lactate production within tumor cells. Lactic acid has adverse effects on the functionality of effector T cells, reducing their proliferation and diminishing the production of IFN-γ. Additionally, it results in a decrease in pH levels within the TME, further contributing to the suppression and development of resistance to immunotherapy [10-14].
Similarly, understanding the influence of hypoxia in the different cells within the TME is crucial. Hypoxia enhances the activity of immunosuppressive cells in the TME. Tumor-associated macrophages (TAMs) recruitment and differentiation to the TME is facilitated by hypoxia. Various soluble factors, including C–C motif chemokine Ligands 2 and 5 (CCL2, CCL5), colony-stimulating factor 1 (CSF1), vascular endothelial growth factor (VEGF), and semaphorin 3A, attract TAMs via chemotactic signals to the tumor. These cells promote the progression, angiogenesis, and metastasis of different cancers, including melanoma. Hypoxia further inhibits the motility of TAMs; hence, they accumulate in hypoxic tumor areas. Myeloid-derived suppressor cells (MDSCs) inhibit the immune cells’ activity. These are recruited to the TME via releasing of various cytokines, including CCL26, G-CSF, and IL-6, which are expressed due to hypoxia. Moreover, multiple immunosuppressive factors, such as PD-L1 and TGF-β1, promote cancer growth. Tregs can be recruited to the TME via IL-23, produced by TAMs, which expresses interleukin-10 (IL-10) and transforming growth factor-beta (TGF-β) to further inhibit the effector T-cell responses and cytotoxic lymphocyte capacity of killing tumor cells [15-22].
On the other hand, hypoxia displays an inhibitory effect on immunostimulatory cells. During hypoxic states, TCD8+ cell proliferation and cytotoxic effect are diminished due to the upregulation of PD-L1 on NK cells and thus weaken the anti-cancer immunity. NK cell activation and IFN-γ, necessary for an effective antitumor response, are also suppressed in hypoxia. This reduction of NK cell activation is produced because of the reduced expression of surface receptors, such as NKp46, NKp30, NKp44, and NKG2D. Similarly, hypoxia reduces DCs’ ability to process and uptake cancer antigens, reducing T-cell priming, and downregulating CD40, CD80, and MHCII due to increased production of IL-10 and VEGF [15-22].
At the end, lactic acid buildup, derived from melanoma cells, in the context of hypoxic states, diminishes the activation and antitumoral effect of cytotoxic T cells and NK cells, inhibiting IFN-γ signaling and increasing the expression of PD-L1 on tumor cells and immunosuppressive cells within the TME. This will induce resistance to PD-1/PD-L1 blockade by reducing T-cell recognition and immune activation in the TME. Melanoma tumor cells use this mechanism to escape immune surveillance. Together, these above-mentioned factors create an immunosuppressive TME that will ultimately reduce the effectiveness of ICI, such as PD-1 inhibitors, contributing to treatment resistance in melanoma [15, 21, 22].
1.1.4 P-Selectin Glycoprotein Ligand-1 (PSGL-1): A Critical Player in T-Cell Exhaustion Within TME
Recently, it was discovered that PSGL-1, a glycoprotein crucial for cell adhesion and inflammation, plays a vital role in regulating T-cell responses within the TME. PSGL-1 was identified as a potential regulatory point, inhibiting T-cell receptor (TCR) signaling in exhausted CD8+ T cells, leading to decreased pro-inflammatory IL-2 and increased PD-1 levels [13].
1.1.5 Immunosuppressive Mechanisms and Fibroblast-Mediated Resistance in Cancer Immunotherapy
In addition, immunosuppressive cells, like Tregs, MDSCs, M2 macrophages, and N2 neutrophils, hinder immune cell function [23]. However, the fibroblasts activated by TGF-β in the TME lead to the development of cancer-associated fibroblasts (CAFs). These CAFs release TGF-β and IL-6, which impede the function of DCs responsible for presenting antigens, thus hindering the priming of T cells directed at the tumor [23-25]. Additionally, this complexity is further exacerbated by the heterogeneous nature of the TME in melanoma. Heterogeneity, defined as the differences between cells or subpopulations of cells within a tumor, in the TME in melanoma is a predisposing factor to developing resistance to ICI. The TME is a dynamic ecosystem that comprises an extracellular matrix (ECM), stromal cells, soluble molecules, fibroblasts, and endothelial cells. Together these factors, among others, interact with each other to promote melanoma progression and metastasis. The most important cell type in the tumor stroma is the CAFs, producing secretory molecules that lead to tumor growth, angiogenesis, inflammation, and drug resistance. Hence, an increased number of CAFs in the melanoma TME is associated with a poor prognosis and a high risk of metastasis. Heterogeneous melanoma, different antigens, and immune infiltrate can also be found. Moreover, diverse and highly heterogeneous tumors are associated with a reduction of antitumor cells, including TCD4 and TCD8, effect and activation, and increased recruitment of more immunosuppressive cells, which will ultimately induce tumor resistance. Of note, IFN-γ secreted by cytotoxic T cells binds to its receptor in tumor cells to activate apoptosis signaling and tumor growth suppression. On the other hand, TCD4 cells can display both anti- and pro-tumorigenic activity [25-28] (Figure 1).
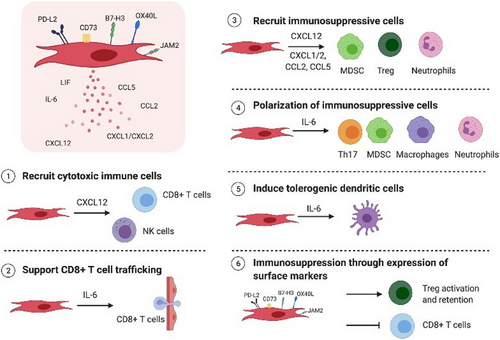
1.1.6 Navigating Immunotherapy Resistance in Melanoma Treatment: Advances and Challenges
Melanoma, a prominent and aggressive skin cancer, constitutes around 5% of malignant tumors in the United States [29, 30]. Malignant melanoma displays significant diversity and is highly resistant to broad treatments like chemotherapy [30]. Recent years have seen advancements in mutational analysis and next-generation sequencing (NGS) that revealed the somatic mutations in genes like BRAF and neuroblastoma rat sarcoma (NRAS) that contribute to unregulated survival and movement, particularly when combined with genetic changes and epigenetic events that facilitate senescence avoidance [30, 31]. The most common mutation in melanoma is BRAF mutation that induces melanoma genesis via the activation mitogen-activated protein kinase (MAPK) pathway (RAS/Rapidly accelerated fibrosarcoma (RAF)/MAPK kinase (MEK)/Extracellular signal-regulated kinase (ERK). This promotes tumor cell proliferation, growth, survival, migration, and immune response evasion via the production of MMP-1, IL-1β, IL-6, IL-8, and TGF-β by melanoma cells. Around 50% of melanomas carry this mutation, especially the V600E variant. Inhibiting BRAF will improve antigen expression and T-cell cytotoxicity and create a favorable TME that augments the efficacy of immunotherapy. Due to the high resistance and toxicity to immune checkpoint inhibitors, BRAFis have been developed. Two different randomized control trials (RCTs) comparing BRAFi, vemurafenib and dabrafenib, demonstrated promising results and improvement in the outcomes of patients with BRAF-mutant unresectable or metastatic melanoma. However, the combination of BRAFi with MEK inhibitors (MEKis) has resulted in better and more consistent outcomes with a better safety profile than BRAFi alone. Moreover, a network meta-analysis of 15 RCTs evaluating systemic therapy for untreated melanoma found higher and better objective response rates in patients treated with BRAFi and MEKi compared to BRAFi alone [32-38].
However, despite notable progress, the emergence of resistance poses a substantial challenge to effectively treating melanoma, where this resistance can be influenced by various factors, originating both within and outside the cancer cell [8, 30] (Figure 2).
1.2 Strategies to Overcome Immunotherapy Resistance in Skin Cancer
- Immunomodulatory agents: The emergence of more potent immune checkpoint inhibitors beyond CTLA-4 and PD-1 inhibitors holds promise for expanding therapeutic options and addressing resistance, reshaping the cancer treatment landscape [38-40]. In a study focusing on aggressive melanoma, researchers targeted the PSGL-1 molecule within the tumor environment. This approach significantly boosted the activity of crucial immune cells, CD4+ and CD8+ T cells, known for their role in cancer defense [38, 39]. Additionally, it reduced regulatory T cells (Tregs), which usually suppress the immune response. The activated CD4+ and CD8+ T cells displayed increased functionality, becoming more potent and prolific. This heightened immune response slowed down the tumor's growth. In essence, targeting PSGL-1 enhanced the immune response against melanoma, offering promise for improving melanoma immunotherapy [38, 39]. Moreover, VISTA, LAG-3, T cell immunoglobulin and immunoreceptor tyrosine-based inhibitory motif (TIGIT), and TIM-3 are promising new targets for cancer therapy [5, 39, 40]. Blocking these pathways, especially LAG-3, TIM-3, and TIGIT, can improve the immune response within tumors [5, 39, 40]. This approach counters the suppressive effects of regulatory T cells and enhances the function of CD8+ and NK cells, with better safety profiles compared to existing therapies like CTLA-4 and PD-1 inhibitors [39, 40]. It is a potential game-changer for cancer immunotherapy. LAG-3 (CD223), expressed in the surface of tumor-infiltrating lymphocytes (TILs), TCD4, TCD8, and Tregs, plays a key role in the TME. LAG-3 interacts with MHC-II, prohibiting the union of this major histocompatibility complex (MHC) molecule to a TCR and TCD4, therefore suppressing the TCR signal. This molecule displays an inhibitory signal via the KIEELE motif in the cytoplasm, and the result of the crosslinking of LAG-3 and CD3/TCR complex can suppress the T-cell proliferation that results in suppressing clearance of tumor cells by the immune system, cytokine secretion, and calcium flux. In melanoma cells, two novel ligands, Gal-3 and LSECtin, interact with LAG-3, modulating the TCD8 function within the TME. Similarly, TIM-3 is highly expressed in tumor DCs. Binding with its ligands, GAL-9, HMGB1, PtdSer, and CEACAM1, which are found in a variety of immune cells, leads to apoptosis of effector T cells, the inability of dying tumor cells to activate an immune response, suppression of TCR signaling, and T-cell exhaustion. Additionally, TGIT is expressed in many types of T cells and NK cells. TIGIT ligands, CD112 and CD155, are mainly expressed in APC and a variety of cancers such as melanoma, lung adenocarcinoma, and pancreatic cancer. By binding together, TIGIT delivers inhibitory signals that suppress innate and adaptative immunity in patients with melanoma. TGIT suppresses the expression of TCR, inhibiting directly the activation and proliferation of TCD8+, and it also induces phosphorylation of CD155 on DCs, improving the production of IL-10 and reducing IL-12. Additionally, TGIT can inhibit CD226 signaling in T/NK cells that promotes immunosuppression. In patients with melanoma, TGIT expression is increased in Tregs, which leads to immunosuppression. It is remarkable that in such patients, expression of CD155 induces resistance to anti-PD-1 therapies and other immunotherapies by inducing degradation of the activating receptor CD226 in TCD8+ [30, 41-45].
- Combination therapies: Combining different immunotherapies, such as checkpoint inhibitors, can sometimes be more effective than using a single agent (Figure 3) [5, 38, 39, 46]. For example, a combination of anti-PD-1 and anti-PSGL-1 antibodies has shown improved response rates in melanoma [38, 39]. Studies show that targeting the PD-1 molecule in the absence of PSGL-1 in the body had a synergistic effect on the immune system's ability to eradicate tumors [38]. They also found that simultaneously targeting both PSGL-1 and PD-1 enhanced antitumor immunity and slowed the growth of melanoma tumors [38, 39]. These findings underscore the potential of combining immunotherapy strategies to improve the immune system's ability to combat cancer [38, 39, 46].
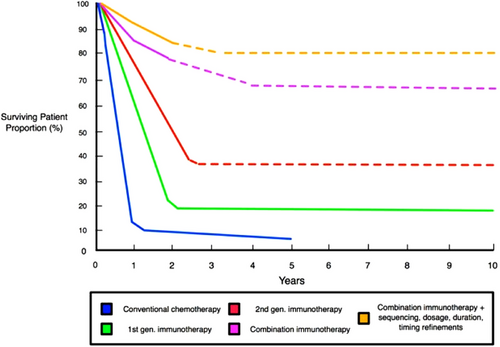
Moreover, combining immunotherapy and targeted therapy is paramount in overcoming resistance to immunotherapy [5, 46, 47]. Researchers discovered that when they reduced CDK5 levels, it triggered an increase in interferon regulatory factor 2 and interferon regulatory factor binding protein 2 [46]. This, in turn, led to a suppression of PD-L1 expression [46, 48]. What's truly remarkable is that this suppression resulted in the rejection of tumors, and the immune response was found to be reliant on CD4+ T cells [46]. This exciting finding opens the door to a potential strategy: combining CDK5 targeting with immune checkpoint blockade to supercharge the immune system's response against tumors [46, 48]. Moreover, by inhibiting CDK4/6, we can further enhance the body's antitumor immunity, empowering T cells to become more effective in eliminating cancer cells [46].
Additionally, the synergistic approach of merging immunotherapy with targeted therapy specifically addresses the HIF pathway, which impedes the immune system's effectiveness by inhibiting T-cell responses and upregulating immune checkpoints such as PD-L1 [46, 47]. Furthermore, the cooperation between HIFs and STAT3 highlights the intricacies of tumor resistance, and inhibition of STAT3 can enhance the effects of immunotherapy [46]. In essence, the synergy between immunotherapy and targeted therapy is a promising strategy to address multifaceted resistance mechanisms, providing a comprehensive approach to combat cancer more effectively [5, 46, 47].
- Cytokine therapy: Extensive preclinical studies have been dedicated to crafting cytokine payloads, with implications for cancer therapy [55]. More recently, various cytokines, including IL-12, IL-15, IL-2, and IL-21, as well as GM-CSF and IFN-α, have shown promise in experiments using mouse models of cancer, where they can amplify and activate immune cell infiltration at specific sites, opening new avenues for exploration [55, 56]. IL-2, for instance, received approval for the treatment of advanced melanoma back in 1998 [55]. It promotes the growth of T cells and aids in antibody production by B cells [55], while also contributing to immune balance through the support of regulatory T cells (Tregs) and participating in activation-induced cell death (AICD) to fine-tune the immune response [55]. In essence, cytokines like IL-2 offer multifaceted ways to activate, control, and balance the immune system for potential therapeutic benefit [55, 56].
In patients with melanoma, the TME is highly immunosuppressive and causes T-cell exhaustion (dysfunction). Cancer cells require high quantities of nutrients to grow and proliferate, which leads to a hypoxic state, acidosis, metabolic stress, and a nutrient-depleted environment that is responsible for the inhibition of T-cell responses [57].
Several factors have been implicated in this: expression of multiple inhibitory receptors, immunosuppressive cells in the TME, suppressive soluble molecules, and nutrient competition. The TME contains several inhibitory cells, regulatory T cells (Treg cells), TAMs, MDSCs, CAFs, adipocytes, and endothelial cells that lead to T-cell exhaustion by the production of several inhibitory products. For instance, Treg cells suppress the activation, proliferation, and survival of T cells by producing IL-10 and TGF-β. These cells also express several molecules, including CC chemokine receptor 4 (CCR4), cluster of differentiation 39 (CD39), and cluster of differentiation 73 (CD73). Additionally, several molecules in the TME promote further T-cell dysfunction such as IL-10, TGF-β, type I IFNs, indoleamine 2,3-dioxygenase (IDO), adenosine, VEGF-A, and interleukin-35 (IL-35) [57-59].
Several interleukins (IL) play a crucial role in the T-cell function, and targeting these could be used as a therapy to promote an inflamed TME. IL-2 stimulates cell proliferation and memory formation, whereas IL-7 signaling is essential for T-cell development and proliferation. In murine models, IL-7 alone has no effect, but using it as an adjuvant improved a vaccine-induced antitumor response. IL-12 induces naïve T-cell differentiation into Th1 cells, stimulates the production of IFN-γ, and improves the cytotoxic activity of TCD8+ and NK cells. T12 produced by Baft DCs can control the progression of metastasis. IL-15 induces the expansion of dendritic, NK, and TCD8+ cells. In vivo studies have demonstrated that this molecule induces DCs to help prime naïve TCD8+ cells to differentiate into antigen-specific cytotoxic T cells. IL-21 prevents T cells from dysregulation, promotes T-cell differentiation and survival, and reduces IFN-γ expression. In patients with melanoma, a Phase II trial of IL-21 monotherapy has antitumor activity in patients with metastatic melanoma with an overall survival of 12.4 months [57-59].
- Personalized medicine: One of the most significant advancements in modern oncology is the shift from an approach that primarily considers the affected organ when making treatment decisions to a more personalized strategy driven by in-depth molecular analysis [60], and melanoma management has emerged as a leading example of personalized medicine's successful application [61]. Precision medicine in healthcare entails tailoring interventions and treatments for individuals who share a common diagnosis. This personalization considers their unique genetic profiles, the specific characteristics of their tumors, the microenvironment of their condition, and individual health factors. The goal is to more accurately predict how patients will respond to treatments and better understand their disease risks [61, 62]. This approach often involves therapies designed to target specific molecular or cellular features, such as genetic alterations or changes in gene/protein patterns, which are expected to benefit specific groups of patients [63]. The realm of personalized medicine has progressed toward pinpointing distinct molecular traits, such as microsatellite instability in solid tumors, somatic or germline mutations like BRCA, BRAF, or ERBB2, and gene fusions such as FGFR and NTRK. These molecular characteristics are pivotal in shaping precision medicine approaches, offering the potential to enhance patient outcomes irrespective of the tumor's location. To maximize the effectiveness of these precision medicine strategies, the critical task at hand is to accurately identify which patient groups are likely to respond positively and which may encounter resistance [61]. In essence, the objective is to strike a balance between achieving an effective response from the tumor and safeguarding the patient's organ function and overall quality of life and to offer more advanced and patient-centric care to individuals facing cancer [62].
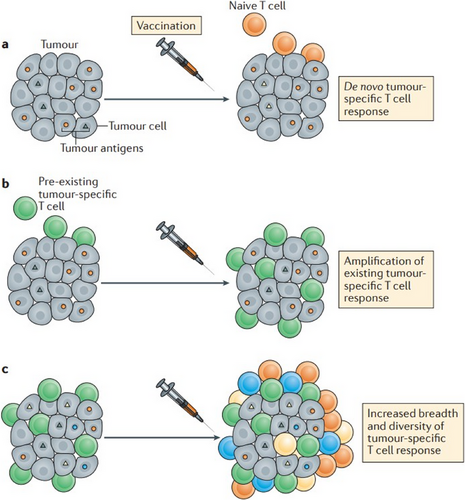
- Therapeutic vaccines: Lately, there has been a notable upswing in the exploration of cancer vaccine therapies that are intricately linked to the TME [64]. These vaccines possess the ability to identify precise antigen targets within tumor cells, inciting the development of fresh antigen-specific T-cell responses against the cancer, effectively guiding the immune system to recognize and engage with the malignancy (Figure 4) [65]. DCs, sourced from the patient's blood or generated in a lab, are loaded with tumor-specific antigens. Once reintroduced into the patient, they activate T cells, including CD4+ helper T cells and CD8+ cytotoxic T lymphocytes (CTLs) [64]. These activated T cells boost the immune response, specifically targeting and eliminating cancer cells with the same antigens [64, 65]. Furthermore, cancer vaccines can fortify and amplify existing T-cell responses that have previously been sensitized to identify the tumor, effectively providing these immune defenders with a potent reinforcement to further assail the cancer (Figure 4) [65]. These vaccines also contribute to enhancing the diversity and breadth of T-cell responses against the tumor (Figure 4). This, in turn, bolsters the immune system's capacity to confront and combat the tumor from various vantage points [65].
As immunotherapy is a ceaseless area of research, many clinical trials are undergoing, and the emerging therapy is continuous. Thus, knowing it helps to overcome resistance and to know other alternatives (Tables 1 and 2).
| Agents and trial identification number with phase | Target | Combined with | Type of tumor | Expected outcome from the trial |
|---|---|---|---|---|
|
IPILIMUMAB [67] NCT02339571 Phase 2/3 |
CTLA-4 | Nivolumab and GM-CSF | Advanced melanoma | To compare overall survival of nivolumab and ipilimumab when given with or without GM-CSF |
|
PEMBROLIZUMAB [66] NCT04708418 Phase 2 |
PD-1 | CMP-001 | Operable melanoma | Effect of pembrolizumab alone or in combination with CMP-001 in surgically operable melanoma |
|
DABRAFENIB [67] NCT04557956 Phase 2/3 |
BRAF | Tazemetostat | Metastatic melanoma | Investigates best dose, possible benefit, and side effect by combining tazemetostat with dabrafenib |
|
DAROMUN NCT03567889 Phase 3 |
Antibody–cytokine fusions as active principle (L19IL2 and L19TNF) | Surgery and adjuvant therapy | Stage 3b/c melanoma | To study efficacy of surgery alone or with daromun adjuvant therapy |
|
BOTENSILIMAB (AGEN1181) NCT05529316 Phase 2 |
Promotes optimized T-cell priming | Botensilimab | Advanced melanoma | Efficacy of botensilimumab alone or in combination with balstilimab |
| Agents and trial identification number with phase | Target | Combination | Type of tumor | Expected outcome from the trial |
|---|---|---|---|---|
PEMBROLIZU MAB NCT05721755 Phase 3 |
PD-1 | Combined with radiation therapy | Metastatic squamous cell carcinoma of head and neck | Safety, tolerability and effects of Pembrolizumab alone versus Pembrolizumab with BNT 113 |
|
NIVOLUMAB NCT03767348 Phase 2 NCT04423029 Phase 1/2 |
PD-1 | Combined with RP1 (genetically modified herpes simplex type 1 virus) Combined with DF6002 |
Locally advanced or metastatic non-melanoma skin cancer Cutaneous squamous cell carcinoma |
Evaluate the safety, tolerability, and side effects of DF6002 alone or in combination with nivolumab |
|
AVELUMAB the CARTA Study NCT04792073 Phase 2 |
PD-L1 | Ablative radiation therapy and immunotherapy | Merkel cell carcinoma | Study of avelumab with or without radiation therapy |
|
TISLELIZUMAB NCT04666688 Phase 1/2 |
IgG4 anti-PD-1 monoclonal antibody |
Combined with LYT-200 | Locally advanced or metastatic tumor | LYT-200 with tislelizumab or LYT-200 with chemotherapy |
1.3 Emerging Immunotherapies
- - BRAF and MEKis have a high overall survival rate, but it does not significantly affect BRAF-mutated melanoma. BRAF and MEKis both act by inhibiting NRAS–ERK pathway, thus resulting in inhibition of gene transcription. Even with BRAF-mutated melanoma, MEKi works and inhibits the pathway.
- - CTLA-4 inhibitor (ipilimumab) with BRAFi (vemurafenib/dabrafenib/trametinib)—CTLA-4 inhibitor is the immune checkpoint inhibitor when combined with BRAFi, blocks at two sites. Thus, making it useful even in BRAF-mutant melanoma. This combination was associated with severe liver or gastric toxicity. Replacing CTLA-4 inhibitor with PD-1/PD-L1 blockers overcame the side effect.
- - PD-1 antibody, spartalizumab dabrafenib, and trametinib lead to 78% survival in advanced BRAF-mutant melanoma. PD-1 antibody acts as immune checkpoint inhibitor.
- - Triple blockade therapy with PD-L1 antibody, BRAF, and MEKi showed better survival outcomes.
- - Combined VEGF inhibitor (apatinib) and IgG4 monoclonal antibody against PD-1.
Bempegaldesleukin (NKTR-214) is a pegylated IL-2 that binds to CD122, preferably than CD25, leading to activation of T cells and NK cells, thus reducing toxicity from Treg activation. It is best tolerated with nivolumab and has good clinical efficacy and improved overall survival rate.
Pegylated IFNa-2b combined with pembrolizumab, T-VEC with pembrolizumab, and T-VEC with ipilimumab were used for treating advanced tumors and showed improvement in overall survival rate. Adoptive cell therapy (ACT) is used for refractory or non-tolerant patients with first-line therapy. Lifileicel (LN-144) cryopreserved autologous TIL therapy. Combining ACT with TIL and IFN-a has a better survival outcome [40] (Table 3).
| Target | Monoclonal antibody against target |
|---|---|
| LAG-3 (lymphocyte activation gene-3, CD223) |
Relatlimab—first FDA-approved anti-LAG-3 mab in combination with nivolumab Ieramilimab added to spartalizumab induces a response in LAG+ metastatic melanoma |
| TIGIT (T-cell immunoglobulin and ITIM domain) | Tiragolumab, domvanalimab, vibostolimab |
| TIM-3 (T-cell immunoglobulin and mucin domain containing molecule-3) | Cobolimab and sabatolimab |
| VISTA (V-domain immunoglobulin suppressor of T-cell activation) | KVA12123, W0180, CI-8993 |
| CD27 | Varlimumab |
| BTLA | Icatolimab |
IDO IO102-IO103 is a dual peptide vaccine for metastatic melanoma [68].
Increase in the understanding of the immunological mechanism involved in melanoma, such as T-cell receptors and also CD 137 and CD 27, is being studied. And more trials are also being conducted on targeting co-receptors such as IDO, TIM-3, and LAG-3 as immunosuppressive therapy. Promising therapy from T-VEC, GM-CSF induces T-cell response. Intramural injection of T-VEC has improved response rate than GM-CSF in a Phase 3 clinical trial [69].
1.4 Monitoring and Early Intervention
Immunotherapy has its own adverse effects on patients. Starting from fever, chills, weakness, dizziness, and nausea, the adverse effects may go up to swelling, palpitations, diarrhea, and allergic reactions. Therefore, to reduce patients going into such complications, they should be treated in a monitored way. Regular monitoring of immunotherapy is essential for identifying the efficacy of the immunotherapy drug.
The most common form of non-invasive monitoring of immunotherapy drugs is by MRI scans, CT scans, and PET scans. Scans taken at regular fixed intervals help in determining changes in the size of the tumor and the progress of the immunotherapy. Newer imaging technologies that include radiotagging antibodies targeting CTLA-4, programmed cell death protein-1, and tumor necrosis factor (TNFSF4, OX40) receptor tagging have made it easier to identify drug distribution and response [70]. This will help in identifying pseudoprogression or mixed response of the tumor. A successful immunotherapy will show a reducing size and reducing spread of the tumor.
Though imaging studies have the limitation of differentiating tumors from local inflammatory responses, a combination of multiple imaging modalities has been promising for the future. Skin cancer has been increasing in our society due to newer lifestyle habits and increased UV radiation. Therefore, screening has become essential for high-risk groups because melanoma has become the eighth biggest cause of cancer-related death. Squamous cell carcinoma is the second most common. Squamous cell carcinoma has shown a significant response with immune checkpoint inhibitor and hypofractionated radiation therapy in advanced stages. Early diagnosis is key to the treatment of this disease; early excision of the tumor with a border of 1–2 mm of normal tissue has a higher chance of complete recovery.
Emerging biomarkers and advanced imaging techniques offer promising tools for monitoring mechanisms related to hypoxia, antigen presentation, and the PD-1/PD-L1 pathway, all of which are essential in understanding and addressing immunotherapy resistance. Imaging methods like [^18F]-FMISO PET scans can be used to non-invasively evaluate tumor hypoxia, which is known to be a factor in immune evasion and treatment resistance. By identifying hypoxic tumor locations, these scans aid in the optimization of treatment plans [71]. Analyzing biomarkers like the expression of MHC molecules and antigen-processing machinery components can also be used to assess the effectiveness of antigen presentation pathways. These indicators shed light on the tumor's capacity to successfully deliver antigens, which is essential for the effectiveness of immunotherapy. Additionally, new insights into the PD-1/PD-L1 axis have uncovered a variety of regulatory mechanisms that affect expression levels and treatment results, including as genetic, epigenetic, and post-translational alterations. The ability to anticipate responses and more precisely customize immunotherapy therapies may be improved by integrating these new methods and biomarkers into clinical practice [72].
1.5 Case Studies and Success Stories
Numerous case reports have shown that primary prevention plays a major role in treatment of skin cancers than their early detection. A recent study showed that in patients with melanoma who underwent treatment with ipilimumab as well as placebo, it was observed that there was a recurrence-free survival of 26.1 months in the ipilimumab group as compared to 17.1 months in the placebo group [73].
There have been outstanding advances in immunotherapy that have generated favorable clinical responses and even remision in skin cancer patients [70]. Even with these groundbreaking progress in immunotherapy, there is always a possibility of development of resistance even with these novel treatments.
A recent case study showed that patients who develop resistance to one immunotherapy modality can show successful response when treated with a combination of dual immune checkpoint blockade and radiation therapy. In another recent clinical trial, cancer patients were randomly assigned to receive nivolumab or ipilimumab or a combination of both drugs, and response was seen after a duration of 6.5 years after treatment. The subjects who received nivolumab–ipilimumab in combination had a lower mortality rate and had no progression of disease [70].
A combination treatment that targets BRAF and MEK pathways, specifically vemurafenib and cobimetinib, was also approved in 2015 to treat patients having metastatic or unresectable melanoma with BRAF V600E or V600K mutation to reduce risk of development of resistance [74]. T-VEC is another viral oncolytic immunotherapy used as monotherapy or in combination with immune checkpoint inhibitors to attenuate resistance mechanisms in tumors [75, 76]. A recent trial is also investigating the role of combination therapy with targeted therapies like dabrafenib (a BRAFi) and trametinib (an MEKi) with immunotherapy agents like anti-PD-1 therapy, pembrolizumab, to counter drug resistance [77].
Besides, the KEYNOTE-942 trial has introduced a combination of mRNA-4157/V940 vaccine and pembrolizumab to investigate its role in reduction of the risk of tumor recurrence post-surgery [78].
Lastly, newer treatment modalities, for example, neoantigen vaccines, including DCs, viral vectors, RNA, and peptides, TIL therapy, nonparticle-based combination therapy for melanoma, DNA damage response inhibitors, and lymphocyte-activation gene-3 inhibitors, are emerging therapies that are being explored either as monotherapy or combination strategies to tackle development of drug resistance and discover promising tools in the coming future of cancer research [44, 79-82].
2 Conclusion
Improvements in immunotherapy have given patients with skin cancer amazing clinical responses, but resistance is growing. To determine the immunotherapy drug's effectiveness and progress, regular monitoring is necessary. Numerous factors contribute to the increase in skin cancer cases. Since 1800, immunotherapy has advanced to include, for example, the use of immune checkpoint inhibitors to improve overall patient survival. It is extremely important to comprehend and overcome resistance with immunotherapy where numerous clinical trials are ongoing. A higher overall survival rate and good clinical efficacy are achieved by various combination therapies. By changing TME's metabolism, tumor cells add to immunotherapy resistance. Regulating PD-1/PD-L1 axis by decreasing PD-L1 is used by cancerous cells to evade antitumor reactions and enhance the immune system's ability to combat tumors. Tumor cells receiving immunotherapy may become resistant to the treatment by altering or downregulating IFN-γ signaling pathways, which circumvents the effect of IFN-γ and consequently lowers PD-L1 expression. One way that hypoxia signaling impairs T-cell function is by causing myeloid suppressor cells to express PD-L1. Low-oxygen levels cause lactate accumulation in cancer cells, which causes the TME to become acidified, inhibiting effector T-cell proliferation and decreasing IFN-γ production. A putative regulatory point, PSGL-1, prevents TCR signaling in worn-out CD8+ T cells, which lowers pro-inflammatory IL-2 and raises PD-1 levels. CAFs prevent T cells aimed at the tumor from being primed. Melanoma treatment faces significant challenges due to resistance, influenced by factors within and outside the cancer cell. A few strategies are to be considered when treating skin cancer patients who are resistant to immunotherapy. Targeting PSGL-1 essentially strengthened the immune response against melanoma, suggesting that melanoma immunotherapy could be improved as blocking TIGIT, TIM-3, and LAG-3 pathways. In certain cases, combining various immunotherapies—such as checkpoint inhibitors—can be more beneficial than employing just one medication. In melanoma targeting with immune checkpoint blockade, for instance, combining anti-PD-1 and anti-PSGL-1, or inhibiting CDK4/6 or combining CDK5, has demonstrated improved response rates. Immunotherapy's benefits may be increased by STAT3 inhibition. Cytokines, such as IL-2, provide a variety of strategies for immune system activation, regulation, and homeostasis with possible therapeutic advantages. By focusing on molecular traits, precision personalized medicine strategies accurately identify which patient groups are likely to respond favorably and which may experience resistance. By eliciting a variety of T-cell responses that specifically target tumors, cancer vaccines work together to cause tumors to recede.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.




