Deciphering Aging, Genetic, and Epigenetic Heterogeneity in Cancer Evolution: Toward Personalized Precision Preventative Medicine
Funding: This study was supported by the National Cancer Institute of the National Institutes of Health under award numbers U01CA271830, U01CA271830-03S1 and P30CA014089. Sheng Li is a recipient of Career Development Award (1398-25) of The Leukemia & Lymphoma Society.
ABSTRACT
Background
Cancer's inherent ability to evolve presents significant challenges for its categorization and treatment. Cancer evolution is driven by genetic, epigenetic, and phenotypic diversity influenced by microenvironment changes. Aging plays a crucial role by altering the microenvironment and inducing substantial genetic and epigenetic heterogeneity within an individual's somatic cells even before cancer initiation.
Objectives
This review highlights the clinical significance of epigenetic mechanisms in cancer evolution, focusing on hematopoietic and solid tumors. The review aims to explore opportunities for integrating evolutionary principles and data science into cancer research.
Methods
The review synthesizes recent advancements in omics technologies, single-cell sequencing, and genetic barcoding to elucidate epigenetic mechanisms and aging's role in cancer evolution.
Results
Epigenetic mechanisms' high plasticity generates heritable phenotypic diversity, driving malignant evolution toward poor prognosis. Advances in single-cell sequencing and genetic barcoding enable the precise detection and tracking of biomarkers, allowing early, personalized interventions. Incorporating data science into cancer research has the potential to map, predict, and prevent cancer evolution effectively.
Conclusion
Understanding cancer evolution through novel technologies and data analysis offers a proactive approach to cancer prevention and treatment. By predicting key evolutionary events and leveraging personalized strategies, patient outcomes can be improved, and healthcare burdens reduced, marking a transformative shift in oncology.
1 Introduction
The significant challenges in categorizing and treating cancer arise from its natural ability to change over time. This results in the continuous development of cancer cell populations that are genetically, epigenetically [1], and behaviorally diverse [2, 3]. The process of cancer evolution requires heritable phenotypic variations and natural selection, which is often impacted by drastic tissue microenvironmental changes, such as aging, smoking, and therapy.
In the realm of cancer clonal evolution, it is imperative to recognize the significant role of aging. As individuals age, they accrue thousands of somatic mutations per cell [4]. Given the vast number of cells in an adult body, this mutation rate effectively spans the entire genome on average. The resulting mosaic composition of cells in an aging individual not only fosters genetic heterogeneity but also instigates epigenetic heterogeneity due to mutations in genes regulating epigenetics, for example, TET2 and IDH2 mutations [5-7], and nonmutational epigenetic reprogramming [8-12]. Notably, the phenotypic outcomes of most mutations remain obscure. For example, 97% of TET2 mutations recorded in ClinVar database are classified under the “unknown significance” category [13]. Recent attention among cancer evolution researchers has been directed toward the functional roles of epigenetic heterogeneity in cancer evolution. Traditionally, cancer evolution has been investigated through somatic mutations, particularly with a spotlight on the P53 gene, “the guardian of the genome,” as a source of the inherited variations needed for evolution. Loss of function in P53 has been associated with an increased rate of somatic mutations, contributing to cancer evolution. Epigenetic mechanisms have been shown not only to diminish P53 activity, leading to weakened DNA repair checkpoints and heightened genotypic heterogeneity that fosters selection and cancer evolution [14], but also to serve as a source of phenotypic heterogeneity among cells of the same genotype, facilitating natural selection and cancer evolution, similar to the evolution as a result of genetic heterogeneity. The rise in such heterogeneity, often attributed to mutations in the genes encoding “the epigenome guardians” like TET2 and DNMT3A, has been linked to poor disease prognosis, particularly observed in leukemia [15].
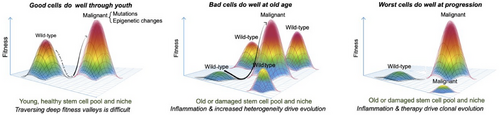
Besides promoting heritable genetic and epigenetic somatic heterogeneity, aging plays a pivotal role in shaping the microenvironment [16] and influencing selection processes. Whether a genetic mutation or an epigenetic alteration leads to a pathogenic state depends on the surrounding milieu [17-19]. The aging environment can either amplify or dampen the selection of mutations [20], and by similar reasoning, it may influence the selection of epigenetic patterns. Therefore, to fully comprehend the role of aging, it is imperative not only to identify genetic mutations or epigenetic patterns associated with pathogenic phenotypes but also to examine their selection dynamics during the aging process. The role of aging and epigenetic heterogeneity in cancer evolution is illustrated in Figure 1.
We are in a favorable era to shift from merely observing cancer evolution to predicting it [21], targeting it [22], and rethinking its treatment, thanks to notable technological advancements. Innovations like single-cell long read-sequencing, for example, ONT and PacBio, and genetic barcoding techniques, for example, cell-tag indexing [23] and LARRY [24], provide unprecedented opportunities. These techniques enable the simultaneous detection of single-nucleotide polymorphisms (SNPs) and epigenetic changes at the single-cell level, allowing for the identification and tracking of the evolution of specific clones. These breakthroughs will ultimately empower us to identify and comprehend the pivotal events in cancer development, ultimately enhancing strategies for cancer diagnosis, treatment, and prevention.
2 Cancer as an Evolutionary Process
For decades, cancer research has primarily delved into dissecting individual genetic or epigenetic changes implicated in cancer initiation and progression, reflecting the somatic mutation theory of cancer [25]. However, this simplified model has limitations, emphasized by the lack of explanatory power of known cancer-related genes. Intriguing results have revealed that certain crucial genes that are known to be oncogenes may function as tumor suppressors in other cases [26-28]. Moreover, it has been shown that modifying the microenvironment can revert cancerous cells or tissues to a noncancerous state [29, 30], regardless of the mutations they harbor. Additionally, numerous studies have identified a significant burden of mutations in many noncancerous tissues, despite the absence of any apparent pathological consequences [31-33]. These and other studies suggest a shift toward viewing cancer as a holistic system, akin to a new species evolution, where both the entire genome (i.e., rather than a few focal changes) and the environmental factors determine the malignant progression of a clone.
Different evolutionary models have been proposed to explain distinct patterns observed in cancer evolution [34]. These models include the linear model [35] where mutations are acquired gradually in a linear fashion, leading to increasingly malignant stages of cancer; the branched model in which clones diverge from a common ancestor and evolve independently within the tumor mass, resulting in multiple clonal lineages [36]; and the neutral model [37], which posits that there are no fitness changes during most of the tumor's lifetime, and random mutations accumulate over time, leading to genetic drift. These three models presuppose a sequential and gradual accumulation of mutations over time. A fourth model, the punctual equilibrium model, proposes that macroevolution is marked by abrupt genetic changes occurring in brief, intense bursts, propelling accelerated tumor progression, interspersed with extended periods of stability. These stability periods are interpreted as genuine interruptions, underscoring the intricate, nondeterministic essence of evolution—making it the most challenging model. The paleontologists Niles Eldredge and Stephen Jay Gould introduced a model in their groundbreaking 1972 publication, in which they contested the prevailing notion of gradual speciation by Charles Darwin, proposing instead this punctuated equilibrium model [38]. Several recent studies in breast cancer [39] and other cancer types lend credence to the alignment of cancer evolution with this paradigm [40-42]. In a recent study [43], patterns of evolution were experimentally observed using the in vitro immortalization model [44]. H. Heng et al. observed two distinct stages of evolutionary patterns in this Li-Fraumeni fibroblast cellular model: punctuated and gradual evolution [43]. All these studies emphasize the significant departure from traditional cancer research approaches and the urgent need for a comprehensive reassessment of cancer modeling, prediction, and treatment in the context of aging microenvironment, as summarized in Figure 2.
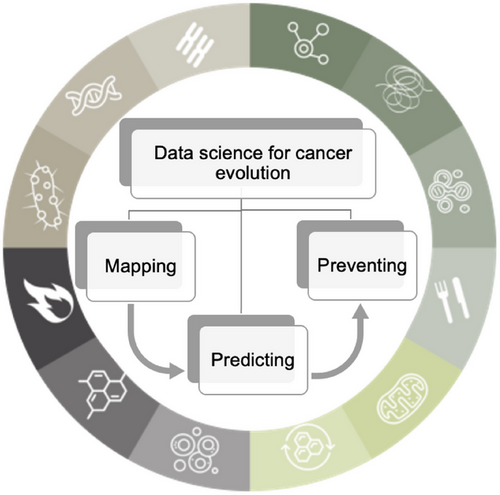
2.1 Mapping Cancer Evolution: The Whole Genome-Epigenome Architecture Combined With the Microenvironment Determines the Phenotypic Outcome
Transitioning away from traditional simplistic models, which focus solely on individual genetic or epigenetic changes, requires extensive biological data collection from both healthy and diseased individuals. These data should encompass a wide array of biological information, including whole genome sequences, transcriptomes, and epigenomes, while recording factors such as age and the microenvironment. The aim is to move toward a more comprehensive understanding of the genome–epigenome architecture, rather than studying isolated changes, in order to accurately elucidate and predict cancer initiation and progression, as discussed further in the following subsection.
This shift has already begun with the adoption of whole genome sequencing and exome sequencing. However, this significant transition needs to be complemented by comprehensive data collection at other levels, particularly focusing on epigenome alterations. The importance of epigenetics in cancer evolution has recently gained recognition. Mutations in genes encoding epigenetic regulators have been shown to trigger epigenetic heterogeneity among genetically identical cells, thereby introducing a novel mechanism of cancer evolution [46]. Epigenetic perturbations are inherited, have high plasticity, and are stimulated by the environment [1]. Such evolutionary mechanisms manifest across various cancer types, including hematopoietic and solid tumors [47-50]. Epigenetic aberrations and alterations in chromatin states confer significant oncogenic properties, fulfilling multiple hallmarks of cancer such as proliferative signaling [51], evasion of growth suppressors [52], invasion and metastasis [53], replicative immortality [54, 55], angiogenesis [56], and resisting cell death and apoptosis [57-59]. Furthermore, their presence has been correlated with poor prognosis in different cancers [60, 61], attributed to the remarkable plasticity of the epigenome, which enables the epigenome to dynamically adapt to changing environmental cues and therapeutic pressures [62]. A plasticity that creates one-to-many genotype-to-phenotype mapping can provide insight into cancer drug resistance that is mostly unexplained by genetics alone [63]. The independent role of epigenome heterogeneity in cancer evolution, its high plasticity leading to poor prognosis and drug resistance evolution, and its reversible nature underscore the effectiveness of simultaneously measuring whole epigenome alterations alongside whole genome changes.
By amassing comprehensive data on whole genome and epigenome perturbations, as pioneered in the EpiMap project [64], spanning health, disease, and aging, particularly with the recent strides in whole genome–epigenome single-cell sequencing, we gain a treasure trove of insights. These data, often disregarded as noise in simplistic somatic mutation theory models, harbor valuable information crucial for unraveling the intricate dynamics of health and disease progression. By leveraging computational and statistical models, we can effectively identify significant mutual information and correlations within these heterogeneous datasets, shedding light on the complex interplay of factors across different stages of health and disease.
2.2 Predicting Cancer Evolution: Age-Dependent Trajectories and Transition States Timing
The new direction in cancer research needs to transition from just observing cancer evolution and mapping data to modeling its evolutionary journey and to inferring early-stage predictions. Aging plays a vital role in this journey, significantly shaping the environment where natural selection operates. Aging leads to various changes in the microenvironment, for example, deregulated nutrient sensing, impaired intracellular signaling, increased cellular senescence, and stem cell exhaustion [16]. These alterations in the microenvironment exert varying selective pressures on evolving clones. Additionally, aging provides the time necessary for gradual evolutionary patterns to unfold. This underscores the importance of longitudinal testing in both healthy and diseased populations. By analyzing longitudinal (time-series) data, we can track how individuals change over time with age, as well as uncover differences and similarities in these patterns among individuals in a population [65]. Such inter- and intraindividual analyses would aid in predicting the paths in both healthy and diseased individuals, as well as in anticipating the intricate factors and timing that lead to a sudden evolution or a transitional state.
We have introduced the various evolutionary patterns in cancer, where they can have gradual or punctual nature, or even a combination of both in two-stage models. To accurately describe each pattern, different models are needed [66]—for instance, the multistate life table model [67], which treats change as a Markov process and calculates transition probabilities between predefined discrete states in the punctuated evolution model. These states are defined by complex factors including phenotype, genome/epigenome perturbations, and age. To establish well-defined states, extensive data collection is necessary to compute accurate transition probabilities. This not only predicts likely transitions but also identifies factors that could lower transition probabilities, offering preventive measures when possible. For gradual evolutionary patterns like linear, branched, and neutral models, continuous distribution functions [68] are more suitable. These functions represent continuous trajectories across various ages, independent of predefined discrete states. Such continuous models are applicable to gradual changes observed in healthy individuals, where somatic mosaicism increases significantly with aging [4], allowing for the prediction of significant upcoming changes. Moreover, viewing cancer through the lens of an evolutionary process necessitates a paradigm shift in treatment approaches, such as employing game theory to model cancer treatment. This modeling of the treatment can minimize a tumor evolution toward drug resistance [69].
Recent efforts have been directed toward modeling epigenome heterogeneity, employing techniques such as the Ising model. This approach has shown success in modeling heterogeneity and extracting insights from the stochastic nature of methylation data, particularly focusing on single-cell whole-genome methylation data [70, 71]. This model has also been applied to analyze 3D genome structure [72]. By analyzing clinical single-cell whole-genome methylation data from acute lymphoblastic leukemia (ALL), this model demonstrated success in modeling methylation heterogeneity in ALL and predicting its diver [73]. Methylation heterogeneity within different genes was calculated using Shannon entropy across the different samples. Through network analysis of the perturbed genes, researchers identified the UHRF1 gene as a central node driving epigenetic heterogeneity and ALL evolution.
Investigating the combined genetic mutations and epigenetic configurations that facilitate the transition from wild type to malignant clones, as well as identifying epigenetic configurations associated with therapy resistance, holds significant potential for targeting cancer evolution and shaping disease trajectory. The reversible nature of epigenetic alterations further underscores the importance of targeted interventions aimed at restoring normal epigenetic states and reversing cancer-associated changes [46].
2.3 Preventing Cancer Evolution—Unlocking Nature's Successes
Rather than solely studying cancer evolution, there is an urgent need to expand our focus toward preventing this evolution, given the growing global aging population. Human families exhibit significant diversity in their resistance to cancer; while some families rarely experience cancer, others have a higher predisposition to it [74]. This epidemiological evidence suggests that certain genetic backgrounds are more adept at impeding cancer evolution [75]. By studying families with lower incidences of cancer, researchers can identify genetic factors and molecular pathways that confer resistance to cancer evolution. The Albert Einstein College of Medicine's “Longevity Gene Project” is a distinctive study involving long-lived, cancer-free individuals aged between 95 and 112 [76]. Through the analysis of SNPs in these healthy elderly participants and their children, researchers aim to uncover genetic factors contributing to their long lifespans. Another notable research initiative is the Aging Atlas study [77]. Its objective is to furnish researchers with extensive multiomics data, encompassing genomics, epigenomics, transcriptomes, proteomes, metabolomics, and pharmacogenomics, both at population and single-cell levels. This endeavor aims to investigate age-related changes across various biological levels [77].
Moreover, investigating longevity among different species would bring more insight into cancer prevention [78]. Notably, the spectrum of cancer resistance varies considerably among our relatives across the tree of life. Certain mammals, such as the naked mole rat, have evolved to be highly resistant to cancer. Leveraging the successful mechanisms of species that have effectively prevented cancer adds a unique dimension to understanding cancer evolution. Numerous studies attempted to learn the cancer prevention mechanisms from the species over the evolutionary spectrum that independently evolved to be almost resistant to cancer, for example, certain mole rats [79, 80], bats [81], elephants [82], and whales [83]. Extensive research has explored the resistance mechanisms of the naked mole rat, revealing its stable epigenome, hypersensitivity to cell contact inhibition, unique immune system, efficient DNA repair pathways, and other distinctive resistance mechanisms [84-90]. Elephants were shown to have multiple copies of the TP53 gene responsible for enhanced apoptosis and duplications in other genes related to apoptosis, cell cycle, DNA damage repair, cell resistance to oxidative stress, and TP53 regulation [82, 91–94]. Whales, including the bowhead whale that lives more than 200 years, have shown positive selection in genes related to cell cycle and DNA repair, as well as pathways associated with cell proliferation, metabolism, and apoptosis [83, 95, 96]. A substantial portion of these mechanisms are associated with either stable genome and epigenome or with controlled microenvironment, which blocks cancer evolution mechanisms—heritable variations and natural selection. This highlights the significance of focusing on epigenetic and genetic diversity as well as the microenvironment as a strategy to impede cancer evolution.
Each of the aforementioned studies tried to learn the resistance mechanisms within individual species, which may only be relevant to those specific species. However, to effectively translate these inhibition mechanisms to humans, we need to learn the global mechanisms that have independently evolved among phylogenetically distant resistant species, which are likely applicable to various organisms, including humans. We previously identified genes whose conservation either positively or negatively correlated with a species’ proxy estimate of cancer resistance [97]. Resistance to cancer has been estimated based on Peto's paradox, where cancer incidence rate does not increase with the species size or lifespan, despite the need for a greater number of cell divisions. It follows that the intrinsic level of cancer resistance in a given species needs to roughly counteract its risk of cancer development due to cell division, which, according to a simple cancer development model, is proportional to the sixth power of lifespan multiplied by the adult weight [93, 98, 99]. Another measure of cancer resistance, termed “maximum longevity controlled for adult weight,” was developed based on the well-established correlation between lifespan and body size [100]. Fist, a linear regression was performed between species’ lifespans and body weights. Then, for each species, this measure was calculated from the residual obtained by regressing out the weight from the lifespan. Genes exhibiting correlated conservation with estimates of cancer resistance were found to be enriched in pathways primarily associated with tumor suppression, notably those involved in DNA repair. Given the remarkable plasticity of epigenetic alterations, a promising approach to prevent cancer evolution involves understanding how to preserve a stable epigenome. It is noteworthy that epigenetic heterogeneity can occur even among genetically identical cells, where the source of a specific epigenetic pattern is nongenetic, yet the level of heterogeneity is genetically determined, as we [7] revealed that somatic mutations drive epigenetic heterogeneity in leukemia patients with poor prognoses. The complete genetic underpinnings of epigenetic heterogeneity remain elusive. However, there is a significant potential to learn the genetic underpinnings of a maintained epigenome or the “epigenetic stabilizers” from cancer-resistant species. For instance, one of the mechanisms employed by the naked mole rat to prevent cancer evolution involves the highly maintained epigenome, as evidenced by studies showing slower progression of their DNA methylation clocks [101-104]. In an era witnessing an exponential increase in genomic and epigenetic data, the epigenome and single cell data of different species will be available [105, 106], introducing an opportunity to predict global epigenetic stabilizers with potential implications for humans. This presents a significant avenue for the development of preventive medications that emulate the mechanisms of action of these epigenetic stabilizers.
We believe that a lot of cancer protective mechanisms can be learnt by generating cross-species comparative analysis across various layers of biology, including whole genomes, gene expression, alternative splicing, regulatory genome, epigenome, and more. This will hold huge potential of transferring the resulting knowledge to enhance our resistance beyond the natural confines of our genetics.
3 Discussion
3.1 The Transition Toward Preventive Approaches Became Possible by Bringing Cancer Evolution Into Oncology Combined With Rigorous and Reproducible Data Science
Advancements in single-cell whole-genome/epigenome sequencing, coupled with technologies and algorithms, have significantly enhanced our ability to trace and predict the history of clonal evolution. One notable achievement is the development of the GRITIC algorithm by the Van Loo group, which can predict the timing of tumor somatic mutations [107] utilizing single cell whole genome data. Technologies like mitoMuVEH facilitate clonal identification and genetic sequencing at the single-cell level [108]. These advancements have ushered in a new era, enabling a shift from reactive cancer treatment approaches to proactive cancer prevention strategies. By allowing early predictions of pathogenic cell subpopulations that could lead to malignancy, these technologies support early and targeted interventions. This paradigm shift requires early intervention and personalized approaches, made feasible by the integration of cancer evolution science into oncology practice. The vast amount of data generated by these technologies, combined with advancement in analytical approaches as discussed in Section 2.3, underscore the potential for revolutionizing cancer prevention efforts and improving patient outcomes.
In this transition, several levels of data comparisons need to be leveraged. First, longitudinal data analysis is imperative, encompassing both interindividual comparisons to glean insights from the diverse trajectories, such as tumor evolution, therapy success, drug resistance, or even a success to avoid a predicted tumor, and intraindividual analyses to predict personalized evolutionary trajectories as a function of genome/epigenome configuration and aging. Additionally, we explored the potential for learning from cross-species comparisons (Figure 3). Given the huge variations in cancer resistance and longevity among other species, there is much to be gleaned from understanding both species-specific and general evolved mechanism of cancer resistance. This knowledge base, enriched by the higher genetic diversity and cancer resistance variations found in even phylogenetically nearby species, surpasses the limited variations present within the human population. An excellent instance of translating insights from long-lived species into life-extending interventions is exemplified by the research led by Vadim Gladyshev's lab. They conducted multitissue RNA-seq analyses across 41 mammalian species, identifying longevity signatures and exploring their connection with transcriptomic biomarkers of aging, as well as established lifespan-extending interventions [109].
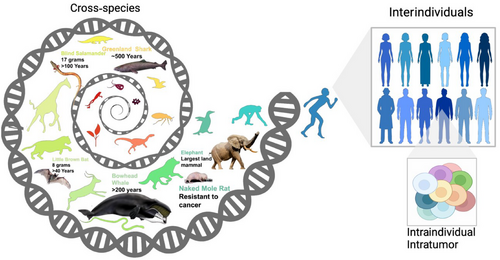
Learning from these three hierarchical levels of comparative genomics can facilitate the development of preventive measures and interventions at the earliest possible stages, thereby improving patient outcomes and reducing healthcare burdens. Transparent AI models [110] that are developed based on the integration of the three hierarchical levels of genome and epigenome architecture [7] are also expected to play a critical role in predicting cancer evolution.
3.2 Bone Marrow Diseases: A Viable System of Investigating Evolution and Heterogeneity
Understanding and preventing cancer evolution necessitate analyzing the tremendous genetic and epigenetic changes induced as we age at the single-cell level. One major challenge in this context lies in obtaining longitudinal single-cell data capable of capturing this vast heterogeneity among cells, especially to identify specific cells with early signs of pathogenicity in the aged individuals [4, 111]. Nonetheless, an accessible and fruitful avenue for exploring this heterogeneity is through hematopoietic stem cells (Figure 4), given the ease of accessing blood samples combined with targeted, error-corrected sequencing techniques [111, 112].
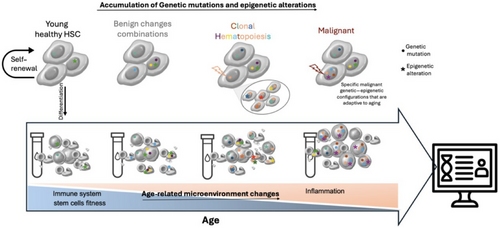
Blood cells have a rapid turnover rate, providing a quick selective advantage for mutations compared to cells with longer turnover rates, highlighting the significance of phenotyping these mutations. Additionally, the effects of these mutations are widespread, with differentiated cells fulfilling various functions such as oxygen transport, immune defense, and blood clotting. In recent years, clonal hematopoiesis of indeterminant potential (CHIP), which reflects the clonal expansion of an early hematopoietic progenitor marked by a putative driver mutation, has drawn significant attention [113, 114]. CHIP has been linked to various age-related diseases such as leukemia and cardiovascular diseases, presenting a new key player for investigation [115]. Remarkably, the top three mutated genes in clonal hematopoiesis are the epigenetic regulators encoding genes TET2, DNMT3A, and ASXL1 [116], underscoring the importance of studying epigenetic evolution and the ability to predict pathogenic epigenetic changes in advance. This has become feasible in humans with the assistance of clonal history inference approaches such as GRITIC [107] and mitoMuVEH [108].
3.3 The Ising Model: An Advocated Method for Modeling Genetic and Epigenetic Heterogeneity as a Function of Aging
Modeling heterogeneity is critical in gaining insights into the stochastic changes in the epigenetic landscape or genome architecture of cancer cells that drive oncogenic evolutions and uncovering potential pathogenic signaling pathways and therapeutic targets. Recent studies [71, 72] have quantitatively defined the epigenetic landscape employing unconventional approaches, such as the adaptation of the Ising model from physics that was traditionally used to understand the behavior of magnetic systems. In their adaptation, the researchers replaced the traditional up and down states of a magnet's spin with the methylated and unmethylated states. Such approach accommodates statistical interdependencies between methylation sites, focusing on the probability dynamics of methylation rather than the stochastic dynamics of methylation itself. These studies highlight the significance of this approach, as it adeptly handles the extensive statistical information inherent in methylation data, surpassing the limitations of conventional marginal methods. To accurately infer dynamic correlations among epigenetic patterns as a function of age and disease, similar approaches need to be followed in modeling other types of epigenetic data, such as ATAC-seq or RRBS.
Similarly, the generalized Ising model or the Potts model, derived from quantum statistical physics [117], should have great potential for applications in cancer and single-cell genomics chaos. This model has demonstrated efficacy across various biological domains, such as predicting protein interaction sites [118], determining the 3D structure of protein sequences [119], and assessing the pathogenic impact of mutations at the residue level [120]. With recent advancements in single-cell whole-genome sequencing technologies, it has become feasible to apply this model to single-cell data.
Additionally, while previous studies [71, 72] have analyzed the epigenome at a single time point, cancer evolution is an adaptive and longitudinal process. Therefore, it is imperative to invest additional effort into modeling the epigenetic and epigenetic heterogeneity as a function of age and environment. In the Ising model, this can be achieved by treating the temperature (T) parameter in the model as a variable to represent aging, rather than assuming it constant. By modeling temperature variation, we can not only forecast the longitudinal trajectory of epigenetic evolution but also anticipate the transition points like the transition between gradual and punctual evolutionary patterns. This prediction mirrors how the Ising model is utilized in physics to predict the critical temperature for the transition from one phase to another in materials, such as ferromagnetic materials.
Fourth, potential application of modeling heterogeneity using the Ising model could allow prediction of compensatory pathogenic epigenetic alteration or genetic mutations, providing additional clues for prevention. Identification of a pathogenic epigenetic pattern or a genetic mutation in the absence of a pathogenic phenotype in a cell implies the existence of another compensatory pathogenic pattern, which can serve as a viable preventative target when accurately predicted.
4 Summary
In this perspective, we have reviewed cancer as an evolutionary process driven by both inheritable changes and selective pressure from the microenvironment. We also examined how aging contributes to the accumulation of heritable changes and alters the surrounding environment. Furthermore, we discussed how recent technical advancements, when combined with computational models and data from cancer-resistant species or individuals, can help bridge the gap between cancer evolution science and its application in cancer therapeutic and cancer prevention, as summarized in Table 1.
| Cancer Evolution | Category | Key finding | Example studies | Implications |
|---|---|---|---|---|
| Cancer evolution requires heritable variations and selection | Genetic heterogeneity | Somatic mutations in TP53 enhance genetic diversity and drive tumor evolution. | [14] | Early detection and targeted therapy |
| Epigenetic heterogeneity | Mutations in epigenetic regulators enhance epigenetic heterogeneity in cells with identical genetics, contributing to cancer evolution, poor prognosis, and drug resistance. | [1, 5-12, 15, 46, 63] |
|
|
| Aging impacts selection | Aging increases the heritable genetic and epigenetic variations and reshapes the microenvironment. | [4, 17-20, 45] |
|
|
| From observing, to predicting, treating, and preventing cancer evolution | Technological advancement |
|
[21-24] |
|
| Toward cancer prevention—multiomics and big data |
|
[75-97] | Leveraging genetic factors and molecular pathways that confer resistance to cancer evolution within the super resistant individual and species | |
| Computational models |
|
[67, 68, 70-73] |
|
Through resolving the interplay of the key players—aging, epigenetic heterogeneity-tuning mutations, and the combined genetic mutations and epigenetic patterns that either suppress or enhance pathogenic evolution—we gain the ability to foresee the gradual malignant evolution or the likelihood of developing pathogenically burst changes and their expected occurrence timeframe. Armed with these predictions and a thorough understanding of preventive and therapeutic targets, personalized medical interventions can be strategically devised. This “manipulating evolution” approach involves targeting either the predicted genetic variants tuning epigenetic heterogeneity, the epigenetic patterns themselves, or the microenvironmental conditions that enable selection for these epigenetic-altering mutations or the resulting patterns. The future promise of this approach is profound: it opens the door to early, precise interventions that could prevent cancer from developing or progressing. With at least nine FDA-approved drugs for epigenetic therapy already available [1], we are on the cusp of a new era in oncology where proactive, personalized strategies can significantly improve patient outcomes and transform cancer care. As research and technology continue to advance, the potential to fine-tune these interventions and expand the arsenal of epigenetic therapies offers a promising horizon for combating cancer more effectively than ever before.
Author Contributions
Lamis Naddaf: conceptualization, writing–original draft, visualization, writing–review and editing. Sheng Li: conceptualization, writing–original draft, visualization, writing–review and editing, supervision.
Acknowledgments
We acknowledge the support of the National Cancer Institute of the National Institutes of Health under award numbers U01CA271830, U01CA271830-03S1 and P30CA014089. Sheng Li is a recipient of Career Development Award (1398-25) of The Leukemia & Lymphoma Society.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




