Considerations for the Use of the DNA Damage Marker γ-H2AX in Disease Modeling, Detection, Diagnosis, and Prognosis
Funding: We would like to acknowledge the Australian Government for the Research Training Program Stipend which provided funding to support one of the authors of this research (H.H.).
ABSTRACT
Background
DNA double-strand breaks are known to be the most lethal kind of DNA damage as they cause genomic instability. Visualization and quantification of these breaks are possible via staining of the phosphorylated histone H2A variant H2AX at serine 139 with the anti-phospho-histone H2AX (γ-H2AX) antibody.
Methods
The literature was searched with the following keywords: DNA damage, double-strand break, PBMC, DNA repair, H2AX, γ-H2AX.
Discussion
This review discusses various methods for the quantification of γ-H2AX and the use of γ-H2AX in cell culture, tissue biopsies, and peripheral blood mononuclear cells. The current research into basal DNA damage as quantified by γ-H2AX is discussed in relation to cancer.
Conclusions
This review suggests that γ-H2AX has the potential for use in the prediction of cancer risk when applied to healthy tissue and peripheral blood mononuclear cells.
1 Introduction
Every cell of the body experiences up to 100,000 DNA damage lesions every day [1]. This damage can be categorized as exogenous (environmental factors) or endogenous (cellular metabolic processes) [2, 3]. Exogenous damage occurs via exposure to ultraviolet radiation, ionizing radiation, and chemical agent, whereas endogenous damage occurs during cellular metabolism [2, 3]. The most lethal form of these breaks, DNA double-strand breaks, affects both strands of the double helix structure of DNA. If left unrepaired, these breaks can lead to cell death, chromosomal aberrations, mutations, and cancer development. When a DNA double-strand break is acquired, three phosphatidylinositol 3-kinase-like kinases: ataxia telangiectasia mutated (ATM), ataxia telangiectasia and Rad3-related (ATR), and DNA-dependent protein kinase (DNA-PK), catalyze phosphorylation of histone H2A variant H2AX at serine 139 (Figure 1) [4]. This forms γ-H2AX which is critical for the signaling of DNA repair proteins such as breast cancer gene protein 1 (BRCA1) and p53 binding protein (53BP1) to gather at the location of damage, forming DNA damage foci [5, 6]. These foci are present with double-strand breaks and disappear following their repair, allowing them to be utilized for the identification of DNA damage and repair. There are a variety of methods to assess DNA damage and repair (including DNA double-strand breaks); however, this review will identify the potential and limitations of using γ-H2AX for this purpose.
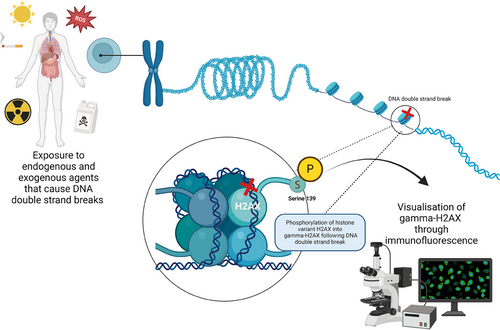
1.1 Evaluation Methods of DNA Double-Strand Breaks
Studies have developed several methods for the visualization and quantification of DNA damage. These include the micronucleus test, single cell electrophoresis (comet assay), halo assay, immunofluorescence microscopy, and flow cytometry.
1.1.1 Micronucleus Test
Micronucleus tests are a commonly completed analysis as there is no inherent requirement for culturing of cells and their availability to test varying cell types and tissues [7]. Ease of experimentation is also a large factor of selection of a micronucleus test with analysis done via visualization of morphology. Although no specific cell type is required for micronucleus analysis, cells are required to be dividing and the visualization of double-strand breaks requires completion of an entire cell cycle which is a negative aspect of this method. In addition, micronucleus tests are also labor- and time-intensive as they require visual scoring of cell numbers which limits the feasibility for use clinically [8].
1.1.2 Single-cell Electrophoresis (Comet Assay)
Single-cell electrophoresis, also commonly known as the comet assay, is a frequently used method for the identification of DNA damage. The comet assay approach is often selected due to the simplicity of the assay, its ability to be utilized on single cell populations and to assess both single- and double-strand breaks [9-11]. The technique utilizes electrophoresis for the assessment of DNA damage with a spherical mass of undamaged DNA termed the “head” of the comet. As damaged DNA migrates through the electrophoresis matrix, streams of DNA which loops around breaks form a “tail” which can be measured to determine the amount of DNA damage [9, 10]. However, the comet assay does have limitations associated with the technique as it is not able to detect damage associated with small deletions, damage that corresponds to fixed mutations or DNA fragments from apoptosis and necrosis which may result in inaccurate results [12, 13]. The technique is also labor-intensive and can prove to be tedious if large volumes of samples are being processed, making it an unlikely candidate for future clinical applications or more dynamic time-based assays.
1.1.3 Halo Assay
The halo assay is beneficial in DNA damage studies as it has the capacity to analyze DNA damage within singular cells. The method uses propidium iodine which intercalates with the DNA for visualization of breaks. Following the addition of propidium iodine, the DNA forms a supercoiled structure which following lysis forms results in “halos” being formed around individual cells which are used to measure chromatin fragility [10]. The halo assay however is limited to only detecting unrepaired DNA damage limiting its usage within repair studies [10].
1.1.4 Immunofluorescence
Visualization of DNA double-strand breaks with γ-H2AX staining methods is possible within a few minutes after the completion of the staining protocol and can be implemented in dividing and non-dividing cells [8, 14]. Furthermore, it has also been noted that the use of γ-H2AX is more reliable and reproducible and is more sensitive at detecting genotoxic compounds than the micronucleus assay [15-18]. New technologies have allowed for the emergence of γ-H2AX quantification via sequencing. However, because this technique is relatively new, it has been noted that there is no current standard method, statistical model, or interpretation [19]. Despite still being a frequently used method, manual eye detection and quantification of γ-H2AX is error-prone as it is a subjective measure that may differ between individuals, microscope, and camera equipment used [20, 21]. In addition, the method is time-consuming and may prove difficult when many foci are present [22]. Automation of foci counting and scoring through software (e.g., FociCounter, CellProfiler, ImageJ) is able to mitigate this issue [20]. Even with the implementation of automated foci scoring, quantification of γ-H2AX foci is difficult when foci overlap which may result in an underestimation of foci. However, flow cytometry is not subject to these issues as the technique quantifies fluorescent intensity of γ-H2AX in the nucleus of thousands of individual cells and reports the mean fluorescent intensity of the sample [14, 20]. Furthermore, this method of analysis allows for a greater volume of cells to be analyzed in comparison to microscopy; however, in order ensure only the correct population is measured, gating is required to only measure from cells which adhere to predetermined requirements [23]. Though once gating requirements are established, all future experiments can follow identical requirements negating variability between users. In addition, there is also the possibility of cross reactivity of antibodies within a sample; however, this can be mitigated by using singular staining and gating techniques.
1.2 Potential for γ-H2AX Utilization in Cancer Diagnostics and Prognostics
Genomic instability and inefficient DNA repair is a fundamental element of cancer and ageing. Inefficient or defective repair of DNA damage may lead to mutations and an unstable genomic environment which promotes rapid evolution, invasion, metastatic, and drug-resistant cancers. Therefore, assessment of genomic instability via γ-H2AX may provide an effective tool in cancer diagnostics and for evaluating prognosis and the risk of cancer.
1.2.1 Recent Cell Culture and Animal Models of Cancer and Use of γ-H2AX
The use of γ-H2AX staining has been substantially used in predictive modeling of disease, cell viability, compound assessment, pharmacodynamics and toxicity studies in animal and cell culture models. Increased γ-H2AX has been shown to be associated with triple negative breast cancer in comparison to non-triple negative breast cancer when modeled in cell lines [24, 25]. Cell line modeling of ovarian cancer has also shown increased γ-H2AX foci in the BRCA2 mutant cell line PEO1 in comparison to BRCA-wild type cell lines, suggesting that impaired DNA repair can be assessed using the marker [26]. Radiation-induced reduction in cell viability has been shown in colorectal cell lines (WiDR and DLD-1) along with a high γ-H2AX expression which exhibits evidence that high γ-H2AX is related to radio sensitivity [27].
In animal models, prolonged exposure to gamma radiation for 24 h in mice has also shown strong correlations with unrepaired DNA double strand viewed via γ-H2AX foci and radiogenic lung cancer [28]. Additionally, foci within liver tissues have been reported to clear to baseline levels by 24 h after X-ray exposure (absorbed dose 5 Gy) [29]. Interestingly, tissues appear to have different susceptibility to accumulation of γ-H2AX foci post radiation. In both 4 and 24 h following exposure to radioactive indium-111 chloride, extensive differences in γ-H2AX foci within hepatocytes and non-hepatocytes within liver tissue were observed between mice that were and were not exposed [30]. In converse, mice testis tissue did not show a significant difference in γ-H2AX foci between mice exposed to indium-111 chloride compared to controls [30].
1.3 Basal γ-H2AX in Malignant Human Tissue
Many mutations and deletions responsible for cancer development occur in the same chromosomal region as the H2AX gene, making staining of the phosphorylated form γ-H2AX, a potential biomarker for cancer formation and progression [31-33]. A small faction of γ-H2AX is present in healthy cells and has been shown to be markedly increased throughout disease progression. In biopsied liver samples for example, γ-H2AX was increased compared to healthy tissue in chronic hepatitis and cirrhosis [34]. Furthermore, liver tissues with cirrhosis along with tumors have showed increased γ-H2AX compared to those without [34].
Overexpression of γ-H2AX has been observed in a range of malignant tissues including those from the bile duct, breast, bladder, cervix, colon, kidney, and lung [27, 31, 32, 35]. Analysis of a range of malignant tissue types has continually shown that the upregulation of γ-H2AX expression is associated with poor prognosis [4, 24, 32, 33]. In breast cancer tissue, elevated endogenous γ-H2AX staining was associated with triple negative breast cancer, poor differentiation, and poor prognosis [24, 36]. Moreover, decreased staining of γ-H2AX has been shown in early-stage cancers (I) comparative to later stage cancers (II and III). Correspondingly, elevated staining of γ-H2AX within breast cancer tissue was shown to correlate with increased tumor size, grade, and lymph node involvement [4]. Within cervical tissue, gradual increases in γ-H2AX expression were observed between healthy tissue and low-grade cervical squamous intraepithelial lesions with low-risk human papillomavirus (HPV) type. Further elevation of γ-H2AX was observed in low-grade cervical squamous intraepithelial lesions with high-risk HPV type [37]. In colorectal cancer tissue, mRNA expression and western blots of H2AX were increased compared to healthy adjacent tissue taken from the same patient [27]. Expression of γ-H2AX within colorectal tissue directly correlates with prognostic features. As seen in other tissue types, high expression of γ-H2AX within colorectal tissue correlated with fast proliferation, increased aggression, metastasis, and poor prognosis [27, 32]. In contrast, low γ-H2AX expression in colorectal cancer tissues has been associated with less aggressive tumor phenotypes and slow proliferation [27, 32].
Whilst a study by Mei et al. [38] which utilized immunohistochemistry staining of γ-H2AX linked increased DNA damage as measured by γ-H2AX to a high risk of cancer recurrence, Saravi et al. [26] identified increased overexpression in histologically normal adjacent tissue comparative to malignant ovarian cancer tissue in their immunohistochemistry studies. The conflicting results warrant further investigation with an alternative method of γ-H2AX detection as the authors acknowledge that the qualitative measure may affect scoring result [26].
1.4 Predicting Cancer Risk with γ-H2AX in Healthy Human Tissue
The most clinically relevant susceptibility tests utilizing DNA damage markers are those that involve non-malignant cells. Investigations into the use of exfoliated oral cells, hair follicles, and peripheral blood mononuclear cells (PBMC) have all shown promise for potential use in clinical susceptibility studies. Selection of surrogate tissue must be carefully considered, but γ-H2AX quantity may vary between tissues. Oral cells have the advantage of being easily collected with potential for large-scale sampling and clinical applications as the samples are able to be collected by the patient without the supervision of a health professional [39]. In addition, they have also been demonstrated to be highly sensitive to low doses of ionizing radiation [40]. However, implementation of exfoliated oral cells into a clinical setting is unlikely due to highly variable γ-H2AX expression following irradiation. Yoon et al. [39] however notes that these variations may be influenced by discrepancies in technique as time between oral cell collection and irradiation sometimes varied between participants. The multiple cell types and sub-populations of buccal cells present in the oral cavity also further complicate the quantification of γ-H2AX [41]. Varied numbers of γ-H2AX foci have been demonstrated in different shaped nuclei of buccal cells in multiple studies. Furthermore, it has been noted that a large population of buccal cells are nonviable, necrotic, or apoptotic at collection, suggesting that the tissue is too diverse for implementation as a tissue for susceptibility studies [39, 41, 42].
Hair follicle testing has also been promoted as a possible surrogate tissue for susceptibility screens as they are proliferative and do not have the requirement of the use of a proliferative agent in studies that utilize chemotherapeutic agents [43]. Additionally, hair follicles have been shown to retain γ-H2AX up to 4 days post irradiation treatment, showing potential for their use in testing for radiation exposure [44]. Ultimately, the practicality of implementation of hair follicles in clinical DNA damage studies is highly contended. First, few studies utilize hair follicle testing as there is a high risk of contamination when tweezers are used [45]. Second, the stress associated with hair follicle removal has been documented to influence DNA damage scoring [46]. However, additional stress factors may be mitigated in studies that implement faster extraction processes such as through the addition of the use of air suction [45]. Another alternate method of collection includes removal of a scalp sample with hair follicles and then dissection under a microscope [47]. Whilst additional stressors may be controlled during the collection process, hair cycle and age have also been reported to influence DNA damage response and γ-H2AX formation which may further introduce variations into assessment [48]. Finally, hair follicle testing may be limited in patients with hair loss [49].
Peripheral blood mononuclear cells are an ideal source given they are non-malignant and relatively easily accessible through a blood sample. As many diagnostic laboratory samples are currently obtained via venepuncture, the utilization of a peripheral blood mononuclear cells poses potential for automation and therefore is more feasible for diagnostic purposes [50]. In addition, the use of blood is advantageous as there is no added variable of cell cycle, as unstimulated lymphocytes are non-cycling [43, 51]. However, laboratory-based stimulation of lymphocytes may be required when assessing response to chemotherapy due to many requiring cellular proliferation as part of their mechanism of action. Despite additional technical considerations needed when working with peripheral blood, it is currently considered the standard surrogate tissue for DNA damage studies [52].
The use of PBMC as a surrogate tissue for γ-H2AX analysis has been successful in a range of studies, although conflicting associations between the use of the marker and cancer risk have been reported. Several studies have demonstrated no significant difference in basal γ-H2AX level within blood between non-cancer and patients with a range of cancers [53-56]. Many studies utilize γ-H2AX ratio for assessment of γ-H2AX following DNA damage exposure with significant differences observed between healthy and cancer patients. More specifically, T-lymphocytes collected from patients with squamous cell carcinoma of the head and neck showed significantly less γ-H2AX than healthy controls following 2 h of 10 µM etoposide treatment [53]. Conversely, increased γ-H2AX ratio within blood cells post DNA damage induction has been shown to correlate with increased esophageal adenocarcinoma, lung, bladder, prostate, and colorectal cancer risk [52, 54–57].
In bladder cancer, conflicting results have been observed regarding γ-H2AX level within lymphocytes. In a study by Fernández et al. [52], a high incidence of γ-H2AX within lymphocytes that were treated with 2.5 Gy irradiation was observed in bladder cancer irrespective of age, biological sex, and smoking status. The group also found that increased γ-H2AX scores were correlated with increased bladder cancer risk [52]. Conversely, Turinetto et al. [58] observed an inverse relationship between high basal γ-H2AX and decreased reoccurrence risk which was hypothesized to be due to possible DNA repair adaptations in high basal DNA damage. Within this study, event-free survival in bladder cancer was also correlated with a high level of basal γ-H2AX; however, no correlation was seen in overall survival [58]. Similarly, Liu et al. [53] also observed an inverse relationship between γ-H2AX post DNA double-strand break inductions with 10 µM etoposide treatment for 2 h. No significant differences in γ-H2AX level in T-lymphocytes from healthy donors and patients with squamous cell carcinoma of the head and neck (SCCHN) were observed at baseline. However, following etoposide treatment, T-lymphocytes from SCCHN patients were reported to have a less γ-H2AX than the healthy samples [53]. To utilize the method for susceptibility studies however, normal tissue must be able to reflect the changes seen in malignant tissue before a malignancy occurs. To date, studies that have been able to demonstrate a link between γ-H2AX foci and expression within PBMCs and tissue response have largely investigated response to radiation therapy and the risk of normal tissue toxicities, hypersensitivity reactions, and future radiation induced cancers.
1.5 The Use of γ-H2AX in Prediction of Patient Response to Radiation and Chemotherapy
Whilst the use of radiation is effective in cancer treatment, surrounding healthy tissue is also subjected to radiation during treatment which may lead to long-term health effects in the healthy tissue due to normal tissue toxicity [59]. Typically, normal tissue toxicities are associated with high dose radiation; however, patients with genetic defects in DNA repair of double-strand breaks may experience normal tissue toxicity at lower doses of radiation, leading to radiation-induced health conditions such as cancer, cardiovascular disease, cataracts, neurodegeneration, fibrosis, and inflammation [60, 61]. In addition, in some cases of hypersensitivity such as in children with ataxia telangiectasia mutations, mortality has been reported [61]. Radiation hypersensitivity reactions have been successfully predicted via the irradiation of patient's peripheral blood lymphocytes prior to therapeutic radiation treatment. Methodologies have varied from whole-body radiation and assessment of lymphocytes post-radiation to in vitro irradiation of lymphocytes prior to or in synch with therapeutic radiation. However, in many methodologies, there has been a distinct link between increased γ-H2AX, impaired DNA damage response, and hypersensitivity reactions to radiation. Recently, the rate of γ-H2AX foci decay ratio has even been suggested to be the “most critical determining factor of late radiation toxicity” [62].
In patients with a genetic mutation identified as a variant of unknown significance, potential response to radiation therapy is often unknown. Hammarsten et al. [63], however, have recently demonstrated that γ-H2AX staining can indicate hypersensitive patients. Following irradiation of T-lymphocytes collected from patients with variants of unknown significance in DNA repair genes PRKDC, DCLRE1C, NBN, and MSH6 Hammarsten et al. [63] were able to detect and quantify radiation hypersensitivity with γ-H2AX in patients with PRKDC and DCLRE1C variants. Furthermore, deficient γ-H2AX removal was also detected in patients who were not radiation sensitive but had a variant of unknown significance (PRKDC & NBN) [63]. Similar trends have been seen in adult populations with increased retention of γ-H2AX foci 24 h post irradiation (2 Gy) of peripheral blood lymphocytes in patients who experienced hypersensitivity reactions during radiation treatment [60].
Increased retention of γ-H2AX foci after in irradiation of peripheral blood has been observed in a number of pediatric cancer studies [64, 65]. More specifically, correlations between increased retention of γ-H2AX foci, poor DNA repair within irradiated (1 and 2 Gy) lymphocytes, and adverse reactions within pediatric patients have been shown [65]. Pediatric cancer patients have also been demonstrated to have a decreased rate of removal of DNA damage and γ-H2AX foci in comparison to healthy pediatrics [64, 65].
In geriatric cancer patients with non-resectable hepatic tumors, γ-H2AX foci within PBMC were shown to significantly increase from baseline to 1 h and 1 week post selective internal radiotherapy [66]. Impaired immune function was also noted in these patients with an inverse relationship between γ-H2AX and lymphocyte proliferation 1 h post selective internal radiotherapy (114 Gy) [66]. Furthermore, in adults, there have also been observations of no significant increases in γ-H2AX foci in lymphocytes from esophageal cancer following chemoradiotherapy treatment with a cumulative dose of up to 60 Gy [67]. No direct correlations between γ-H2AX within lymphocytes following in vivo irradiation (up to 70 Gy) and later severe mucositis in head and neck patients have been found [68].
Recently, promising new research has demonstrated potential of protection of double-strand breaks and γ-H2AX foci accumulation within lymphocytes from in vitro irradiation when melatonin is ingested 1−2 h prior to radiation exposure [69]. Gamma-H2AX has also been used to monitor a patient's response to exposure to X-ray. Although exposure to X-ray is unavoidable for some procedures, it is widely recognized that exposure should be limited where possible. Following exposure to X-ray during cardiac catheterization, increases in γ-H2AX foci in peripheral T-lymphocytes were observed in all patients comparative to their pre-exposure sample [70]. Beels et al. [70] have hypothesized that current methods of estimated carcinogenesis risk from X-ray exposure may be underestimates and γ-H2AX foci may be a better indication of risk. Recent investigations into the effects of therapeutic proton beam use in cancer treatment compared to X-ray have significantly more DNA damage measured by γ-H2AX foci when compared to X-ray 30 min after exposure [71]. Similarly, the use of computed tomography (CT) scans has also shown to increase γ-H2AX foci at an average of 102% per cell in lymphocytes [72]. Interestingly, studies have shown that employees exposed to radiation (radiation workers) do not have a significantly different number of γ-H2AX foci in an untreated sample when compared to non-radiation workers [73, 74]. This suggests that protective measures for radiation workers are sufficient in protecting from workplace-induced DNA damage [73]. These findings demonstrate that γ-H2AX is an effective discriminatory marker for assessing DNA damage and repair following injury and potential treatments.
The use of radiation is a downfall for a susceptibility test due to the relative unavailability of a radiation source to many diagnostic laboratories. Other studies have also investigated the use of γ-H2AX in a clinical context conversely using the assay to predict response to chemotherapy. Cancers associated with BRCA1 and excision repair cross complementation group 1 (ERCC1) are known to be resistant to platinum-based treatments (e.g., cisplatin) and has led to in vitro models of chemotherapy prior to implementation to the patient. In PBMCs peripheral blood mononuclear cells collected from healthy and ovarian cancer patients, Bamias et al. [75] demonstrated an increased rate of intrinsic γ-H2AX in ovarian cancer patients samples compared to the healthy samples. Furthermore, they also found that patients with platinum sensitivity had a higher rate of intrinsic DNA damage measured by γ-H2AX compared to those with platinum resistance [75]. In the PBMCs of patients with recurrent ovarian cancer, increased γ-H2AX staining following prexasertib (cell cycle checkpoint 1 inhibitor (CHK1i) treatment was shown to correlate with treatment-induced lymphodepletion [76].
The majority of studies reviewed have shown γ-H2AX to be a reliable marker to detect DNA damage and repair, mostly in response to assessing the therapeutic window of radiation and chemotherapeutic side effect-induced damage. However, there appears to be more scope for the use of γ-H2AX as a biomarker to assess DNA damage and repair in cells and tissues in response to general lifestyle insults such as ageing, poor diet, chronic disease, and hormonal differences (biological sex) [51, 77]. Differences in baseline DNA damage and repair were observed due to age using γ-H2AX and confocal microscopy which was confirmed by comet assay [51]. This demonstrates γ-H2AX's utility in discriminatory assessment of an individual's differences in DNA damage and repair responses. Therefore, γ-H2AX could be used as a biomarker to assess DNA damage and repair kinetics at baseline, following chemotherapeutically induced double-strand breaks and to determine double-strand break repair in PBMC's and tissues.
2 Conclusion
In conclusion, it is evident that there are abundant applications for the use of γ-H2AX staining. Diagnostically, quantification of basal γ-H2AX has been demonstrated to be an effective tool in the diagnosis of malignant tissue [27, 31, 32, 35]. Prognostically, the use of γ-H2AX has been shown to be effective in the prediction of radiosensitive patients and has potential to serve as a marker for cancer prognosis and susceptibility indication [27]. This review has also highlighted the potential use of γ-H2AX in the assessment of genomic instability and prediction of patients likely to experience DNA damage and treatment-resistant tumors that repopulate.
In addition, studies have only examined γ-H2AX in subtypes of cancer and not in cancer as a whole disease, leaving a gap for assessment of how cancer influences γ-H2AX level in surrogate tissue regardless of original tissue of origin. Furthermore, to date, no studies have assessed how cells may respond to DNA damage in the years and decades post cancer leaving ample opportunity for investigation. There is potential scope to develop and expand on the use of γ-H2AX as a biomarker to assess both background and challenged DNA damage and repair kinetics ex vivo in a cell culture platform to predict ageing and cancer risk.
Author Contributions
Conceptualization: H.H., W.P., P.N., and A.F. Writing–original draft: H.H. Writing–review and editing: H.H., P.N., and A.F. Supervision: W.P., P.N., A.F., and A.F.
Acknowledgments
We would like to acknowledge the Australian Government for the Research Training Program Stipend which provided funding to support one of the authors of this research (H.H.).
Open access publishing facilitated by Central Queensland University, as part of the Wiley - Central Queensland University agreement via the Council of Australian University Librarians.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The authors have nothing to report.




