Trending toward gero-electroceuticals that target membrane potential for reprogramming aging and lifespan
Abstract
Ion gradients across cell membranes generate voltage potentials that are involved in a wide range of biological processes. According to the membrane hypothesis of aging, aging is inextricably linked to a decrease in resting membrane potential (Vmem). Alterations in ion channel activity and membrane fluidity caused by aging disrupt bioelectric homeostasis, increase intracellular calcium and potassium concentrations, induce abnormal mechanistic target of rapamycin (MTOR)- and AMPK-regulated metabolism and energy dissipation, and decrease proliferation and regeneration. Failure to maintain ion channel activity and membrane potential leads to cell senescence or death. There is evidence that by manipulating ion channel activities, a cryptic memory can be recalled to restore lost proliferative or regenerative abilities. Reversal or prevention of senescence, aging phenotypes, and longevity may be achieved by fine-tuning mitochondrial membrane polarization. Therefore, there is optimism that deciphering the bioelectric codes that govern cell functions will lead to the development of new gero-electroceuticals that restore cell function and prevent tissue loss during aging.
1 INTRODUCTION
The relationship between electricity and biological response was first elegantly shown in the late 18th century by Luigi Galvani who demonstrated that electric stimulation causes muscle contraction.1 Since then, it has become evident that bioelectricity is essential for many biological processes including neuron firing, muscle contraction, sensation, cell excitability, differentiation, motility and migration, division, intracellular signaling, and gene expression. Changes in the resting membrane potential regulate a myriad of downstream actions by modulating intracellular chemical signals and signaling cascades. Bioelectric fields drive intricate shape changes during developmental tissue remodeling as well as regulate a host of appropriate responses to unfavorable environmental conditions.
The term “bioelectricity” refers to the endogenous electric currents that are generated by living organisms. Gradients of ions across cell membranes create voltage potentials. The electric potential of membranes (Vmem) is a strong electric field (∼1.0 × 106 V/m) of approximately −10 to −90 mV. Vmem is established by the coordinate actions of numerous membrane ion channels, transporters, and pumps (Figure 1A).2 The movement of Inorganic ions and charged molecules across gap junctions enables electrical coupling and the integration of cellular voltage gradients into tissue-wide bioelectric fields (Figure 1B–D).
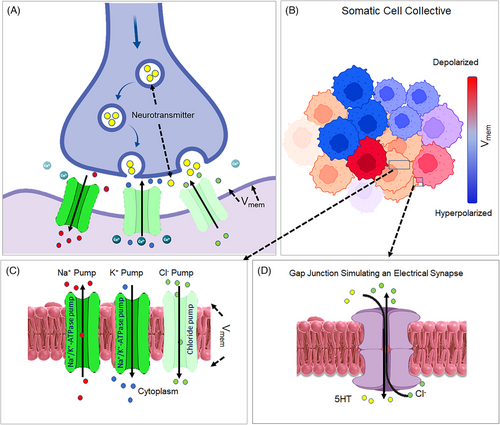
When the membrane potential rises above the resting Vmem threshold, the quiescent normal cells become hyperpolarized, whereas falling membrane potential results in a depolarized state.3 Changes in Vmem and dissemination of electrical charges in coupled cell networks enable cells to uniformly and coherently respond to environmental cues.4 Additionally, bioelectricity stores information that can be propagated in cell collectives or be disseminated horizontally by somatic-to-germline transfer.5
This review discusses the importance of bioelectric code dysfunctions that are permissive to the development of an aging phenotype. We propose that deciphering the bioelectric codes that are corrupted in aging can be used to create novel gero-electroceuticals for treatment of aging and associated diseases.
2 BIOELECTRIC DYNAMICS IN AGING
The membrane potential varies significantly in different cell types, and it is altered during transit through the cell cycle, by mechanical properties of cells, environmental cues, and aging. The membrane hypothesis of aging posits that aging occurs when ionic strength in cellular water content exceeds a critical value beyond 400 mEq/kg. This leads to the condensation of chromatin and loss of activity of DNA-dependent polymerase and other enzymes, and protein synthesis decreases.6
The bioelectric dynamics in cells and whole organisms deteriorate with aging. For example, aging is associated with an increase in total l-type Ca2+ channel activity and voltage-activated calcium (Ca2+) influx.7 Moreover, changes in lipid membrane fluidity drive down resting potassium permeability and allow the intracellular accumulation of this cation.6 There is evidence that aging and senescence cause changes in the resting membrane potential that can impair cellular functionality, proliferative capacity, regenerative potential, and mitochondrial function, leading to senescence and cell death, and limiting the lifespan. This change might be due to the loss of responsiveness to mechanistic target of rapamycin (mTORC)-1 and autophagy.8 Alterations of membrane polarization in senescent cells, mediated by K+ channels, reduces activity of PI3K/Akt pathway and restores sensitivity of mTORC1 in senescent cells to serum starvation.9 Similarly, membrane depolarization of mitochondria and, consequently, mitochondrial dysfunction in senescence and aging is mediated by enhanced activation of the mitochondrial permeability transition pore (mPTP), likely due to ROS overproduction.10, 11 These changes do not appear to be tissue specific and occur in diverse cell types that experience senescence such as CD8+ T cells, central nervous system.12, 13
Patterns of resting potentials within tissues provide mechanistic linkage with molecular genetics and biochemical pathways. Thus, it follows that the modification of the membrane bioelectricity carries the potential to restore pathways that have gone awry in human aging. The electroceutical toolkit for prevention or repair includes neurostimulator medical devices as well as a variety of ion channel drugs, many of which are approved for human use. Some of these pharmaceutical drugs can be repurposed as gero-electroceuticals to address a host of age-related disorders. For example, some of these electroceuticals are tailored to revive lost proliferative or regenerative potentials, some modulate cancer cell proliferative, invasive, or metastatic traits, whereas others show palliative impact on age-related diseases.
3 ELECTROCEUTICALS THAT RESTORE LOST PROLIFERATIVE AND REGENERATIVE POTENTIALS
3.1 Restoring proliferation by modulation of Vmem
Senescent and aging stem cells lose their proliferative potential and this results in organ-wide loss of mass and extensive tissue atrophy. Reversal of this loss-of-function is necessary for replenishing tissues that are lost by aging. It appears that altering Vmem is quite effective in causing proliferation in both normal and malignant cells. Normal cellular collectives collaborate toward an organ-level structural order, which promotes a default level of cell function and proliferative activity by maintaining a normal electric potential across the networked plasma membranes. Vmem fluctuates and appears to be required for G1/S and G2/M transitions during cell proliferation. Changing intracellular ion concentrations is sufficient to activate DNA synthesis and proliferation in otherwise non-proliferating neurons.14, 15 Conversely, altering the extracellular ion concentration can stop G1/S progression and proliferation in a variety of cells.16 However, there are a host of other factors that alter the ion transfer and membrane polarization and change the outcome of proliferation. The formation of gap junctions would deplete sodium channels, reduce sodium permeability, and stop DNA synthesis. Growth factors, however, can competitively bind to channel-connexon elements, cleave gap junctions, increase sodium permeability by influencing sodium channels, and trigger DNA synthesis.3, 15 Thus, it is expected that the manipulation of Vmem, sufficient to induce proliferation, requires a thorough understanding of all the elements that fine-tune the ion transfer and polarization state of cells as they transit through the cell cycle.
3.2 The impact of depolarization and hyperpolarization on proliferation
The broad consensus is that rapidly dividing cells, including stem cells and embryonic or cancer cells, are more depolarized than nondividing, differentiated cells.16, 17 As a result, a cell-specific Vmem threshold may discriminate normal quiescent cells from cells that are in a highly proliferative state.3 There are, however, several caveats to the observation that depolarization is the only bioelectric code necessary for promoting cell division. Hyperpolarization situations, such as those caused by Epidermal Growth Factor (EGF)-mediated Ca2+ influx and prolonged increases in [Ca2+]i, can promote mitogenesis.18 Rather than being dependent on a crucial Vmem value, the effect of hyperpolarization appears to be cell-context sensitive. For example, in the central nervous system of frog embryo, hyperpolarization can induce widespread proliferation or apoptosis depending on the cell type.19 Hyperpolarization itself can change the outcome of cell division by intercepting the Ca2+ influx by the closure of voltage-gated calcium channels.20 Thus, for induction of cell division, it is expected that the manipulation of Vmem must be precisely adjusted based on the target cells with the caveat that the changes in membrane polarization cannot be disentangled from changes which occur in the [Ca2+]i.
3.3 Restoring lost regenerative potential
Loss of regenerative capability in stem cells is a well-defined feature of aging. Alteration of the resting membrane potential delivers instructional messages in a variety of settings to promote cell growth and proliferation, including embryogenesis, regeneration, and cancer progression.21 Bioelectric field gradients control proliferation and regeneration, organ development, organ size, left-right patterning, and wound healing by intercepting the default transcriptional responses across cell-collectives or tissue fields by fine-tuning the flow of ions such as Ca2+ and by altering Vmem. In fact, bioelectric memory gradients overlay with transcriptional gradients in the highly regenerative planarian flatworm.22 In these animals, the Wnt-β-catenin signaling inducers are expressed anteriorly, whereas its inhibitors accumulate posteriorly establishing an anteroposterior (AP) gradient.23 This spatial transcriptomic landscape is mirrored by a gradient in the membrane potential, which is equally important for maintaining the AP directionality.
There is a growing optimism that Vmem and bioelectric fields can be manipulated to restore lost regenerative potentials. As described in the following section, knowledge of this restoration has been gleaned from examining regeneration in flatworms, and by inducing changes in wound healing and restoring regeneration in severed organs under non-regenerative conditions.
3.3.1 Organ regeneration
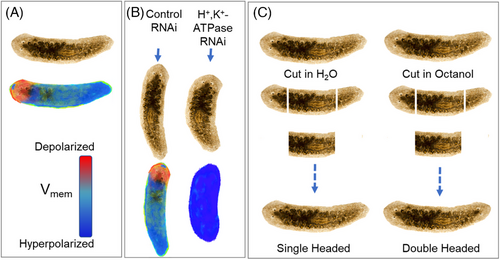
In adult flatworms, when the default spatial bioelectric field gradient is disrupted by severing the body of the animal, changes in the bioelectric field drive the rebuilding of the damaged organ and restore the default bioelectric state. After a planarian is cut in half, the headless animal develops a tail, whereas the tailless animal acquires a head. This implies the existence of a bioelectrically driven memory state (Figure 2A).23-25 The importance of bioelectric fields in shaping the morpho-space also has been demonstrated in the development of shape-form mutants (Figure 2B). When the head-tail direction of membrane potential is disrupted by RNAi to H+/K+-ATPase, regeneration fails and results in shrunken heads (Figure 2B). Disrupting this bioelectric memory, for example, by treating headless and tailless planarian trunks with octanol, results in aberrant formation of double-headed animals.22, 23 (Figure 2C). Disrupting the basal Vmem, however, has been used to entirely regenerate severed body parts by spatially and temporally regulating proliferation or apoptosis in various situations.20, 26-28 Thus, it seems that planaria successfully repair their cut heads and tails by maintaining A–P directionality in their tissue-wide transcriptional and bioelectric fields. Simulating similar changes in bioelectric fields should open the opportunity to restore tissues that atrophy as a result of aging.
3.3.2 Wound repair
The cellular regeneration potential appears to be preserved by a cryptic memory, which can be activated by manipulating ion channel activities.22, 29 Wounds, for example, might be thought of as a disruption of endogenous bioelectric circuits, as evidenced by the formation of depolarization at the wound edge. Along the wound edge, local depolarization causes normally nondividing cells to proliferate and migrate.27, 30 Depolarization relays mitogenic signals, causes cytoskeletal alterations required for migration, and induces shape changes essential for wound repair. This concept is validated by nonspecific depolarization of the plasma membrane potential of epithelial cells that triggers distinctive cytoskeletal rearrangements.27, 30 However, when depolarization reaches certain thresholds, such as with V-ATPase loss-of-function, regeneration in severed tail buds in Xenopus halts, leaving them in a non-regenerative’ refractory’ state.31 An inflow of sodium ions occurs in the regenerative bud 18 h after amputation. This event requires, and does not progress without, the requisite phase of Vmem repolarization that is dependent on NaV1.2 sodium channel expression in the mesenchymal cells.32 The available evidence, therefore, provide credence for the idea that simulating the bioelectric code that recalls cell division is an appealing method for inducing tissue repair in non-regenerating aging tissues.
3.3.3 Regeneration in non-regenerative conditions
The feasibility of restoring regeneration has already been validated by effectively regenerating amputated appendages in non-regenerative species such as Xenopus by modifying Vmem and bioelectric fields.33, 34 The stimulation of H+ flux is sufficient to induce axonal patterning and tail extension even under otherwise non-regenerative conditions.31 Hyperpolarization also plays a key role in the control of organ development including formation of the eye in Xenopus.28 Although hyperpolarization does not necessarily interfere with instructional regenerative signals, it can derail the outcome of organ regeneration. For example, inducing hyperpolarization in planaria after removing their heads results in the production of ectopic heads.35 When the head of the flatworm is severed, depolarization via H,K-ATPase and disruption of Vmem result in a small head and large pharynx indicating that membrane polarization must be adjusted as a final brake to ensure that organs do not exceed an ideal size limit.35
The disruption of tissue-wide bioelectric fields also allows for regeneration, even in organisms that have lost the ability to replace a lost organ. Regenerative development, for example, can be initiated through the coordinated engagement of V-ATPase and NaV1.2.31, 33, 34 Ion imaging and molecular-genetic approaches have indicated that, with the exception of a few islands containing depolarized cells, the cells in the uncut tail of Xenopus are polarized under normal conditions. However, 6 h after amputation, V-ATPase activity increases, and by 24 h, the regeneration bud is repolarized, with an island of depolarized cells developing just anterior to this bud.31, 33, 34 Compared with uncut or regenerating tails, non-regenerative “refractory” tail buds are strongly depolarized.
Thus, V-ATPase and NaV1.2, repolarization of Vmem and the activity of H+ pump and sodium influx, are considered minimal requirements for regulating regenerative activity for effective wound repair and for activating refractory or non-regenerative tissues. These novel strategies, when combined with existing biochemical approaches, potentially may allow for regeneration under the non-regenerating condition of aging.34
4 CANCER TAMING ELECTROCEUTICALS
4.1 Depolarization of membranes and hyperproliferation in cancer cells
Cancer, a common aging disease, causes dysregulation of bioelectric fields. Cancer cells decouple from the bioelectric cues that maintain the delicate tissue order and function.25 Cancer cells function as independent autonomous agents, free of the constraints imposed by bioelectric fields in normal tissue networks. Although hyperpolarization reduces the susceptibility to tumorigenesis, the plasma membranes in cancer cells, such as MCF7 or MDA-MB-231, are more depolarized compared to the resting membrane potential maintained by the neighboring and networked normal cells. Overexpression of calcium channels in cancer cells and influx of Ca2+ is thought to be essential to tumorigenesis and hyperproliferation of cancer cells.36-38 By depolarizing their cell membranes, tumor cells initiate and enter a hyperproliferative state where they continue infinitely to divide, invade, and metastasize to distant sites.22, 25, 35, 38-40 Depolarization can even send instructive signals to cancer cells that render them metastatic.41, 42
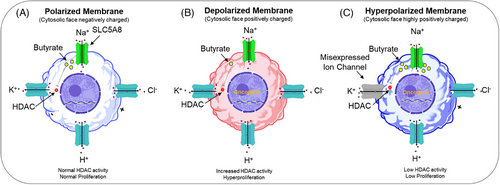
Thus, it can be argued that the modification of the polarization state of cancer cells by pharmacological inhibitors of calcium influx pathways can be used to inhibit cancer proliferation and or invasion.25, 35, 38 Given that depolarization promotes cell division, one expects that hyperpolarization should halt this action. Indeed, hyperpolarization produced through molecular and/or pharmacological targeting of H+, K+, or Cl− ion channels enhances the activities of SLC5A8, a sodium/glucose co-transporter family member. This activation permits excess entry of butyrate into the cytoplasm. By blocking histone deacetylase, excess intracellular butyrate causes cell cycle arrest and inhibits tumor formation (Figure 3A–C).22, 35
5 LIFE EXPANDING MITOCHONDRIAL ELECTROCEUTICALS
5.1 Mitochondrial protonmotive force, a key to mitochondrial homeostasis
There is sufficient evidence that support the concept that cellular decline in aging is reliant on changes in mitochondrial protonmotive force (PMF) and loss of lysosomal acidification that likely leads to the failure to remove the damaged mitochondria.
Mitochondrial dysfunction is regarded as one of the nine major contributors to aging and senescence.43 By employing an electrochemical gradient across the inner mitochondrial membrane, the PMF in mitochondria drives generation of ATP. The proton gradient is made up of two potential energy sources that are connected: an electrical mitochondrial membrane potential (ΔΨm) and a pH differential (∆pH).44-46 PMF, lysosomal acidification, and respiration deteriorate with aging in numerous experimental paradigms. PMF preservation via calorie restriction (CR) preserves respiration rates while reducing proton leak and hydrogen peroxide production.44-46 In aged cells, inhibiting adenine nucleotide translocase (which transfers ADP into mitochondria for ATP synthesis) protects the PMF, reverses respiratory failure, and rejuvenates mitochondria.47 Lysosomal acidification appears to be a determinant of lifespan. For example, during natural aging, the vacuolar acidity declines and such an altered vacuolar pH drives mitochondrial dysfunction, whereas preventing this decline is sufficient to extend lifespan in replicatively aged yeast.45
Mitochondrial membrane depolarization (MMD) is recognized as a common feature of aging in microorganisms such as yeast.45, 48-50 MMD in aging is clearly detrimental as it is associated with a decrease in oxygen consumption, fragmentation of mitochondria, and genome instability.45, 48, 49 Prior to the onset of age-related MMD, vacuolar pH in yeasts increases due to altered activity of the VMA1 and VPH2 genes. These genes normally work with vacuolar H+-ATPase to acidify vacuoles. In yeasts, life can be extended by mere overexpression of VMA1 and VPH2. These over-expressions maintain adequate amino acid import by the vacuole, prevent the age-associated decline in vacuolar acidity, and protect against the development of MMD. Paradoxically, through as yet unknown mechanisms, reduction of mitochondrial membrane potential has shown life-extension property in yeast.51 Furthermore, MMD is engaged by strategies that extend lifespan including CR or deletion of mtDNA.45, 51 A mildly depolarized mitochondrial membrane also has shown antiaging effect in Saccharomyces cerevisiae.52 These beneficial effects can be explained (in part) by the fact that control of mitochondrial permeability is required for autophagy-dependent lifespan extension.53 Opening of the permeability transition pore (mPTP) by genetic means extinguishes autophagy-dependent lifespan extension by CR or loss of germline stem cells. This suggests that the mPTP can transform the outcome of autophagy to a damaging force in mammals, which also suggests that mPTP is proper target for maximizing the benefits of autophagy in aging.53 PMF may also play a role in the regenerative potential of stem cells that declines with age.54
It appears that mitochondrial membrane potential is involved in cell fitness and that adjusting the decrease in this potential renders cells more robust for sustaining long term in vivo persistence and for maintaining enhanced stemness.55 Given that aging is influenced by mitochondrial membrane potential and mild MMD can prolong lifespan, it is necessary to identify gero-electroceuticals that allow setting the bioelectric code parameters that control lifespan by adjusting mitochondrial polarization.56-58 Moreover, restoring the mitochondrial membrane potential of old hematopoietic stem cells is sufficient to restore their youthful phenotype.54
5.2 Lifespan extension by controlling PMF, energy, and nutritional sensors
Energy balance and homeostasis are maintained by the energy sensor AMP-activated protein kinase (AMPK) and by the nutritional sensor mTORC. Defects in this complex system leads to metabolic disorders including obesity and diabetes.59 There is also an intimate link between metabolism, energy and nutritional availability and lifespan.60 This is quite evident in the case of CR, which is known to increase life-span in yeasts, worms, flies, to rodents. CR exerts its effects by virtue of reducing the activity of TOR driven nutrient sensing and increased AMPK, AMP:ATP, and ADP:ATP ratios.60 More specifically, the transgenic expression of AMPK in fat or muscle extends lifespan, whereas its inhibition by RNAi shortens lifespan.60 The hepatic NADH/NAD+ ratio, a latent metabolic measure of oxidative stress, is also an indicator of an unfavorable metabolic characteristic that causes age-related diseases ranging from reduced glucose tolerance and insulin resistance to mitochondrial disease.61
PMF must be fine-tuned for healthy lifespan extension and prevention of metabolic diseases associated with aging. There is sufficient evidence to suggest that there is a delicate balance between the PMF, AMPK energy, and mTOR nutritional sensing.62, 63 For example, exit from pluripotency and cell fate commitment can be regulated by membrane potential via calcium and mTOR.64 Inhibiting mTOR by genetic or pharmacologic means promotes mitochondrial function by encouraging mitophagy, expression of nuclear mitochondrial proteins, maintenance of mitochondrial protein concentrations, and oxidative function.65, 66 The preservation of mitochondrial function increases lifespan in yeasts, fruit flies, worms, and mice.67 Longevity in Caenorhabditis elegans is also dependent on AMP-activated protein kinase (AMPK) by antagonistically controlling metabolism and aging.68 The effects of CR can be replicated by either inhibiting mTOR with rapamycin or by targeting AMPK and mitochondrial respiration with metformin.65, 66 Restoring the PMF by the AMPK preserves viability, redox homeostasis, and normal mitochondrial signaling.69 However, counterintuitively, optogenetic photoactivation of PMF in vivo in C. elegans reverses the positive effect of AMPK activation during nutritional restriction, whereas reducing PMF promotes adaptation to hypoxia and the AMPK-mediated starvation response.70
Furthermore, in replicatively aged S. cerevisiae, increased vacuolar pH, which leads to mitochondrial dysfunction, also increases life-span.45 Mutation or RNAi knockdowns of the components of the mitochondrial transport chain or PMF dissipation by protonophores have also been shown to extend lifespan in C. elegans.71 Similarly, mild mitochondrial uncoupling in mice that simulates CR enhances tissue respiratory rates, improves blood glucose, triglyceride, and insulin concentrations, decreases ROS and tissue DNA and protein oxidation, and reduces body weight.57
6 ELECTROCEUTICALS FOR REROUTING CELL DEATH TO CELL PROLIFERATION
Cell death is one of the most distinctive features of aging. Receiving a death signal has traditionally been thought to send cells onto a path that leads to death. Cell proliferation and death, on the other hand, appear to be inextricably intertwined with escape routes that convert an apoptotic outcome to a proliferative activity. The process “anastasis” hardwires the cellular machinery to escape from death even when cells have endured caspase activation and DNA damage.72 Similarly, identical bioelectric manipulations and Vmem changes can promote both proliferation and apoptosis. Additionally, hyperpolarization via K+ channel activation, instead of causing proliferation, protects cells against cell death.20, 26, 28, 73 The link between cell proliferation and death can be easily visualized in vivo during eye and limb regeneration in Xenopus.32 Waves of membrane hyperpolarization and transcriptome change and proliferation and cell death must be precisely timed throughout embryogenesis to temporally and spatially control organ development and organ size.74 The signature feature of Vmem depolarization includes joint enrichment of cell cycle and cell death signals.73 Cell death engages and requires changes in ion flux and specifically Ca2+ by the activation of transient receptor potential channels, ER stress, or inhibition of voltage-gated Ca2+ channels.75, 76 The cell shrinkage that is associated with apoptosis is also due to net efflux of intracellular K+ and Cl− and influx of Na+.77
Thus, similar bioelectric codes appear to promote both cell proliferation and death non-exclusively. Decoding these intertwined pathways may lead to new strategies for rejuvenating cells rather than allowing them to commit self-suicide.
7 CONCLUSIONS
Overall, bioelectricity and bioelectric patterns decay with aging and exert far-reaching influence over a wide range of aging phenotypes. Conversely, cell stress, macromolecular damage, aberrant AMPK and mTOR signaling, and senescence derail bioelectrical homeostasis. Deciphering aberrant bioelectric codes that promote aging and elucidating how bioelectrical field changes integrate with signaling pathways and transcriptional responses provide key insights into the aging process. Ion channel regulation and membrane potential homeostasis by mTOR and AMPK exemplify linkages between bioelectricity and signaling pathways.78 It is critical to identify pathways that enable voltage membrane patterns that override transcriptional, genomic, and epigenomic responses and to comprehend how bioelectric memory is written—although this likely occurs by inducing a stable epigenetic imprinting.34
There is a gap in our knowledge concerning how dysregulated bioelectric signaling drives senescence and aging phenotypes. Preliminary data encouragingly suggest that targeted modification of membrane potential may enable tissue/organ repair and regeneration. Theoretically, ion channel modifications and corrupted bioelectric codes could be reprogrammed to spatio-temporally regulate Vmem. Elucidating bioelectric patterns of aging may enable innovative therapies for halting or even reversing an aging phenotype, as well as for inducing regeneration, preventing cancer or adopt electroceuticals for cancer treatment that do not require genetic engineering. These insights, together with the development of computational models, combined with the repurposing of existing FDA-approved drugs, have great potential to lead to the development of gero-electroceuticals that combat aging, reverse replicative senescence, and bring cancer cell proliferation to a halt by normalizing bioelectric homeostasis (Figure 4).27, 79-81 The non-locality of bioelectric information also offers the possibility of surrogate-site diagnostics and treatment.
Among the potential gero-electroceuticals are ivermectin, which targets glycine- and glutamate-gated chloride channels, and monensin, which acts as a sodium ionophore. These drugs restore regeneration. Capsaicin, a TRPV1 agonist, induces axonal growth; and fluoxetine, a serotonin transporter inhibitor, suppresses melanoma.81 Together, there is a growing optimism that ion channel drugs might be tailored to generate gero-electroceuticals that alleviate aging and age-related disorders.
AUTHOR CONTRIBUTIONS
Siamak Tabibzadeh contributed to funding acquisition, conceptualization, preparing and writing of the paper and generation of figures and Olen R. Brown contributed to writing and editing.
ACKNOWLEDGMENTS
This work was supported by a grant (FBS-2022) from Frontiers in Bioscience.
CONFLICT OF INTEREST STATEMENT
The author does not declare any commercial or financial or any other potential conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
Data are available to readers.




