Detecting cellular senescence in vivo: Imagining imaging better
Zachary M. Rabinowitz is partially supported by the UF Graduate School Preeminent Scholarship.
Abstract
Methods to detect cellular senescence have become increasingly important, even more so in living animals and humans. This cellular state has been found to play fundamental roles in physiological processes as well as functioning detrimentally toward the advent or progression of pathological conditions. Importantly, the study of senescence involvement in these processes in vivo cannot be done without living-friendly technologies enabling senescence detection. Furthermore, senotherapies or therapies that selectively kill senescent cells have emerged as a new therapeutic strategy for aging and age-related diseases such as atherosclerosis, cancer, and neurodegenerative disorders and require tools to evaluate their use in vivo. As of now, our in vivo senescence detection toolkit includes genetically engineered reporter mouse models and small molecule imaging probes. Herein, we will focus on the detection of senescence in vivo, including a summary of its challenges, current detection methods and strategies, and a perspective on overcoming the current obstacles.
1 INTRODUCTION
Before 1961, it was believed that natural animals cells derived from the organism (i.e., ex vivo) may proliferate perpetually under the right conditions of culturing.1 This idea originated by Dr. Alexis Carrel, who sought to develop new techniques to prolong the life of tissues indefinitely ex vivo.2 However, his techniques were irreproducible by others.1 In 1961, Hayflick and Moorhead were the first to formally report that normal human diploid cell strains ceased cellular proliferation after going through 40–60 doubling populations (termed the “Hayflick limit”).3 This groundbreaking observation marked an end to the prior belief that vertebrate cells may divide indefinitely in culture. The end of a normal cells’ proliferative life span, in which cell division is eventually arrested, is brought about by a process termed “replicative senescence.”3
Cellular senescence is a state of irreversible cell-cycle arrest with altered metabolic activity (Figure 1). This state is different from quiescence, which is when cells temporarily exit the cell cycle but remain capable of re-entering upon stimulation. There are numerous triggers beyond replicative exhaustion, including intrinsic and extrinsic insults to the cell, that induce different types of cellular senescence. These various triggers include but are not limited to oncogenic activation, DNA-damage and mutations, reactive oxygen species (ROS), strong mitogenic signals, and hypoxia.4, 5 The mechanism through which cells exiting the cell cycle and become senescent involve the activation of the tumor suppressor pathways involving p53/p21, p16lnk4a/Rb, as well as others that may have not been realized.6-9 Once senescence is fully established, cells may feature four interdependent hallmarks,5 including (I) cell-cycle arrest, (II) DNA, protein, and lipid damage, (III) senescence-associated secretory phenotype (SASP), and a (IV) deregulated metabolism.5 Furthermore, senescent cells exhibit resistance to apoptosis and can be observed in culture due to their morphological change (i.e., enlarged and flattened).8
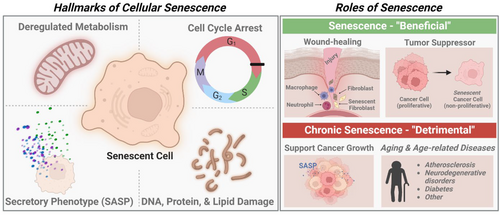
Cellular senescence is a double-edged sword because it is physiologically fundamental, however, it is pathologically relevant.8 Physiologically, senescence plays an important role in normal development, tissue homeostasis and repair, wound healing10, 11 and acts as a tumor suppressor mechanism.7, 8 On the other hand, the accumulation of senescent cells can delay the repair and regeneration of tissue and contribute to tissue and organismal aging.8 Additionally, the secretion of inflammatory SASP factors can drive the advancement of age-related diseases and the decline in homeostatic immune function.9, 12 SASP factors can also induce secondary senescence, where neighboring cells may become senescent through paracrine signaling, thereby furthering the development of a senescent milieu.13
The distinct cellular features of senescent cells allow researchers to identify biomarkers that can be utilized to detect senescent cells in vitro, ex vivo, and in vivo. The desire to detect and selectively kill senescent cells and develop tools to do so comes from its physiological and pathophysiological significance.7 General methods for senescence detection evaluate senescence biomarkers or biomolecules linked to the hallmarks of senescence using qPCR, western blotting, immunohistochemistry, or chemical staining. Most commonly, to identify senescence, cells or tissues are stained with X-Gal, a β-galactosidase (β-gal) substrate-based reagent that yields a blue insoluble indigoid dye once activated by senescence-associated β-galactosidase (SA-β-gal).14 X-Gal staining is often coupled with other senescence markers including G1/S cell-cycle checkpoint inhibitors (i.e., p21, p53, p16), DNA-damaged induced γH2AX, and SASP proteins (i.e., IL-6, IL-8, MMPs).15 Additionally, Sudan Black B (SBB) has been used to identify lipofuscin, an aggregate of oxidized proteins.16
Detecting senescence in vivo (i.e., live animal and human imaging) is particularly appealing as we can learn more about senescence involvement in pathological processes in real time and thus, overcome the need for invasive methods and animal sacrifice to extract cells and tissues from the organism for detection. Furthermore, real-time detection of cellular senescence within tissues in vivo can guide the use of senotherapies, therapies that selective kill senescent cells. This can significantly impact drug discovery efforts as clearance of senescent cells has been shown to promote an antipathological effect.17 Although, in cancer treatment, induction of senescence via chemotherapy, radiotherapy, and targeted therapy has been a therapeutic strategy for several cancers,4 the accumulation of induced senescent cells have been linked to tumorigenesis, therapy resistance, and additional side effects via the SASP, if not efficiently cleared by the immune system.4 Thus, killing senescent cells emerges as an important consideration for anticancer therapies. Detection of senescence, therefore, is appealing yet remains extremely challenging. In this perspective, we will focus on detection of senescence in vivo, including a summary of its challenges, current detection methods and strategies, and a perspective on overcoming current obstacles.
1.1 Considerable challenges of detecting senescence in vivo
1.1.1 Heterogeneity of senescence
The phenotype of cellular senescence is highly heterogeneous, which depends on multiple factors including the type of the senescence-inducing trigger, cell type, physiological location, and the favored molecular pathways within the cell.6, 13, 18 Thus, the choice of biomarker derived from the hallmarks of cellular senescence needs to be carefully considered for a given context. For example, the SASP, a highly dynamic and complex characteristic with the secretion of cytokines, chemokines, growth factors, metalloproteases, and extracellular vesicles, may facilitate an understanding of senescence biological effect through its detection. However, the SASP profile differs depending on the senescence trigger and induction mechanism as well as the cell type.8, 13 Consequently, thorough investigation into senescence of the chosen tissue or organism is imperative prior to designing and developing tools for accurate analysis and interpretation of data.
1.1.2 Lack of specific biomarkers
None of the aforementioned senescence biomarkers can serve as a standalone marker for senescent state because they are not exclusive to senescence.5 For example, SASP factors are not exclusive to senescent cells. Immune, endothelial, and cancer cells also secrete SASP factors. Also, although cell-cycle-arrest proteins such as p21CIP1 are essential to implementation of senescence, they are also upregulated during quiescence.19 Thus, detection of SASP, cell-cycle arrest proteins, and others alone may lead to false-positive results in senescence detection. To increase confidence in detection, the multibiomarker approach or the simultaneous assessment of multiple hallmarks has been used for a more accurate detection of senescent cells. However, the multibiomarker approaches has been limited to in vitro or ex vivo assays due to the challenge of detecting multiple senescence biomarkers in vivo using the current toolkit.15
1.2 Current approaches to detect senescence in vivo
1.2.1 Senescence reporter mouse models
Cell-cycle arrest is among the most defining hallmarks of senescence. Toward cellular senescence, the two pathways that largely mediate cell-cycle arrest include the p16lnk4a/RB and p53/ p21CIP1 axes. These cell-cycle arrest proteins bind and inhibit cyclin-dependent kinase 4/6 (CDK4/6) activity, which then promotes retinoblastoma (RB)-dependent cell-cycle arrest.8 Importantly, persistent expression of p16lnk4a is critical for the maintenance of cellular senescence.20 Consequently, p16lnk4a has been considered a reliable biomarker for the detection of senescent cells in vitro and in vivo.21 However, dependable measurement of p16lnk4a protein induction either by its’ transcriptional mRNA or translational protein levels remains challenging. mRNA and protein levels of p16lnk4a does not always correlate20 and the lack of reliable murine p16lnk4a antibodies decreases confidence in the ability to detect the protein ex vivo using techniques such as western blot or immunohistochemistry.15, 22 On the other hand, the role of p21CIP1 in senescence remains controversial21 and its reliability as a senescence biomarker is debatable. To illustrate, although the expression of p21CIP1 may be important for cellular senescence initiation, its expression is transient after senescence initiation and does not persist during the maintenance phase.21, 23 Additionally, not all cells that express p21CIP1 are senescent. As mentioned previously, p21CIP1 upregulation occurs in the implementation of cellular quiescence,19 and thus may lead to false-positive detection of bona fide senescent cells in vivo.
To detect senescence via these pathways in vivo, reporter mouse models have been developed where a reporter gene, such as firefly luciferase (fLUC), Renilla luciferase (rLUC), or monomeric red fluorescent protein (mRFP), is expressed under the control of the promoter of p16lnk4a or p21CIP1 using either transgenic or knock-in approaches.15 These reporter genes allow for imaging whole animals using bioluminescence or fluorescence imaging. Additionally, ablator constructs or constructs that allow for killing of cells once expressed under the control of the promoter of the target gene may be inserted along with reporter genes, which can be used, for example, to validate the disappearance of in situ senescent cells and study the effects of selectively killing senescent cells in vivo. In this perspective, we will summarize the reporter mouse models targeting either p16lnk4a or p21CIP1 that have been evaluated to detect cellular senescence in living mice. More recently, a reporter mouse model targeting the β-gal encoding gene, GLB1, referred to as Glb1+/m-Glb1-2A-mCherry was generated by Jie Sun et al.,24 which interestingly was shown to predict lifespan expectancy of middle-aged mice and could monitor senolytic treatment (i.e., dasatinib plus quercetin) in a mouse model of bleomycin-induced lung epithelial cell senescence via fluorescence imaging. However, for the purpose of this perspective, we will instead focus on β-gal-targeting small molecule probes for senescence detection in vivo, which will be discussed later. Additionally, those reporter mouse models that were described for imaging applications ex vivo such as INK-ATTAC, p16tdTOM, p16-Cre/R26-mTMG, p16-CreERT2- tdTom, p16-CreERT2-DTR- tdTom, and p21-Cre/+;tdTom/+ are also summarized in Table 1 but will not be discussed in detail due to the focus of the perspective.
| Mouse model | Target gene | Strategy | Reporter gene | Ablator construct | Reported imaging applications | Ref. |
|---|---|---|---|---|---|---|
| Human p16lnk4a | p16lnk4a | Transgenic | fLUC | - | In vivo | 25 |
| INK-ATTAC | p16lnk4a | Transgenic | eGFP | FKBP-Casp8 | Ex vivo | 17 |
| p16LUC | p16lnk4a | Knock-in | fLUC | - | In vivo | 26 |
| P16-3MR | p16lnk4a | Transgenic | rLUC/mRFP | HSV-TK | In vivo | 11 |
| p16tdTOM | p16lnk4a | Knock-in | TdTOM | - | Ex vivo | 27 |
| P16-Cre/R26-mTMG | p16lnk4a | Knock-in | eGFP | - | Ex vivo | 28 |
| P16-CreERT2- tdTom | p16lnk4a | Knock-in | TdTOM | - | Ex vivo | 29 |
| P16-CreERT2-DTR- tdTom | p16lnk4a | Knock-in | TdTOM | DTR | Ex vivo | 29 |
| p21-Cre/+;LUC/+ | p21CIP1 | Transgenic | fLUC | - | In vivo | 6 |
| p21-Cre/+;tdTom/+ | p21CIP1 | Transgenic | TdTOM | - | Ex vivo | 6 |
| p21-Cre/+;LUC/DTA | p21CIP1 | Transgenic | fLUC | DTR | In Vivo | 6 |
| Glb1+/m-Glb1-2A-mCherry | GLB1 | Knock-in | mCherry | - | Ex vivo/in vivo | 24 |
- Abbreviations: DTR, diphtheria toxin receptor; eGFP, enhanced green fluorescent protein; FKBP-Casp8, FK506-binding-protein-caspase 8 fusion protein; fLUC, Firefly luciferase; HSV-TK, truncated herpes simplex virus 1 thymidine kinase; mCherry, mCherry red fluorescent protein; mRFP, monomeric red fluorescent protein; rLUC, Renilla luciferase; TdTOM, tandem-dimer tomato.
p16lnk4a reporter mouse models
In 2009, Yamakoshi et al. developed the first p16lnk4a reporter mouse model (Table 1) using a transgenic approach.25 A large genomic DNA segment of the human chromosome containing the entire Ink4a/Arf gene locus was engineered to express a fusion protein of human p16lnk4a and fLUC to monitor human p16lnk4a gene expression using bioluminescence imaging under various physiological conditions in living mice. A concern of this mouse model would be that human p16lnk4a and mouse p16lnk4a are regulated differently. However, the researchers demonstrated that human p16lnk4a was induced in mouse embryonic fibroblasts derived from p16-luc mice under two settings of senescence induction, serial and ectopic expression of oncogenic Ras, suggesting the applicability of this mouse model for studying the senescence program via human p16lnk4a expression in vivo.25
Later, Burd et al. generated the first p16lnk4a reporter mouse model (Table 1) using a knock-in approach.26 The p16LUC knock-in allele was produce by knocking the complementary DNA of fLUC, followed by an SV40 polyadenylation signal, into the translational start site of the endogenous mouse p16lnk4a locus, Cdkn2a. Using this reporter mouse model, they showed that activation of p16lnk4a directly correlated with chronological age of mice in a lifelong assessment, suggesting the accumulation of senescent cells with age; however, there was high variability in total body p16lnk4a expression in their cohort. Others have utilized this reporter mouse model to identify environment toxicants that induce senescence30 and the tissue specificity of senescent cell accumulation during aging.31
In 2014, the Campisi lab developed a p16lnk4a reporter mouse model (Table 1) with the additional ability to selectively kill senescent cells in vivo, which is referred to as p16-3MR mouse model (Table 1).11 To do so, they created a transgenic mouse line in which the p16lnk4a promoter drove the expression of trimodality reporter fusion protein, 3MR, containing the functional domains of a synthetic rLUC for detection of senescent cells in vivo, mRFP for detection of senescent cells ex vivo, and truncated herpes simplex virus 1 thymidine kinase (HSV-TK) for selective elimination of senescent cells in vivo. HSV-TK expressed through p16lnk4a promoter in senescent cells can lead to apoptosis upon ganciclovir treatment, which is converted into a toxic DNA chain terminator by HSV-TK. Importantly, ganciclovir possesses high affinity for HSV-TK and low affinity for murine and human TK, giving great selectivity for killing cells that express HSV-TK. Using this model, the Campisi lab illustrated that senescent cells and the SASP were important in tissue repair, although their presence was transient.11 This mouse model is a suitable model to study cellular senescence in vivo because of the dual ability to detect senescent cells longitudinally and selectively kill senescent cells with temporal control. Others have utilized the p16-3MR mouse model to study cellular senescence such as asking whether clearance of senescent can promote health in models of various diseases. For example, the Zhou lab used this model to ascertain whether their identified potent senolytic drug, ABT263, could act as a senolytic in vivo.32 Besides these notable examples, a number of other mouse models have been developed to detect senescence via p16lnk4a gene expression.17, 27-29
p21CIP1 reporter mouse models
Compared with p16lnk4a, the role of p21CIP1 in senescence in the in vivo setting is less well studied and remains controversial. It was not until 2021 that Xu and coworkers reported the first p21CIP1 reporter mouse model (p21-Cre/+;LUC/+) (Table 1) and the first p21CIP1 dual reporter/ablator mouse model (p21-Cre/+;LUC/DTA) (Table 1) using a site-specific transgenic approach.6 They produced a p21-Cre mouse line containing a p21 promoter that drives a Cre recombinase (Cre) fused to a tamoxifen-inducible estrogen receptor domain, thereby allowing for induction of Cre upon addition of tamoxifen or 4-hydroxytamoxifen. To generate the p21-Cre/+;LUC/+ mouse model, they crossed the p21-Cre mouse with floxed knock-in fLUC mice, which allows the detection of p21high cells in living mice using bioluminescence imaging. The researchers showed that p21-Cre/+;LUC/+ mice exposed to three different senescent triggers, doxorubicin (a potent chemotherapy drug that damages DNA), obesity, and aging, elicited a significant bioluminescent signal. They also verified that p21high cells exhibited the SASP (i.e., Il6 and Cxcl1), enlarged size, SA-β-Gal activity, reduced proliferation, and loss of Lamin B1, suggesting that this p21CIP1 reporter mouse model is a suitable model to detect senescence. To generate the dual reporter/ablator p21 mouse model (p21-Cre/+;LUC/DTA), they crossed p21-Cre/+;LUC/+ mice with floxed diphtheria toxin A (DTA; protein synthesis inhibitor) mice. This mouse model allows for the specific killing of p21high cells via DTA-induced apoptosis and bioluminescence detection to see if all p21high cells are removed. Using this mouse model, the authors found that old mice (23 months) with p21high cells selectively killed exhibited significantly higher maximal walking speed, grip strength, hanging endurance, daily food intake, and daily activity than old mice without the removal of p21high cells.
Reporter mouse models are powerful tools to study cellular senescence in vivo; however, generating a mouse model using transgenic and knock-in approaches requires a considerable amount of time, cost, and effort. Also, to investigate different diseases, developing models using the reporter mouse models with a specific disease is necessary, further complicating the model development and validation. Moreover, genetically modified animal models are great research tools, but not translatable toward the detection of senescence in humans.
1.2.2 Small molecule-based imaging probes
One of the most notable features of cellular senescence is the increased lysosomal biogenesis and activity, such as lysosomal hydrolytic enzyme β-gal (SA-β-Gal). In most mammalian cells, the lysosomal activity can only be observed at an optimal pH of 4.0, however, due to the highly elevated GLB1 gene encoding β-gal in senescent cells, this activity can be observed at the suboptimal pH of 6.0. Initial evidence describing SA-β-Gal activity as a biomarker for senescence was reported in replicative-induced senescent human fibroblast cells in vitro and in aging skin in vivo using X-Gal staining.33 SA-β-Gal activity was not found detectable in quiescent fibroblasts or terminally differentiated keratinocytes,33 possibly providing a more selective detection of senescence cells over quiescent cells in vivo.34, 35 However, like other senescence biomarkers, high lysosomal β-Gal activity is not unique to the senescent state, posing a specificity issue.36 For example, high lysosomal β-Gal activity has been found in nonsenescent cell types such as early-passage adult melanocytes,33 macrophage-like U937 and HL60 cells,37 and several primary metastatic ovarian cancer cells lines.38 Moreover, cellular conditions such as serum starvation or high-confluency results in high lysosomal β-Gal activity.39 Overexpression of β-Gal has been suggested to not be required for the induction and maintenance of senescence.40 Yet, detection of SA-β-Gal activity is currently the most widely used and well-established method for senescence detection in vitro and in vivo.34, 41 Previously, assessing β-gal activity is useful in the field of molecular biology where it is commonly used as a reporter gene (LacZ), and the imaging probes developed to detect the reporter gene can potentially be explored for their application in senescence research.42 Also, there have been other lysosomal enzymes proposed to be biomarkers for the senescent state,43, 44 and their association with senescence in various tissue or disease types remains to be validated. In this perspective, we will summarize the small molecule probes targeting β-gal (Figures 2 and 3) that have been evaluated or have the potential to detect cellular senescence in vivo.
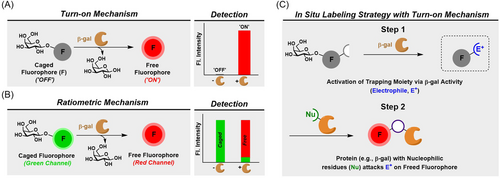
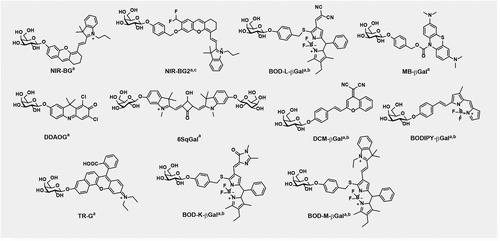
Optical probes for senescence imaging in vivo
In 2019, our lab developed an activatable turn-on near-infrared (NIR) probe for SA-β-gal referred to as NIR-BG (Table 2 and Figure 3) for the detection of DNA-damaged induced senescent cells in vivo.45 Once NIR-BG is activated by β-gal, the NIR fluorescence emission at 708 nm is enhanced 130-fold. In model cells, NIR-BG was able to detect β-gal activity in LacZ-expressing CT26.CL25 cells. NIR-BG was able to differentiate chemotherapy-induced senescent HeLa (human cervical cancer) and MCF7 (human breast cancer) cancer cells versus nonsenescent HeLa and MCF7 cells. Importantly, NIR-BG was also applied in living mice to image DNA-damage-induced senescence in xenograft human tumors and was able to detect chemotherapy-induced SA-β-gal in real time.45
| Probe | Detection approach | Fluorophore ex/em (nm) | Reported imaging applications | Ref. |
|---|---|---|---|---|
| NIR-BG** | Turn-on | 680/708 | CT26.CL25 (LacZ) cells, senescent HeLa and MCF7 cells, CT26.CL25 tumor (mice), senescent HeLa tumor (mice) | 45 |
| BOD-L-βGal** | Turn-on, ratiometric | 651/730 | Senescent VSMCs, Atherosclerotic ApoE−/− mice | 46 |
| NIR-BG2** | Turn-on | 675/709 | Senescent HeLa cells and tumor (mice) | 47 |
| MB-βGal* | Turn-on | 620/690 | Senescent HeLa and MEF cells | 48 |
| DDAOG | Turn-on | 646/659 | 9L-LacZ cells and tumor (mice) | 49 |
| 6SqGal | Turn-on | 600/640 | HEK293T-LacZ cells, pCMV-SPORT β-gal (mice) | 50 |
| DCM-βGal | Turn-on, ratiometric | 535/685 | HEK293T-LacZ cells, OVCAR-3 cells, LoVo tumor pretreated with avidin β-gal (mice) | 51 |
| BODIPY-βGal | Turn-on, ratiometric | 660/730 | SKOV-3 cells, A549 tumor preinjected with exogenous β-gal (mice) | 52 |
| TR-G |
Turn-on, Two-photon# |
550/638 780/638# |
OVCAR-3 cells and tumor (mice) | 53 |
| BOD-K-βGal | Turn-on, ratiometric | 633/715 | SKOV-3 cells and tumor (mice) | 54 |
| BOD-M-βGal | Turn-on, ratiometric | 723/853 | SKOV-3 tumor (mice) | 54 |
- These probes were utilized for detecting cellular senescence in senescent animal models and in senescent cells** or just in senescent cells*. The excitation/emission wavelengths for two-photon imaging#.
In 2020, Chen et al. developed a ratiometric NIR probe called BOD-L-βGal (Table 2 and Figure 3) for imaging atherosclerotic vasculature in vivo by detecting β-gal in senescent vascular smooth muscle cells.46 In the absence of β-gal, the probe exhibits fluorescence with an emission maximum peak at 580 nm when excited with 488 nm. In the presence of β-gal, the fluorophore is freed, eliciting a new fluorescence signal at 730 nm when excited at 651 nm. Given that BOD-L-βGal was able to detect β-gal ratiometrically, the authors applied the probe in senescent vascular smooth muscle cells. Confocal cell imaging using ratiometric detection showed that the probe was able to differentiate senescent vascular smooth muscle cells and nonsenescent vascular smooth muscle cells. Additionally, the probe was formulated into nanoparticles for imaging of senescent atherosclerotic vasculature in ApoE−/− mice. Notably, after injection via the caudal vein, the probe displayed an overall higher NIR fluorescence signal in atherosclerotic mice versus control mice.
A general challenge with activatable small molecule probes is that the released imaging moiety (e.g., fluorophore) can diffuse away from the site of activation, hindering the spatial resolution. This is specifically frustrating when attempting to detect enzymatic activity in cells or in vivo because of the ultimate loss in the signal-to-noise ratio due to diffusion and subsequent excretion. In 2021, our lab developed NIR-BG2 (Table 2 and Figure 3), featuring a caged moiety for in situ labeling installed on the fluorophore.47 Once activated by β-gal, the highly reactive species (i.e., a quinone methide) can label nucleophilic residues of nearby proteins in situ. We showed that this probe could label β-gal itself after activation in vitro and provided a greater signal-to-noise ratio in chemotherapy-induced senescent HeLa cells. Furthermore, NIR-BG2 was able to provide real-time imaging of drug-induced senescence in xenograft tumor models, offering significantly higher detection sensitivity than NIR-BG without the capacity for in situ labeling.
A small molecule probe that can both detect and eliminate senescent cells would be a useful tool to investigate the role of senescent cells in vivo. In 2022, Yang et al. developed an activatable, turn-on NIR probe for β-gal with a 170-fold turn-on ratio based on the methylene blue (MB) fluorophore, termed MB-βgal (Table 2 and Figure 3).48 In the presence of β-gal, MB can be freed to exhibit fluorescence in the far-red region peaking at 690 nm, and MB can serve as a photosensitizer for a photodynamic therapy upon irradiation at 660 nm. Using MB-βgal, the authors demonstrated the visualization and selective killing of senescent HeLa and MEF cells.
Previously, a number of fluorescent probes were developed for the detection of β-gal encoded by LacZ reporter gene or expressed in certain tumors in animal models, and potentially these probes can be applied in senescence models. For example, Corey et al. developed a water soluble probe (now referred to as DDAOG), activatable by β-gal (Table 2 and Figure 3).55 Once the probe is hydrolyzed by β-gal, the 7-hydroxy-9 H-acridin-2-one fluorophore is released and an approximate 50 nm bathochromic shift (608 → 659 nm) in the fluorescence emission spectrum is observed.49 In 2004, the Weissleder lab adapted this far-red probe to image β-gal activity in living mice.49 DDAOG was able to penetrate into cells (i.e., 9L gliosarcoma cells) and was able to detect β-gal activity in 9L cells containing the LacZ gene (9L-LacZ). Then, DDAOG was administered intravenously into mice that bared coimplanted 9L and 9L-LacZ tumors in the mammary fat pad. The results showed specific detection of the 9L-LacZ tumor over the 9L tumors in early as 10 min after probe administration; the signal peaked out at 40–60 min and was completely depleted 5 h after administration.
In 2012, Oushiki et al. developed 6SqGal (Table 2 and Figure 3).50 The structure of 6SqGal contains two β-galactosyl groups conjugated to 6SqOH, a NIR polymethine dye that turns on due to interaction with proteins. Thus, removal of two β-galactosyl groups by β-gal unleashes the free dye, 6SqOH, to interact with β-gal or other proteins to enable its fluorescence enhancement. It is important to note that 6SqGal can be turned on in the presence of bovine serum albumin (BSA) without the need for β-gal. Thus, β-gal-independent staining might arise, leading to unspecific staining. HEK293-LacZ cells incubated with 6SqGal showed bright, diffuse fluorescence staining whereas HEK293 cells treated with 6SqGal showed little fluorescence. Furthermore, 6SqGal was administered intravenously into mice that contained either a liver-targeting β-gal-encoding plasmid or a negative control plasmid. Fluorescence images acquired sequentially for 30 min revealed that the liver of mice containing the liver-targeting β-gal-encoding plasmid showed fluorescence enhancement in a time-dependent manner. The average fluorescence intensity observed in the liver was also significantly different in mice containing the β-gal-encoding plasmid versus the ones with the negative control plasmid. However, it is important to note that the fluorescent images do show that the probe possibly elicits nonspecific staining in other organs.
In 2016, Gu et al. developed DCM-βGal (Table 2 and Figure 3), a dual ratiometric and turn-on NIR probe targeting β-gal.51 Once DCM-βGal is hydrolyzed by β-gal, the dicyanomethylene-4H-pyran (DCM) fluorophore is liberated, turning on the fluorescence. The probe is excited at 450 nm, and its fluorescence signal can be detected ratiometrically using the intensity of light generated at 685 nm over that of 500 nm. DCM-βGal was applied to detect β-gal activity in living HEK293T-LacZ cells and OVCAR-3 cells using both ratiometric and turn-on detection. Furthermore, they applied their probe in vivo to detect β-gal activity in human colorectal cancer LoVo cell xenografts in mice given a commercial tumor-targeting reagent avidin-β-gal, which specifically localizes β-gal to tumor. After intratumoral injection, serially acquired fluorescence images of living mice showed real-time detection of β-gal-expressing tumors in vivo. They revealed that the signal was captured in the liver, suggesting that the probe was distributed to and cleaved within the liver even though it was injected intratumorally.
In 2019, a BODIPY-based ratiometric/turn-on NIR fluorescent β-gal probe was developed (Table 2 and Figure 3, BODIPY-βGal).52 The intact probe, BODIPY-βGal, elicited a robust fluorescence emission at 575 nm. Upon cleavage of BODIPY-βGal, the masked BODIPY-OH is released, resulting in an enhanced fluorescence emission peak at 730 nm and a decrease in the initial fluorescence intensity at 575 nm; this event occurred in a β-gal activity-dependent fashion. The probe was applied to ratiometrically track β-gal activity in living human ovarian cancer cells expressing β-gal (i.e., SKOV-3). The probe was able to detect β-gal in SKOV-3 cells in a ratiometric manner. BODIPY-βGal was also applied in vivo for the real-time visualization of β-gal activity in tumors. In a human lung xenograft tumor cell mouse model, injection of BODIPY-βGal intratumorally showed a robust fluorescent signal at 730 nm in tumors preinjected with β-gal. Tumors that were not preinjected with β-gal did not show any significant fluorescence at 730 nm.
In 2020, Li et al. developed TR-G (Table 2 and Figure 3), a two-photon, far-red fluorescent probe targeting β-gal.53 The structure of TR-G includes a far-red fluorophore Rho linked to a β-galactosyl moiety. In the presence of β-gal, the probe is hydrolyzed, releasing Rho and consequently, producing an enhanced fluorescence response at 638 nm in a β-gal activity-dependent manner. TR-G was shown to be selective over other biological species in vitro. TR-G was able to detect β-gal activity in living OVCAR-3 cells and in OVCAR-3 tumor-bearing mice after hypodermic injection. Notably, TR-G was also able to stain OVCAR-3 tumors ex vivo and be imaged by two-photon microscopy.
In 2020, Chen et al. developed two β-gal activatable, ratiometric, and turn-on NIR probes, termed BOD-K-βGal and BOD-M-βGal (Table 2 and Figure 3).54 After incubation of BOD-K-βGal with β-gal, the NIR fluorescence emission at 715 nm is enhanced, while the emission at 565 nm is decreased. This probe was evaluated to detect β-gal activity in SKOV-3 cells and in SKOV-3 tumor-bearing mice. On the other hand, BOD-M-βGal after incubation with β-gal the NIR fluorescence emission at 853 nm is enhanced and emits some light in the NIR-II region (1000–1200 nm). Thus, the authors utilized the probe for NIR-II imaging, which offered deeper tissue penetration than NIR-I.
In summary, optical probes have been used extensively in molecular imaging of various molecular targets in various physiopathological conditions.56 Also, optical probes can be easily adapted to various animal models. In preclinical studies with small animals, optical probes are preferred due to their convenience, low cost, high sensitivity, spatiotemporal resolution, and safety profile.57 However, optical fluorescence imaging typically suffers from low penetration depth (subcentimeter), meaning that physical barriers such as skin and tissue will interfere with detection. For imaging of cells in culture, penetration depth is not a matter of importance and thus, fluorescent based probes with short excitation/emission wavelengths (<600 nm) may be utilized. On the contrary, depth of penetration is a highly important parameter for in vivo imaging. To overcome this limitation, the optical based probes discussed herein contain NIR or far-red fluorophores, providing deeper penetration depth as well as other advantageous imaging properties such as reduced tissue photodamage and background autofluorescence43 for applications in live animal (e.g., rodents) imaging. However, optical probes are constrained toward clinical use in humans for deep-tissue imaging due to its limited penetration depth, even in the case of NIR or far-red fluorophores, although there have been applications in humans where penetration depth is less of a concern such as fluorescence-guide surgery.58 Importantly, small molecule based probes can be adapted for a wide variety of imaging modalities, including those that are translatable to clinical use in humans.57
Clinically relevant small molecule probes for senescence detection
To facilitate clinical translation, small molecule probes based on modalities such as positron emission tomography (PET), single photon emission computed tomography (SPECT), or magnetic resonance imaging (MRI) can be developed as desired. The basic principles of each modality, advantages, and major limitations have been reviewed in detail elsewhere57 and will not be reiterated here. To our knowledge, clinically relevant small molecule probes for senescence detection are scarce, representing a significant gap in our senescence detection toolkit. Yet, there have been a few reported small molecule probes targeting β-gal designed for MRI or SPECT/PET imaging. Here, we will summarize the notable MRI and PET probes reported in the literature that may have the potential for in vivo imaging of senescence via β-gal detection.
MRI probes
In 1997, the Meade group reported the first activatable, turn-on MRI contrast agent developed for β-gal referred to as Egad (Table 3 and Figure 4).59 This probe is based on Gd3+ for MRI detection. In the absence of β-gal, the β-galactosyl moiety proposedly blocks water from binding to the remaining coordination site on the Gd3+, which renders the probe in the “OFF” state. Once β-gal cleaves the β-galactosyl moiety, water can bind to the coordination site of Gd3+ allowing the probe to be irreversibly turned “ON.” However, Egad demonstrated poor contrast (i.e., 20% change in relaxivity after being exposed to β-gal) and was inadequate for robust in vivo imaging. Therefore, in 2000, the Meade group developed α-EgadMe (Table 3 and Figure 4) to increase the signal contrast in response to β-gal. This probe features an α-methyl group on the appending arm of the tetraazacarboxylic macrocycle to restrict the rotation of the blocking β-galactosyl moiety, thereby ensuring the probe in the “OFF” state. α-EgadMe was introduced through microinjection into Xenopus laevis embryos and was able to detect β-gal activity via T1-weighted MRI.60 Additionally, the Meade group developed β-EgadMe (Figure 3), which features a β-methyl group on the appending arm of the tetraazacarboxylic macrocycle.61 Interestingly, the β-EgadMe probe for “smart” MRI detection is rendered in the “OFF” state, not by the blocking β-galactosyl moiety, but rather due to endogenous carbonate binding to Gd3+.62 After β-gal activation, the cleaved hydroxyl group of the β-galactosyl moiety displaces the carbonate, increasing the relaxivity.62 However, these probes developed by Meade suffer from low reactivity with β-gal, and are unable to penetrate cell membranes, thus delivery methods of EgadMe must be developed to realize its potential for applications in small animals.62
| Probe | Imaging moiety | Modality | Reported imaging applications | Ref. |
|---|---|---|---|---|
| Egad | Gd3+ | MRI | In vitro | 59 |
| α-EgadMe | Gd3+ | MRI | Xenopus laevis (LacZ) | 60 |
| β-EgadMe | Gd3+ | MRI | Xenopus laevis (LacZ) | 61 |
| [Gd(DOTA-FPG)H2O] | Gd3+ | MRI | CT26-LacZ tumors (mice) | 63 |
| PFONPG | 19F | MRI | PC-3-LacZ cells, LNCAP C4-2-LacZ cells | 64 |
| OFPNPG | 19F | MRI | MAT-Lu-LacZ cells, MTLn3-LacZ cells, PC3-LacZ tumors (mice), MCF7-LacZ tumors (mice), | 65-67 |
| PCF3ONPG | 19F | MRI | MCF7-LacZ cells | 68 |
| [123I/125I]Iodo-PETG | 123I/125I | SPECT | COS-7 (LacZ) cells, SK-N-SH tumors preinjected with Ad-CMV-βgal/GFP (mice) | 69 |
| [18F]-2c | 18F | PET | HEK293T-LacZ cells | 70 |
| [11C]-3c | 11C | PET | HEK293T-LacZ cells | 70 |
| [18F]FPyGal | 18F | PET | N/A | 71 |
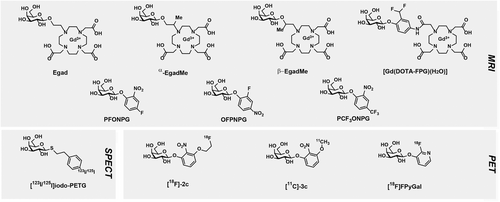
In 2004, the Mason lab developed p-fluoro-o-nitrophenyl-β-D-galactopyranoside (PFONPG) (Table 3 and Figure 4), a fluorinated analog of the well-known colorimetric and spectrophotometric biochemical agent, o-nitrophenyl β-D-galactopyranoside (OPNG), for the detection of β-gal via 19F MRI.64 The advantage of activatable 19F MRI probes for detection in cells and in vivo is that there is essentially no naturally occurring endogenous 19F signal and thus, no competing, background signal. However, the spatial resolution of 19F MRI is inferior to that of 1H MRI. Activatable 19F MRI probes are detected by changes in the 19F chemical shift value resulting from enzymatic activity. PFONPG could enter cells and was able to differentiate β-gal-expressing prostate cells (PC-3 or LNCAP C4-2) versus wild-type controls through the observed change (>3.6 ppm) in chemical shift of the 19F signal of the intact probe, PFONPG, and the released moiety, o-nitrophenol. However, a major limitation of PFONPG for in vivo use is that the released moiety, o-nitrophenol, is cytotoxic and is not trapped within cells. Additionally, in 2004, the Mason lab synthesized six additional fluorine-bearing analogs of OPNG and described their ability to detect β-gal in cells.66 In this study, o-fluoro-p-nitrophenyl-β-D-galactopyranoside (OFPNPG) (Table 3 and Figure 4) was found to be able to detect β-gal activity in β-gal-expressing MAT-Lu rat prostate cancer cells (MAT-Lu-LacZ) and MTLn3 breast cancer cells (MTLn3-LacZ). Notably, the released moiety of OFPNPG was less toxic than that of PFONPG. In a 2007 report, intratumorally injected OFPNG was able to differentiate β-gal-expressing prostate tumor (PC3-LacZ) xenografts65 and MCF7-LacZ breast tumor xenografts67 in mice. In another study, the Mason lab synthesized a series of trifluoromethyl (CF3)-bearing analogs of OPNG to enhance the signal-to-noise ratio by threefold, compared with a single fluorine atom. Despite an enhanced signal compared to OFPNPG, their lead compound, PCF3ONPG (Table 3 and Figure 4), and the freed moiety after β-gal cleavage, PCF3ONP, revealed a much smaller chemical shift difference (1.14 ppm), which is not likely feasible for detection in vivo.67, 68 The Mason lab has developed several other MRI probes for detecting β-gal activity, but some depend on the codelivery of ferric ions to chelate with the released moiety for MRI contrast.72-75 In 2007, Chang et al. developed a smart MRI contrast agent targeting β-gal with the aim to improve the enzyme-mediated relaxivity enhancement. The MRI probe [Gd(DOTA-FPG)(H2O)] (Table 3 and Figure 4) takes advantage of an β-gal-activatable “trapping” group (i.e., difluoromethyl moiety) linked to the Gd3+ chelate to react with nearby proteins such as human serum albumin (HSA) or β-gal after activation. The Gd3+ chelate-protein adduct will produce a higher relaxivity than the Gd3+ chelate alone due to a resulting larger rotational correlation time value of high molecular weight molecules such as macromolecules.76 In vitro, they reported that the probe's relaxivity when incubated with β-gal (2 μM) and HSA (0.5 mM) is about approximately fourfold higher than in the presence of only β-gal (2 μM) as well as in the absence of the two proteins. Importantly, they applied their probe to detect β-gal in Balb/c mice bearing both CT26/β-gal tumors and control CT26 tumors. After intravenous injection (0.3 mmol/kg), the results indicated that this probe could act as an MRI contrast agent for detecting β-gal in vivo. However, as other similar MRI probes failed to show contrast in vivo—most likely due to their low cell permeability, the authors did not explicitly discuss how the probe crossed the cell membrane. In vitro, it was shown that [Gd(DOTA-FPG)(H2O)] when incubated with HSA alone led to a approximately twofold higher relaxivity, compared with [Gd(DOTA-FPG)(H2O)] alone. They suggested that this was a result of the probe noncovalently bound to HSA. Presumably, their probe could have entered cells via the help of HSA, as albumin-based systems have been extensively used for intracellular delivery of drugs.77
SPECT and PET probes
In 2004, Lee et al. developed a radioiodine-labeled competitive inhibitor against E. coli β-gal, termed [123I/125I]IodoPETG (Table 3 and Figure 4), for the imaging of β-gal in LacZ-expressing tumors in mice using SPECT.69 The thiol group substitution hinders the β-galactosyl moiety from being hydrolyzed, and thus inhibits the enzymatic activity of β-gal. IodoPETG (Ki = 43.7 μM) showed a fivefold higher inhibition constant (Ki) than the native PETG (Ki = 7.9 μM), without iodine present on the phenyl ring. Using SPECT, the probe was able to visualize LacZ-transduced tumors, presumably via noncovalently binding to β-gal, retaining the signal intracellularly. Thus, inhibitor-based probes such as IodoPETG do not detect enzymatic activity, but rather the expression of the enzyme. The tissue/muscle uptake ratio of probe was only ∼1.4-fold higher in LacZ-transduced tumors versus control tumors. Additionally, the authors revealed that the biodistribution data suggested a poor rate of transport into tumors as the tumor uptake was similar to blood activity.
In 2008, Celen et al. synthesized and evaluated derivatives of ONPG labeled with 18F or 11C for imaging β-gal in Lac-expressing tumors in mice using PET. Cell experiments suggested that both these probes could enter cells, with [18F]-2c (Table 3 and Figure 4) exhibiting a higher cell uptake in a shorter-time frame in β-gal-expressing cells versus control cells compared to [11C]-3c (Table 3 and Figure 4). Thus, [18F]-2c was the only probe promoted to further in vitro and in vivo experiments. Importantly, further investigation of this probe revealed that unfortunately, the majority of the hydrolyzed product diffused out of the cells after β-gal cleavage. In their PET imaging of β-gal-expressing tumors, intravenous injection of [18F]-2c failed to accumulate in β-gal-expressing tumors, which they reasoned to be due to poor passive diffusion of their probe into tumors. This was similar to what was observed for iodoPETG, and thus this issue represents a general problem for this type of probes.
In 2018, a 18F-radiolabeled probe targeting β-gal, termed [18F]FPyGal (Table 3 and Figure 4), for PET imaging of senescence was patented.71 The inventors stated that [18F]FPyGal could accumulate selectively in senescent cells as cleavage of the probe through β-gal activity would result in trapping and enrichment of 18F signal within the senescent cells, allowing for cellular senescence detection by PET. Currently, the Phase I/II clinical trial is in the recruiting stage to assess its safety, radiation exposure, and diagnostic accuracy for tumor imaging in oncological patients (NCT04536454).
Overall, the ability of MRI probes to provide contrast is based on their ability to cross cell membranes, engage with the intracellular enzyme target (e.g., β-gal) to enhance their relaxivity, and have their imaging moiety retained at the site of activation. Due to poor penetration into cells, MRI agents are generally limited to the extracellular space.57 Thus, formulations or structural modifications to improve their cell-penetrating ability is warranted for detection of intracellular targets. For example, Keliris et al. developed a dual-labeled β-gal-targeting MRI probe, Gd-DOTA-k(FR)-Gal-CP, which incorporated a cell-penetrating peptide into the structural design to enhance the MRI probe's cell permeability.78 Additionally, enhancing sensitivity is essential because MRI probes generally require high concentrations (>0.1 mM) to observe contrast—this large concentration poses toxicity issues as most common MRI probes are based on gadolinium (III) (Gd3+), which is toxic if not chelated.62 To observe image contrast using SPECT and PET probes, the probe must enter the target organ/tissue, be cleaved by or bind to the target intracellular enzyme to trap the signal moiety within the cell, and any remaining probe must be rapidly cleared from blood circulation. As radioisotope cannot be turned on or off in the presence of the target molecule, the PET contrast arises from specific uptake and retention. Some PET probes suffer from poor cellular uptake and may require delivery systems to reach their intracellular targets. Furthermore, strategies to alleviate the ability of the cleaved radiolabeled moiety of PET probes to diffuse away from the site of activation may be used as needed. Our lab has been developing PET and MRI senescence probes that can potentially be translated for human use with enhanced sensitivity using a self-immobilizing strategy.79
2 CONCLUSION AND PERSPECTIVE
We believe that molecular imaging probes and genetically engineered mice are powerful tools to study cellular senescence in vivo. With molecular imaging probes, different imaging modalities and strategies may be utilized and thus, the need for specific criteria can be adopted for specific applications. For example, in preclinical studies, optical probes are the most convenient, but for clinical use, their applications are limited. Therefore, clinical-translatable probes with imaging modalities such as MRI, SPECT, and PET are favorable. The current arsenal of molecular imaging probes to detect senescence in vivo is based on the detection of a single biomarker such as β-gal. However, the significant specificity and heterogeneity challenges with the known biomarkers for detection of senescence in vitro and in vivo remain. To gain more confidence in specific senescent cell detection, in vitro assay protocols may utilize a combinatorial approach to detect multiple senescent biomarkers simultaneously through various methods such as qPCR, western blotting, and staining.5, 7, 15, 18, 80 The key question is how to use a combinatorial approach for designing small molecule imaging probes to be used for a more specific senescence detection in living animals. Answering this question will unveil the future of next-generation probes for specific senescence detection in living systems. One possibility is to use a “multicaged” probe design, where successive activation of the probe by multiple targets is required to “turn-on” the imaging moiety. In addition to alleviating the risk of false-positive signals, the resulting probes are expected to show lower background fluorescence, aiding the accuracy of detection.81 Using modular chemistry, our lab has been developing a platform for the generation of specific senescence probes for imaging senescence in different scenarios, such as during cancer therapy, inflammation, diabetes, and neurodegenerative diseases. Beyond sensitivity and selectivity, other vital characteristics of small molecule imaging probes that needs not to be overlooked are good pharmacokinetics (i.e., bioavailability, stability) and low toxicity. Optimizing these parameters is necessary during the design process and development of probes.
AUTHOR CONTRIBUTIONS
Lina Cui: Formal analysis; validation; writing—original draft; writing—review & editing. Zachary M. Rabinowitz: Formal analysis; writing—original draft; writing—review & editing.
ACKNOWLEDGEMENTS
This work is supported by research grants to Prof. L. Cui from the University of Florida, and the National Institute on Aging (R41AG081007). Z.R. is partially supported by the UF Graduate School Preeminent Scholarship.
CONFLICT OF INTEREST STATEMENT
Prof. L. Cui is the founder of SenoTrac Biotechnology, a private company developing senescence imaging probes for various applications. The University of Florida has filed patent applications for a variety of senescence imaging probes.
Open Research
DATA AVAILABILITY STATEMENT
Not applicable.




