Acute myeloid leukemia in elderly patients: New targets, new therapies
Abstract
Prior to the past few years, the development of new therapies for acute myeloid leukemia (AML) has been disappointingly slow. For several decades, the standard therapy for AML has consisted of intensive induction chemotherapy, and potentially a subsequent hematopoietic stem cell transplant. Unfortunately, older patients are less responsive to, and are frequently unfit to tolerate, such intensive chemotherapy. Given that a majority of AML patients are elderly, this population has been most affected by the lack of newer less toxic therapies. However, in recent years, the treatment landscape for AML has dramatically shifted with the approval of many new drugs. As summarized in this review, several of these new drugs are targeted agents that are better tolerated than standard chemotherapy and could substantially benefit elderly patients. Although drug resistance remains a major concern, the treatment options for elderly AML patients are more numerous than ever before, bringing new promise for improved patient outcomes.
1 ACUTE MYELOID LEUKEMIA
Acute myeloid leukemia (AML) comprises a heterogenous group of malignant disorders characterized by aberrant clonal expansion and accumulation of immature myeloid precursors (myeloblasts) in the bone marrow (BM), peripheral blood, and other tissues. The expansion of myeloblasts occurs at the expense of normal production of myeloid lineage cells including erythrocytes, monocytes, granulocytes, and megakaryocytes.1
1.1 Diagnosis and clinical presentation
The median age at AML diagnosis is 67 years old, with approximately 30% of diagnosed patients above the age of 75 years.2 While having a myeloblast count of ≥20% in the BM or blood is generally considered a key diagnosis criteria (with the exception of AML with recurrent genetic abnormalities),3, 4 defining AML subtypes requires further analysis, such as immunophenotyping and cytogenetics. A wide range of genetic abnormalities can occur during various stages of leukemogenesis, implicating the complexity and heterogeneity of the disease.5, 6 Although the clinical presentation of AML at diagnosis varies in each case, patients with AML generally present with symptoms related to pancytopenia, including fatigue and weakness on exertion due to anemia, recurrent infections from neutropenia, and occasional bleeding due to thrombocytopenia.1 There have been accumulating incidences of other complications, including extramedullary leukemic infiltration of soft tissues, lymphadenopathy, and some serious complications, such as hyperleukocytosis7 and intracranial bleeding8 that can ultimately lead to death.
1.2 Incidence and survival rate
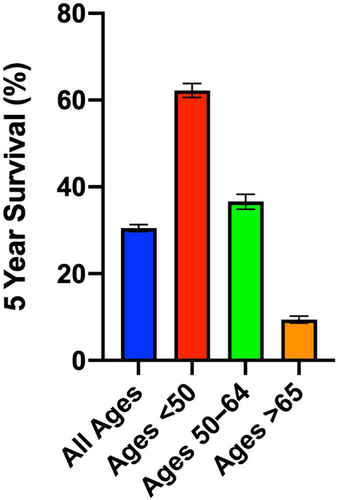
According to the data from the Surveillance, Epidemiology, and End Results (SEER) program of the National Cancer Institute, the estimated number of new cases of AML in the United States during 2022 is 20,050, while the estimated number of deaths from AML is 11,540. Since the 1970s, age-adjusted rates for new AML cases and deaths have been stable and an increasing trend in 5-year relative survival rate has been observed. Compared to other hematological malignancies, however, AML has the lowest 5-year survival rate. According to the data from SEER 17 2012–2018, the 5-year survival rate for AML was 30.5%, compared to 70.8% for acute lymphocytic leukemia, 87.9% for chronic lymphocytic leukemia, and 70.4% for chronic myeloid leukemia. An improvement in curing AML has been observed in young patients (<40 years of age), but not in older patients, as they tend to have significantly less tolerance to intensive therapy and more comorbidities compared with young patients.9 Indeed, 5-year survival for elderly AML patients (>65 years) is dismal compared with that of younger patients (Figure 1).
1.3 Mutational complexity of AML
Evidence from studies looking at mutational complexity of AML has suggested that a wide range of driver and secondary mutations are required for the development of the disease. According to the mutational analysis of 19 genes (FLT3, NPM1, DNMT3A, NRAS, CEBPA, TET2, WT1, IDH2, IDH1, KIT, RUNX1, MLL-PTD, ASXL1, PHF6, KRAS, PTEN, TP53, HRAS, and EZH2) from patients with newly diagnosed AML who were enrolled in the Eastern Cooperative Oncology E1900 clinical trial (n = 398 patients), Patel et al. identified diverse mutation combinations involving frequently co-occurring mutations or mutations that were mutually exclusive.10 It is of importance to note that there has been accumulating evidence that supports the role of clonal heterogeneity and tumor evolution in recurrence and therapeutic resistance in many different types of cancer.11 The complexity and heterogeneity of AML pose challenges to therapeutic advances, leading to extensive efforts to understand the order of mutational events during leukemogenesis. Whole-genome sequencing data have shown that the mutational spectrum between AML cells and normal hematopoietic stem and progenitor cells (HSPCs) is very similar, suggesting that the majority of the somatic mutations in AML are preexisting background mutations in the HSPCs, and AML is not a disease caused by hundreds of mutations, but only a few initiating events including mutations in NPM1, IDH1, IDH2, TET2, RUNX1, ASXL1, PTPN11, DIS3, KIT, SMC3, and STAG2.12 In addition, a recent independent study using high-throughput single-cell DNA sequencing in 154 AML samples not only provided a validation for the clonal relationship among AML driver mutations, but also revealed novel clonal relationships, such as between TP53 and PPM1D.13
Using whole-genome sequencing or whole-exome sequencing, Ley et al. analyzed genomes of 200 clinically annotated adult cases of de novo AML.5 From this study, they found that nearly all samples had at least one nonsynonymous mutation in one of nine categories of genes, including signaling genes (59%), DNA methylation-related genes (44%), chromatin-modifying genes (30%), the gene encoding nucleophosmin (NPM1) (27%), myeloid transcription factor genes (22%), transcription factor fusions (18%), tumor suppressor genes (16%), spliceosome complex genes (14%), and cohesion complex genes (13%). While they identified 23 genes that were recurrently mutated, only four genes were mutated in patients with frequencies of 20% or higher: FMS-like tyrosine kinase 3 (FLT3) (28%); NPM1 (27%); and DNA methyltransferase 3A (DNMT3A) (26%) and isocitrate dehydrogenase 1 or 2 (IDH1 or IDH2) (20%). These four genes have distinctive protein functions, types of mutations and their consequences, and different prognostic impacts (Table 1). Among these, FLT3 mutations are most common in adult patients with AML and are particularly associated with poor prognosis, often in combination with other genetic abnormalities. While the frequency of each mutation varies depending on the patient population being studied, Metzeler et al. compared the frequency of mutations in younger patients (<60 years old) and elderly patients (>60 years old). In their study, they found a significantly higher frequency of IDH2 mutation in elderly patients than younger patients (18% vs. 11%), whereas other mutations did not show significant differences between these populations (elderly patients vs. younger patients: 27% vs. 32% for internal tandem duplication mutation in FLT3 [FLT3-ITD]; 32% vs. 34% for NPM1; 35% vs. 29% for DNMT3A; and 7% vs. 7% for IDH1).14
| Gene | Protein functions | Type of mutations and consequence | Prognosis | References |
|---|---|---|---|---|
| FLT3 | Receptor tyrosine kinase | In-frame internal tandem duplications (ITDs) in the juxtamembrane domain | ITDs are adverse, TKDs are unclear | 15-18 |
| Missense mutations in tyrosine kinase domain (TKDs) | ||||
| Constitutive activation leading to aberrant downstream signaling | ||||
| NPM1 | Ribosome biogenesis, chromatin remodeling, and DNA repair | Out-of-frame insertions at the C terminus | Favorable, (especially in the absence of FLT3-ITD) | 19 |
| Loss of nuclear localization signal and gain of nuclear export signal | ||||
| DNMT3A | De novo methylation of cytosines in CpG dinucleotides | Missense mutations in methyltransferase domain or truncations | Generally adverse (especially with concomitant FLT3 mutations | 20, 21 |
| LOF | ||||
| Others | ||||
| IDH1 and IDH2 | Decarboxylation of isocitrate to α-ketoglutarate and conversion of NADP+ to NADPH (IDH1: cytoplasmic; IDH2: mitochondrial) | IDH1: Missense R132 mutations | Highly variable | 22, 23 |
| IDH2: Missense R140 and R172 mutations |
1.4 FLT3 mutations in AML
FLT3 is a transmembrane receptor tyrosine kinase. An extracellular FLT3 ligand binds and activates FLT3, promoting proliferation, cell survival, and differentiation through its downstream signaling pathways including PI3K, mTOR, RAS, ERK, and JAK/STAT.24 Internal tandem duplication (FLT3-ITD) mutations are replicated sequences with variable sizes in the juxtamembrane domain and/or tyrosine kinase domain 1 (TK1) and represent the majority of FLT3 mutations (25% of mutations in AML). While FLT3-ITD has been generally associated with poor prognosis, some FLT3-ITD-related variables such as the length or insertion site of FLT3-ITD have been shown to be associated with worse overall survival and relapse-free survival.16, 25, 26 Point mutations in the tyrosine kinase domain (FLT3-TKD), on the other hand, comprise about 7% of mutations in AML and its prognostic impact has not been clearly defined due to conflicting results between studies.24 Both FLT3-ITD and FLT3-TKD mutations constitutively activate FLT3 activity, leading to aberrant activation of multiple downstream signaling pathways.
Comparative analyses of AML samples from patients at diagnosis and relapse have shown that FLT3-ITD mutations that were originally undetectable at diagnosis can become detectable later at relapse, suggesting that clones with FLT3-ITD mutations may undergo clonal expansion and become a dominant population at relapse after the selective pressure of chemotherapy.27-30 Indeed, primary samples from relapsed AML patients demonstrated more significant sensitivity to FLT3 inhibitors than samples obtained from AML patients at diagnosis in vitro, suggesting that AML cells at relapse are more dependent on FLT3 signaling.31 In addition, patients who had new FLT3-ITD mutation at relapse were shown to have shorter overall survival,32 as well as significantly worse prognosis even after allogenic hematopoietic stem cell transplant (alloHSCT) than those who maintained wild-type FLT3.33, 34
Taken together, many studies have demonstrated the prognostic impact of FLT3 mutations in both newly diagnosed and relapsed/refractory AML. As FLT3 mutations can be detectable throughout the course of disease progression from diagnosis to relapse, comprehensive and effective testing for FLT3-ITD mutations at multiple time points may be necessary for better outcomes by allowing early intervention with FLT3-ITD-targeted therapies.35
1.5 IDH mutations in AML
Isocitrate dehydrogenase (IDH) is an enzyme that has been shown to play a role in the Krebs cycle, wherein it catalyzes the oxidative decarboxylation of isocitrate, resulting in alpha-ketoglutarate (αKG) and carbon dioxide. The oxidative decarboxylation requires NADP+, which subsequently gets converted to NADPH, providing reducing energy for redox homeostasis as well as facilitating other metabolic reactions such as fatty acid synthesis.36, 37 The isoforms IDH1 and IDH2 encode proteins located in cytosol and mitochondria, respectively.
Mutations in IDH1 and IDH2 occur at conserved arginine residues within the active site of enzyme (IDH1: R132 locus, IDH2: R140 and R172 locus, more commonly at R140).38 These mutations block the normal conversion of isocitrate to αKG, and instead cause a reverse reaction where αKG is converted to 2-hydroxyglutarate (2HG).39 As a result, the “oncometabolite” 2HG competitively inhibits αKG-dependent enzymes, including TET2, resulting in a wide range of consequences such as DNA and histone hypermethylation, aberrant gene expression, and leukemogenesis.40-42 While IDH mutations comprise about 20% of mutations in AML, studies have shown that the prevalence of IDH mutations significantly correlates with increasing patient age.14, 43-45 The impact of IDH mutations on AML prognosis is highly variable and context dependent. Notably, Prassek et al. demonstrated that no elderly patients (>75 years of age) with IDH1-mutated AML reached complete remission (CR), making the overall survival of this group significantly shorter than that of elderly IDH1 wild-type patients who received induction chemotherapy.46 In a separate study, on the other hand, Patel et al. showed that IDH mutations co-occurring with NPM1 mutations (without FLT3-ITD mutations) confer favorable outcomes in younger patients (<60 years of age).10 While IDH2 mutation alone was shown to confer a favorable prognosis in the meta-analysis by Zhou et al.,45 Papaemmanuil et al. showed that patients with concomitant IDH2 and DNMT3A mutations had a significantly poorer prognosis.47
1.6 BCL-2 in AML
BCL-2 and its family members are pro-survival proteins that block the mitochondrial pathway of apoptosis. BCL-2 family proteins bind to and sequester factors that promote mitochondrial outer membrane permeabilization (MOMP), preventing apoptosis and promoting cell survival.48 While mutation of BCL-2 is uncommon in cancer in general, BCL-2 is commonly overexpressed in AML and is associated with resistance to chemotherapy, a lower CR rate, and poor overall survival.49 Moreover, BCL-2 has been shown to be important for maintaining oxidative phosphorylation in AML leukemia stem cells (LSCs), which has been shown to be critical for LSC survival.50
2 AML THERAPY
Treatment of AML via conventional chemotherapy typically comprises two phases: induction and consolidation. The goal of induction therapy is to achieve a CR, usually by intensive chemotherapy. Induction therapy is usually immediately followed by consolidation therapy. As a postremission therapy, consolidation therapy uses similar or slightly lower doses of drugs than induction therapy. Key factors of the treatment course (number of cycles, durations, and drug dosages/regimens) are determined by patients’ age and comorbidities.1
Since the introduction of the intensive regimen combining anthracycline and cytarabine in the 1970s, this combination regimen, known as the “7+3” regimen (7 days of cytarabine + 3 days of daunorubicin or idarubicin), has been considered as the standard therapy in AML. While the outcomes for this regimen have been promising with about 70% CR for younger patients (<60 years of age), it has been poorly tolerated by the elderly patient population that comprises the majority of patients with AML or patients with comorbidities.51, 52 Only recently have treatment strategies for AML made significant progress, with the development of molecularly targeted therapies and the implementation of personalized medicine, as there has been an increased understanding of the genomic landscape and pathophysiology of AML (Figure 2).

3 TREATMENT OPTIONS FOR ELDERLY AML PATIENTS
3.1 Standard chemotherapy
For younger patients (<60 years of age), the primary focus of treatment is to achieve a cure with induction + maintenance therapy, and failures of treatment are often due to relapse from CR rather than treatment-related mortality or development of resistance.1 As mentioned before, the outcomes of 7+3 standard therapy have been effective for about 70% of this group of patients, and an increasing body of research suggests modifications of this regimen can lead to better outcomes. However, these modifications will not be applicable to elderly patients (>60 years of age) as their tolerance to intensive therapy is significantly worse than younger patients. Because of their lack of tolerance and presence of other comorbidities, treatment for elderly patients is usually managed differently compared to young patients. For elderly patients without high-risk karyotypes or myelodysplastic syndrome (MDS), however, intensive induction therapy can be considered as CR rates of 70% were achieved in the study by Knipp et al.52 A novel liposomal formulation of cytarabine and daunorubicin, CPX-351, has shown superior overall survival compared to standard 7+3 therapy in fit, older patients with newly diagnosed secondary AML.53 However, venetoclax + azacytidine therapy (discussed below) demonstrated comparable efficacy in older AML patients, with a more favorable toxicity profile.54
As postremission management, patients who received alloHSCT for consolidation therapy showed significantly increased 5-year overall survival compared to those who received chemotherapy as consolidation therapy (19% vs. 9%). Notably, this comparative analysis revealed that relative rates of relapse or death in patients who received alloHSCT were not associated with cytogenetic risk categories.55 Due to transplant-related mortality, however, the choice of postremission therapy type should be carefully made. Although transplant-related mortality has steadily decreased over recent decades, no substantial progress has been made in reducing the risk of relapse, the major cause of transplant failure.56 The identification of patients who are likely to benefit from alloHSCT requires a comprehensive assessment of many variables to estimate a transplant-related mortality score.57, 58 Historically, elderly patients have not been considered for alloHSCT due to the presence of comorbidities and high risk of mortality.59 While there has been an increasing evidence that demonstrates the benefit of alloHSCT in fit elderly patients after intensive chemotherapy,60, 61 only a small portion of elderly patients receive alloHSCT.60, 62, 63
Fortunately, several molecularly targeted therapies, with fewer side effects compared to conventional chemotherapy, have been recently approved by the Food and Drug Administration (FDA), and three regimens have been approved for newly diagnosed elderly AML patients (>75 years of age) or patients with comorbidities that preclude intensive chemotherapy (Figure 2). These regimens are as follows: (1) venetoclax in combination with hypomethylating agents (HMAs) such as azacitidine, decitabine, or low-dose cytarabine (LDAC); (2) glasdegib + LDAC; and (3) ivosidenib + azacitidine.
3.2 Targeted therapy: BCL-2
In November 2018, the FDA approved the BCL-2 inhibitor venetoclax in combination with HMAs such as azacitidine or decitabine, or LDAC in elderly patients (≥75 years old) with de novo AML or in patients not eligible for intensive induction chemotherapy due to the presence of comorbidities or poor karyotypes.64 This approval was based on several studies in which venetoclax in combination with either azacitidine or decitabine demonstrated remarkable efficacy for older patients not eligible for induction chemotherapy,65-67 and thus HMA with venetoclax has become the standard of care for this elderly population. Additionally, combinatorial therapy of venetoclax and LDAC demonstrated a manageable safety profile, producing rapid and durable remissions in older patients.68 It is noteworthy that traditional risk stratification recommendations, that is, European LeukemiaNet (ELN), that are applicable to conventional chemotherapy do not apply to venetoclax-based therapy.69 Mechanistically, selective inhibition of antiapoptotic protein BCL-2 alone has been shown to promote cytotoxicity in AML cells.70 Currently, venetoclax is almost extensively used in combination with HMA or LDAC for patients who are not eligible for intensive induction therapy. However, the regimen may be optimized by replacing azacitidine for a molecularly targeted therapy or adding a molecularly targeted therapy to venetoclax–HMA (triplet therapy). In particular, this triplet regimen potentially enhances the outcome in patients who have targetable mutations, including FLT3 and IDH1/2, or targetable antigens such as CD33, CD123, and PD-1.35 Moreover, the triplet therapy regimen of low-intensity chemotherapy (LDIC) ± FLT3 inhibitor ± venetoclax showed higher complete response rates (67% vs. 32%) compared to doublet therapy (LDIC ± FLT3 inhibitor) in older/unfit patients with FLT3-mutated AML.71
LSCs have been shown to be selectively dependent on mitochondrial OXPHOS.72, 73 More specifically, LSCs are shown to be uniquely reliant on amino acid metabolism for OXPHOS and survival, and targeting amino acid metabolism using venetoclax and azacytidine induces cell death in LSC populations.74 Furthermore, LSCs from older patients who received treatment with venetoclax/azacitidine showed metabolic perturbations that ultimately lead to suppression of OXPHOS.75 These studies demonstrated that therapeutic elimination of LSCs can be achieved by targeting specific metabolic pathways.
3.3 Targeted therapy: Signaling pathways
The idea of molecularly targeted therapy is to block the survival and growth of cancer cells by using small-molecule inhibitors that specifically inhibit targets, such as tyrosine kinases, cyclin-dependent kinases, proteasome, and poly ADP-ribose polymerase, or antibodies that specifically bind to antigen, cell receptor, or membrane-bound proteins to induce cell death. Molecular-targeted therapy has demonstrated promising outcomes in many cancer types including AML, breast, lung, gastric, and colon cancers in the clinic.76 One of the advantages of molecularly targeted therapy is the effective delivery of drugs with higher specificity and less toxicity compared to conventional chemotherapy.77
Aberrant activation of signaling pathways that promote the survival, growth, and proliferation of AML cells has been shown to be closely associated with recurrent mutations of genes in these pathways. Many different signaling pathways involving FLT3, KIT, RAS, MEK, JAK, PI3K/AKT, mTOR, and PIM have been shown to be overexpressed and/or dysregulated in AML cells and therapeutic inhibition of these pathways can be achieved by a wide range of kinase inhibitors.24, 78-80 The antileukemic activity of these kinase inhibitors has been demonstrated in preclinical models.81-85 Some of these inhibitors had been tested in clinical trials either in combination with other drugs or as monotherapy in patients with AML at different stages. For example, a phase 1b study of mTORC1 inhibitor everolimus in patients with relapsed AML in combination with chemotherapy demonstrated 68% CR with a good safety profile.86 Furthermore, some kinase inhibitors, such as ruxolitinib and dasatinib, are currently in clinical trials for AML (NCT03874052 and NCT02013648 for ruxolitinib and dasatinib, respectively), suggesting emerging interest in developing new therapeutic strategies by targeting mediators of key survival signaling pathways.
Glasdegib in combination with LDAC was approved by the FDA in November 2018 for the treatment of newly diagnosed AML in elderly patients or patients with comorbidities that preclude the use of intensive induction chemotherapy. Glasdegib is a small molecule inhibitor of Smoothened, a key regulator of the Hedgehog pathway, that has demonstrated its therapeutic efficacy in sensitizing LSCs to cytarabine in a xenograft mouse model of primary AML.87 The approval was based on the results of BRIGHT AML 1003 (NCT01546038), an open-label, multicentered phase II trial comparing glasdegib + LDAC combination therapy with LDAC alone. The median age of each group was 77 and 76 years of age, respectively. Compared to the LDAC alone group, patients who received glasdegib + LDAC showed significantly longer median overall survival (8.3 vs. 4.3 months) and much higher CR rates (18.2% vs. 2.6%).88 While common side effects were cytopenias, fatigue, hemorrhage, febrile neutropenia, musculoskeletal pain, and edema, some nonhematologic adverse events, such as abnormal renal values or QT interval prolongation, were observed more commonly in glasdegib + LDAC group than LDAC alone group. Despite these adverse effects, the benefit–risk analysis of the results of BRIGHT AML 1003 supported regular approval of this regimen for elderly patients.88
3.4 Targeted therapy: FLT3
As mentioned previously, FLT3-ITD mutations are among the most prevalent mutations and are particularly associated with poor prognosis. FLT3-ITD mutations have been known to be an independent risk factor for post-alloHSCT relapse.89, 90 Furthermore, even with high-dose daunorubicin (90 mg/m2, three times), patients with AML harboring FLT3-ITD mutations showed a high relapse rate (61%) and poor overall survival (28%).91 The unfavorable outcomes for FLT3-ITD AML suggest the importance of the development of a better therapeutic strategy than standard induction/consolidation therapy or alloHSCT. Clear scientific and clinical evidence that supports the significance of activated FLT3 in leukemogenesis has led to the development of several FLT3-targeted inhibitors.92
First-generation tyrosine kinase inhibitors (TKIs) that target FLT3 include lestaurtinib, sunitinib, sorafenib, and midostaurin. While most of these first-generation FLT3 inhibitors demonstrated limited antileukemic activity and signs of toxicities in patients,93, 94 midostaurin in combination with standard 7+3 chemotherapy in patients with newly diagnosed AML with FLT3 mutations was shown to significantly improve overall survival compared with 7+3 chemotherapy alone in the RATIFY trial.95 Based on this favorable outcome, midostaurin (with standard cytarabine/daunorubicin induction and cytarabine consolidation therapy) was approved by the FDA in 2017 for the treatment of adult patients with newly diagnosed FLT3-mutated AML. Given that first-generation FLT3 inhibitors are multikinase inhibitors, however, the favorable outcomes achieved by midostaurin may not necessarily be solely due to specific inhibition of FLT3.96-98 In addition, patients in the RATIFY trial were much younger (with the median age of 48 years), suggesting the need for additional data for midostaurin in elderly patients or patients with comorbidities.35
The next-generation FLT3 inhibitors (crenolanib, quizartinib, gilteritinib) have demonstrated much greater specificity for FLT3 and higher potency than muti-targeted first-generation TKIs.99 Gilteritinib and crenolanib are type 1 inhibitors that target both the active and inactive conformations of FLT3, whereas quizartinib is a type 2 inhibitor that is only specific for the inactive conformation so that it can only target FLT3-ITD, but not FLT3-TKD.35 Several clinical trials with quizartinib and gilteritinib have demonstrated their effectiveness in refractory AML patients with activating FLT3 mutations, showing significantly higher CR rates and longer overall survival compared to salvage chemotherapy.100-104 Although the results of QuANTUM-R phase 3 trial for quizartinib were promising,102 several concerns regarding the impact of quizartinib resulted in rejection by the FDA for approval in 2019. These concerns were as follows: (1) median survival was only extended 6 weeks; (2) no significant difference in event-free survival between quizartinib and salvage chemotherapy; and (3) dropout rate for the chemotherapy arm.105 Quizartinib is approved in Japan for relapsed/refractory FLT3-mutated AML, and there are still multiple ongoing clinical trials with quizartinib (in combination with standard chemotherapy or other chemotherapy drugs) in the United States (NCT02668653, NCT03989713, NCT04047641, and NCT03135054). In the ADMIRAL study, where efficacy of gilteritinib was compared with that of salvage chemotherapy in patients with relapsed or refractory FLT3-mutated AML, gilteritinib demonstrated much higher CR rates (21.1% for gilteritinib vs. 10.5% for salvage chemotherapy), longer overall survival (9.3 vs. 5.6 months), and longer event-free survival (2.8 vs. 0.7 months).104 The positive outcomes from this study resulted in FDA approval of gilteritinib (as a monotherapy) for relapsed or refractory AML patients with FLT3 mutations in 2018.
Although gilteritinib is now approved as a monotherapy, debate continues about how much clinical impact FLT3 inhibitor monotherapy could make on relapsed or refractory AML patients with FLT3 mutations. There has been emerging evidence showing multiple drug resistance mechanisms to FLT3 inhibitors, even for the next-generation TKIs, suggesting that gilteritinib would more likely exert a substantial effect if incorporated into a broader treatment regimen including chemotherapy, other targeted inhibitors, and/or immunotherapy.106 Indeed, a phase 1 study of gilteritinib in combination with induction and consolidation in patients with newly diagnosed AML (median age of 59.5 years) demonstrated combinatorial treatment of gilteritinib with chemotherapy was well tolerated.107 Furthermore, gilteritinib combined with venetoclax led to high response rates and impressive median overall survival in patients (median age of 63 years) with relapsed/refractory FLT3-mutated AML.108 Multiple clinical trials testing gilteritinib in combination with other treatment regimens are now ongoing (NCT03730012: with atezolizumab, a monoclonal antibody that inhibits PDL-1; NCT04240002: with cytarabine, fludarabine, and granulocyte colony-stimulating factor; NCT05028751: with lanraplenib, an inhibitor that targets SYK, spleen tyrosine kinase).
3.5 Targeted therapy: Epigenetic regulators
Beyond genes involved in signaling pathways, the second most common recurrent mutations in AML are found in genes involved in epigenetic regulation, including DNA methylation and posttranslational histone modifications, such as DNMT3A, TET2, WT1, IDH1, and IDH2.5 Among these, IDH inhibitors have been evaluated in clinical trials of patients with AML. Ivosidenib and enasidenib were approved by the FDA for the treatment of IDH1- and IDH2-mutated diseases, respectively, facilitating clinical trials to investigate the potential option of targeted therapy for treating AML patients harboring mutations in IDH1 or IDH2. A second IDH1 inhibitor, olutasidenib, that inhibits mutant IDH1 was recently approved by the FDA for the treatment of adult patients with relapsed or refractory AML with a susceptible IDH1 mutation.109 A recent clinical trial demonstrated that combinatorial treatment of enasidenib with azacitidine significantly improved overall response rates compared with azacitidine monotherapy in newly diagnosed AML patients with IDH2 mutation.110 Similarly, combinatorial therapy of ivosidenib and azacitidine in patients with newly diagnosed AML with IDH1 mutation who are ineligible for intensive chemotherapy leads to a high rate of clinical response.111 A recent phase 1 study showed that ivosidenib or enasidenib combined with 7+3 induction and consolidation chemotherapy was well tolerated in newly diagnosed AML patients with IDH1 or IDH2 mutations.112 Evaluation of ivosidenib or enasidenib with 7+3 standard chemotherapy in a large phase 3 randomized study is currently ongoing (NCT03839771).
Recently, the FDA approved ivosidenib + azacitidine for treating elderly patients with newly diagnosed IDH1-mutated AML in May 2022. The approval was based on a randomized, multicenter, phase 3 clinical trial (NCT03173248) that included 146 patients with newly diagnosed IDH1-mutated AML, comparing a group of patients who received ivosidenib + azacitidine (n = 72) with patients who received azacitidine alone. The median age of each group was 76.0 and 75.5 years, respectively.113 Compared to the azacitidine alone group, patients who received ivosidenib + azacitidine demonstrated significant improvements in the probability that a patient would remain event free at 12 months (37% vs. 12%), median overall survival (24 months vs. 7.9 months), and CR rates (47% vs. 15%). Common adverse effects were febrile neutropenia (28% with ivosidenib and azacitidine, and 34% with azacitidine alone) and neutropenia (27% vs. 16%, respectively).113
3.6 Targeted therapy: Metabolism
Numerous studies have demonstrated that metabolic adaptation plays a key role in the survival of AML cells, supporting the therapeutic potential of targeting metabolic pathways for treating AML. Recently, a few drugs that specifically target metabolic pathways, enzymes, and metabolites have been actively tested in clinical trials.
It has been shown that enhanced glycolysis in AML cells causes decreased sensitivity to cytarabine, whereas inhibition of glycolysis potentiates the cytotoxicity of cytarabine, providing evidence for the use of metabolic pathways as novel prognostic markers and therapeutic targets for AML.114 AML cells have been shown to be more susceptible to oxidative stress compared to normal hematopoietic cells due to low reserve capacity in the respiratory chain, suggesting a metabolic vulnerability specific to AML cells.115 IACS-010759, an inhibitor of complex 1 of the mitochondrial electron transport chain, was shown to inhibit the growth of AML cells in vivo.116 A clinical trial investigating the efficacy of this oxidative phosphorylation (OXPHOS) inhibitor in patients with relapsed or refractory AML is ongoing (NCT02882321). Glutamine is also known to contribute to OXPHOS (through its conversion to glutamate and subsequently to alpha-KG) and blocking its conversion to glutamate using the glutaminase inhibitor CB-839 has demonstrated killing effects in AML cells.117 Moreover, inhibition of glutaminolysis induces mitochondrial apoptosis, resulting in synergistic killing effects with BCL2 inhibition.118 CB-839 also demonstrated synergism with FLT3 inhibitor quizartinib in FLT3-ITD AML cells by depleting glutathione, thus inducing mitochondrial reactive oxygen species (mitoROS), which promotes apoptotic cell death.119 Furthermore, glutaminase inhibition with CB-839 makes AML cells susceptible to various adjuvant pro-oxidant drugs that exacerbate mitoROS production and apoptosis.120 The clinical trial testing CB-839 in combination with azacitidine in patients with relapsed and refractory or newly diagnosed AML was recently completed, but results have thus far not been reported (NCT02071927).
3.7 Immunotherapy for AML
Our improved knowledge of AML pathogenesis has led to the development of immune therapies for the effective treatment of AML patients. Molecular surface markers including CD33, CD123, CLL1, and CD244 are ubiquitously expressed on AML cells and LSCs, making them promising targets for AML immunotherapy.121 While numerous types of monoclonal antibodies against these AML surface antigens have been tested for therapeutic efficacy both preclinically and clinically, gemtuzumab ozogamicin (GO), a humanized anti-CD33 monoclonal antibody conjugated with calicheamicin, was granted accelerated approval by the FDA in 2000 for relapsed AML patients who are not eligible for intensive chemotherapy.122 Due to an unfavorable safety profile, including the development of hepatic veno-occlusive disease,123 GO was voluntarily withdrawn from the U.S. market in 2010 by the company. However, in 2017, the FDA granted approval of GO for the treatment of adults with newly diagnosed CD33+ AML and for patients aged ≥2 years with CD33+ AML who have experienced a relapse or who have not responded to initial treatment. Recently, sabatolimab and magrolimab, monoclonal antibodies targeting TIM-3 and CD47, respectively, have demonstrated efficacy against AML.124 Moreover, magrolimab, which targets the “don't eat me” signaling protein CD47, has shown early evidence of efficacy as triplet therapy with venetoclax and azacytidine, especially for TP53 mutant AML.125 The main safety issues with monoclonal antibody-based immune therapy are (1) limitations in identifying and targeting antigens that are specifically expressed on AML cells and (2) extramedullary toxicities of monoclonal antibodies.126
Another immune-based therapeutic strategy is to use chimeric antigen receptor (CAR)-T cells. CARs are engineered molecules that contain extracellular antigen-binding domains and intracellular signaling domains that activate T cells.127 Although a few candidates for antigens targeted by CAR-T cells, including CD33, CD123, and FLT-3, have been tested for efficacy, the lack of specificity has been a recurring issue as these antigens are also expressed in normal hematopoietic stem cells (HSCs) as well as other hematopoietic cells, causing myelosuppression.126 Further identification of antigens that are AML specific and the potential targeting of bispecific AML antigens128 will be of high priority for more effective, less toxic immunotherapies.
4 DRUG RESISTANCE MECHANISMS IN AML
Although 80% of AML patients achieve a CR (defined by <5% blast cells in the BM) after induction and consolidation therapy, the majority of them relapse and 5-year survival rates are still less than 30%.129 In particular, elderly patients who are older than 60 years of age show a considerably lower complete response rate to induction therapy (40%–60%), compared to younger patients (60%–85%).130 Unfortunately, AML patients treated with targeted therapy employing inhibitors targeting FLT3, IDH1, or IDH2, almost always relapse unless they have alloHSCT following the therapy.35, 131, 132
Relapse is thought to originate from preexisting resistant clones that survive after therapy. These clones develop into leukemia that is often more aggressive and resistant to therapy, making it even more challenging to treat patients with relapsed AML. The risk of relapse can be estimated by determining minimal residual disease (MRD), a residual leukemic subclone(s) that will eventually reestablish and expand during relapse.133, 134 For example, Ivey et al. found that the presence of MRD can be reliably determined by quantification of NPM1-mutated transcripts in NPM1-mutated AML. Although other mutations associated with preleukemic clones were also detected in patients during ongoing remission after chemotherapy, NPM1 mutations were found to be the most reliable marker for sequential monitoring of MRD to identify impending relapse.135 MRD testing can be performed upon completion of the first round of therapy, and the presence of MRD can be used to determine the next management plan. MRD-positive patients often receive another round of therapy or alloHSCT to further eradicate the remaining leukemic subclones and prevent relapse.129, 136 Although the exact mechanism for how MRD clones develop drug resistance and survive through therapy is not completely understood, a growing body of preclinical and clinical research has revealed a number of cell-intrinsic or cell-extrinsic mechanisms that drive relapse and drug resistance.
4.1 Intrinsic mechanisms of drug resistance
While multiple drug resistance mechanisms induced by the BM microenvironment have been identified (further discussed in the subsequent sections), intrinsic changes/adaptations of AML cells in response to therapy have been discovered as common drug resistance mechanisms. Mutation of the gene encoding the drug target is the most common intrinsic mechanism of resistance to targeted therapy.137 For example, FLT3-mutated patients treated with type 2 FLT3 inhibitors, including quizartinib and sorafenib, that bind to FLT3 only when it is in the inactive conformation have been shown to acquire point mutations in the kinase domain, often at D835 and “gatekeeper” F691 residues. Such point mutations block the binding of type 2 FLT3 inhibitors to their binding sites within FLT3.138-141 By analyzing the co-crystal structure of quizartinib bound to FLT3, Smith et al. demonstrated that the binding of quizartinib to FLT3 relies on edge-to-face aromatic interactions with the gatekeeper F691 residue, and F830 within the highly conserved Asp-Phe-Gly motif in the activation loop, making quizartinib vulnerable to mutations in gatekeeper residues and the activation loop. Indeed, a novel FLT3 inhibitor pexidartinib (also known as PLX3397) demonstrated therapeutic efficacy for quizartinib-resistant FLT3 AML cells harboring FLT3 F691L mutation by retaining its activity against the F691L mutant.142 A phase 1/2 study revealed that pexidartinib was well tolerated in relapsed/refractory AML patients and demonstrated clinical activity in patients harboring FLT3-ITD mutations, as well as FLT3 F691L mutation.143
Similarly, the emergence of multiple secondary site mutations in IDH1 has been identified following IDH1 inhibitor ivosidenib monotherapy in patients with IDH1-mutated relapsed or refractory AML.144, 145 While the co-crystal structure has illustrated the binding pockets of IDH inhibitors,146 the development of a new IDH inhibitor that binds to a binding pocket distant from secondary site mutations would be a feasible pharmaceutical strategy to overcome acquired resistance for treating IDH-mutated AML patients. Indeed, LY3410738, a covalent inhibitor that targets IDH1 by binding to Cys269 in the allosteric binding pocket that is distinct from where ivosidenib binds, has demonstrated improved potency and durability compared to ivosidenib both in vitro and in vivo, suggesting its promising potential for clinical use for targeting secondary site mutations on IDH1 and thus overcoming resistance.147, 148
Acquired modifications that often lead to either reactivation or persistent activation of signaling pathways essential for cell survival following drug treatment have also been commonly observed in drug-resistant AML cells. For the case of patients with FLT3-mutated AML, this type of resistance has been commonly observed in those treated with type 1 inhibitors that target both FLT3-ITD and FLT3-TKD mutations. Some of the resistance-conferring mutations occur in genes/pathways including N-RAS or K-RAS,149, 150 PTPN11,151 PI3K/AKT, and/or MAPK signaling pathways.152 Additionally, upregulation of BCL2 family antiapoptotic factors, such as MCL-1, has been shown to promote FLT3 inhibitor resistance.153 Activation of the MAPK signaling pathway has also been shown to be associated with acquired resistance to IDH2 inhibition. MAPK-activating mutations in several genes including ASXL1 and SRSF2 were shown to be significantly enriched in relapsed/refractory AML patients with IDH2 mutations, leading to resistance to IDH2 inhibitor enasidenib.154 In addition, mutations in hematopoietic differentiation-related transcription factors including CEBP1, RNX1, and GATA2 have been shown to be associated with drug resistance to inhibitors targeting IDH1 or IDH2.155, 156
Mutations of BCL2 that preclude the binding of venetoclax have been identified in patients with relapsed chronic lymphocytic leukemia after venetoclax treatment157; however, such mutations have not been identified in AML patients. Overexpression and/or increased dependence on other BCL2 family members, such as MCL-1 and Bcl-xL, are found to be associated with venetoclax resistance in AML.48, 158 Moreover, the plasticity of the differentiation state in AML has been found to strongly influence sensitivity to venetoclax + HMA therapy, with phenotypically primitive cells being sensitive and more differentiated monocytic cells being resistant.158 Inactivation of TP53 is also associated with resistance to venetoclax-based therapy in AML and MDS.159, 160 Mechanisms of venetoclax resistance have been extensively reviewed elsewhere.48, 161
Other drug resistance mechanisms in AML involve increased drug efflux. It has been shown that increased expression of the ATP-binding cassette subfamily B member 1 (ABCB1), also known as P-glycoprotein or multidrug resistance protein 1 (MDR1), is associated with resistance to different types of drugs including chemotherapy and FLT3 inhibitors.162, 163 Given that ABC transporters mediate multidrug resistance and their expression levels have been shown to be highly associated with prognosis in AML,164-167 therapeutic targeting of ABC transporters may result in better clinical outcomes by overcoming drug resistance. However, targeted ABCB1 inhibitors have not shown significant improvements in the survival of AML patients, suggesting targeting ABCB1 alone may not be sufficient to overcome other potential drug resistance.168
While multiple intrinsic drug resistance mechanisms have been revealed in studies utilizing patient samples, AML cell lines, and various murine models of AML, how these mechanisms are associated with aging is poorly understood. Further investigation to understand intrinsic drug resistance in the context of aging would substantially benefit clinical outcomes in patients with AML, particularly for elderly patients.
4.2 LSCs and resistance/relapse
LSCs, a distinctive subpopulation of AML cells that have stem cell properties (self-renewal and undifferentiated state, and drug resistance), reside in the BM microenvironment. Because of their stemness properties, the cause of leukemic relapse has been considered primarily due to remnant LSCs in the BM following therapy. Both intrinsic properties of LSCs as well as the interaction between LSCs and extrinsic factors associated with BM microenvironment have been proposed to contribute to drug resistance and relapse.169-173 Thus, understanding the underlying mechanism for how LSCs survive under therapy and derive relapse has been an area of extensive research.
Unlike bulk AML cells, LSCs generally reside in a mostly quiescent state, making it difficult to target them by using chemotherapeutic agents.174 There is evidence that has shown that LSCs quiescence can be controlled through growth factors and/or cytokines174 and microRNAs.175 However, LSCs are not always quiescent. Hope et al. demonstrated that LSCs can be reversibly quiescent, and their self-renewal potential is heterogeneous. LSC functional heterogeneity may dictate the course of relapse observed in the clinic where some relapsed AML patients respond to the same chemotherapeutic agents that were originally used to achieve first remission. This scenario can be explained by the reappearance of LSCs that had been initially quiescent and unaffected by the first round of chemotherapy, but later entered an active cell cycle and thus became targetable during the second round of chemotherapy.176 Through comparative analysis of subpopulations from paired diagnosis/relapse samples, Shlush et al. identified two distinctive patterns of relapse. While relapse can originate from LSCs in some cases, other relapse cases may involve subclones of lineage-committed CD33+ leukemia cells that retained stemness transcriptional signatures. This finding suggests that the origin of relapse can be a mixture of LSCs and non-LSCs, emphasizing the importance of developing therapeutic approaches that target stemness to prevent relapse.177
In an effort to develop novel therapeutic strategies to target LSCs, understanding unique metabolic alterations in LSCs has been a major focus in many recent studies. As discussed previously, targeting OXPHOS and mitochondrial metabolism has shown promising results in the elimination of LSCs.74, 75, 178 Given that normal HSPCs have been shown to rely on glycolysis as their primary energy metabolism,72, 179 incorporating OXPHOS targeting drugs in the regimen may be clinically viable as it will allow for specific eradication of LSCs independent of their genotypes with perhaps less deleterious effects on normal hematopoiesis. Indeed, inhibition of OXPHOS in LSCs by BCL-2 inhibition results in decreased ATP levels and subsequent cell death, whereas the bulk of AML cells recompensate ATP production via increased glycolysis, indicating that targeting this metabolic vulnerability of LSCs can be an effective strategy for selective eradication.50 However, like many other types of therapy, the emergence of resistance is anticipated for therapies designed to target metabolic vulnerabilities of LSCs. Jones et al. demonstrated that the reliance of LSCs on amino acid metabolism for OXPHOS is decreased in relapsed patients as a result of increased fatty acid metabolism.74 In addition, Stevens et al. demonstrated that upregulation of fatty acid oxidation obviates the need for amino acid metabolism in LSCs from relapsed patients, and pharmacological inhibition of fatty oxidation restored sensitivity to venetoclax with azacitidine in relapsed LSCs.180 The emergence of resistance highlights the importance of a better understanding of the targetable features of LSCs as well as their resistance mechanisms in relapsed AML.
4.3 Aging and the inflammatory BM microenvironment
The BM is a soft tissue that occupies medullary cavities within the bone. The bone marrow stroma cells (BMSCs) refer to a heterogenous population of cells that provide the stimuli required for the regulation of normal hematopoiesis. The BM microenvironment represents a complex system composed of a collection of different niches wherein the nonhematopoietic cells (such as BMSCs) interact with HSCs to regulate hematopoiesis. Either cell-to-cell interaction or indirect interaction via a variety of soluble factors, cytokines, and chemokines has been shown to play important roles in HSC quiescence, self-renewal, and differentiation.181-184 Moreover, there has been emerging evidence that shows the BM microenvironment not only supports normal hematopoiesis, but also plays essential roles in the initiation and propagation of leukemia cells, as well as drug resistance.171, 185, 186
Inflammation is a physiological process that involves inflammatory mediators such as cytokines and chemokines in response to infection and tissue injury/damage. Given that the majority of elderly patients are affected by chronic inflammatory diseases and inflammation itself is highly involved in altered intercellular communication, one of the hallmarks of aging,187 it would be of importance to understand how chronic inflammation affects hematopoiesis and the BM microenvironment. While systemic upregulation of major pro-inflammatory cytokines, such as interleukin (IL)-6, tumor necrosis factor α, and IL-1α, has been known to drive inflammaging in elderly individuals,188 many studies have shown how these inflammatory cytokines influence normal hematopoiesis, leukemogenesis, and BM microenvironment. Verovskaya et al. demonstrated that the inflammatory milieu in old BM drives remodeling of both the BM niche and hematopoiesis, and pharmacological inhibition of IL-1, a pro-inflammatory cytokine, was sufficient to revert aging of the BM niche and restore the defective regenerative capacity of old HSCs.189 Moreover, IL-1 receptor antagonist (IL-1RA) was shown to significantly inhibit proliferation of AML cells,190 and AML patients with high levels of IL-1RA together with low levels of IL-1β were shown to be strikingly protected against relapse, suggesting the potential therapeutic strategy of targeting IL-1β to lower relapse risk and thus improve survival in AML.191
In addition, there has been a growing body of evidence that demonstrates inflammation-mediated drug resistance in AML. Using 442 primary BM samples, Vadakekolathu et al. showed that while expression of genes associated with inflammation and interferon-gamma (INF-γ) signaling was significantly higher in elderly patients (>60 years of age) relative to younger patients, INF-γ-related mRNA profiles significantly improved the prediction of chemotherapy resistance.192 In a patient-derived xenograft model, Bertoli et al. showed that the transcriptome of residual primary AML cells isolated from the BM of mice treated with cytarabine displayed dramatic upregulation of genes associated with inflammatory responses, including the nuclear factor-κB (NF-κB) network, and combinatorial treatment of anti-inflammatory agent dexamethasone with cytarabine significantly enhanced therapeutic responses in vivo, suggesting that AML cells exhibit in vivo chemoresistance through upregulation of inflammation.193 Similarly, Gebru et al. revealed that a drug-tolerant subpopulation of FLT3-ITD AML cells that survive treatment of lethal doses of FLT3 inhibitors demonstrated upregulation of inflammatory pathways, and combinatorial therapy of quizartinib and dexamethasone significantly enhanced antileukemic activity in both primary AML samples and xenograft mouse models.194 Mechanistically, they showed that FLT3-ITD AML cells confer sensitivity to dexamethasone through glucocorticoid receptor-dependent upregulation of the proapoptotic protein BIM and proteasomal degradation of antiapoptotic protein MCL-1.194 Furthermore, elevated expression of inflammatory/NF-κB pathway genes is associated with venetoclax/azacitidine resistance in monocytic AML.158 These studies suggest that targeting inflammatory pathways could be a viable therapeutic strategy to overcome drug resistance in AML.
4.4 BM stroma-mediated resistance
The role of the BM microenvironment in the development of drug resistance in AML has been extensively studied. Employing either co-culturing of AML cells with BMSCs or conditioned media of BMSCs, a number of in vitro studies have identified multiple drug resistance mechanisms mediated by BMSCs. Crosstalk between AML cells and BMSCs via either direct cell-to-cell contact or soluble factors (cytokines, growth factors, and microvesicles) secreted by stromal cells has been reported to mediate the protection of AML cells from the cell-killing effects of various types of therapies including chemotherapy,195-198 molecularly targeted therapy,199-203 and immunotherapy.204 In addition, BMSCs have shown to promote evasion of immune surveillance by protecting leukemic blasts from natural killer cell-mediated lysis.205
While the mechanisms of BM stroma-mediated protection of AML cells from therapeutic elimination involve a complex interplay of stoma-produced cytokines, chemokines, and adhesion molecules, intrinsic activation of specific pro-survival pathways has been reported. For example, Zeng et al. showed that interactions between stromal-derived factor 1α (SDF-1α) and its cognate receptor CXCR4 promote prolonged activation of ERK and PI3K signaling pathways, leading to resistance of AML cells to apoptosis induced by chemotherapy, and protective effects of BMSCs were abrogated by pharmacological inhibition of CXCR4.206 Other studies also revealed that BMSCs-mediated protection of AML cells from chemotherapy involves persistent activation of Notch signaling,195 Wnt/β-catenin signaling,207 and ERK1/2 signaling.208 Notably, Chen et al. demonstrated that co-culturing of AML cells with human BM stromal cell line HS-5 cells resulted in upregulation of PI3K/AKT signaling, which is essential for resistance to multiple chemotherapeutic drugs including daunorubicin, homoharringtonine, and cytarabine, indicating that the BMSC-induced drug resistance mechanism is not necessarily limited by a drug's mechanism of action.198
Similar BM stroma-mediated protective mechanisms have been observed for molecularly targeted therapy. In the case of FLT3-targeted therapy using FLT3 inhibitors, FLT3-ITD AML cells have been shown to confer resistance to apoptosis induced by FLT3 inhibitors via aberrant activation of MAPK signaling through fibroblast growth factor 2 (FGF2),202 STAT5 signaling,200 ERK signaling,201 and JAK/STAT5 signaling via granulocyte-macrophage colony-stimulating factor and IL-3.203 Each study demonstrated that concurrent inhibition of the upregulated signaling pathway restores sensitivity to FLT3 inhibitors even in the presence of BMSCs or BMSC-derived cytokines or growth factors. Notably, Joshi et al. demonstrated the stepwise evolution of gilteritinib resistance in FLT3-mutated AML by performing multifaceted approaches using whole-exome sequencing, CRISPR-Cas9, metabolomics, and proteomics. In their study, they proposed that the development of early resistant cells was mediated by BM microenvironmental factors such as FGF2, while late resistant cells represent preexisting NRAS mutant subclones that ultimately underwent expansion. Early resistant cells showed distinctive proteomes, metabolic reprograming, and cell-cycle regulation, whereas late resistant cells were highly NRAS dependent, highlighting the complexity of the evolution of drug resistance.209 On the other hand, BMSCs have been shown to mediate resistance to FLT3 inhibitors via enhanced drug metabolism. Chang et al. showed that shRNA-mediated knockdown of cytochrome p450 enzyme 3A4 in BMSCs resulted in the loss of protection against different FLT3 inhibitors (sorafenib, quizartinib, and gilteritinib) when co-cultured with FLT3-ITD AML cells.210 Collectively, these studies suggest that BM stroma-mediated resistance against FLT3-targeted therapy in FLT3-mutated AML cells is multifactorial and context dependent.
Studies from our lab have demonstrated a role in the maintenance of OXPHOS by a novel ATM/mTOR pathway in BMSC-mediated resistance to FLT3 inhibitor therapy.211 We found that AML cells are protected from apoptosis following FLT3 inhibition due to BM-mediated activation of ATM, which upregulates oxidative phosphorylation via mTOR signaling. mTOR is required for the BM stroma-dependent maintenance of protein translation, with selective polysome enrichment of oxidative phosphorylation gene transcripts, despite FLT3 inhibition. Importantly, using primary AML xenograft mouse models, we showed that while BM-resident AML cells were highly resistant to the FLT3 inhibitor quizartinib alone, the addition of the mTOR inhibitor everolimus induced profound reductions in leukemic burden and prevented disease relapse.211 These studies provide a novel mechanistic understanding of BM-based therapeutic resistance and suggest a promising strategy for improved treatment of FLT3-mutated AML.
Apart from the protective effects through soluble factors secreted by BMSCs, direct cell-to-cell contact between BMSCs and AML cells has also been shown to mediate drug resistance. Such direct contact involves adhesion receptors on the leukemia cell surface (such as integrins and/or the very late antigen-4 [VLA-4]) binding to stromal ligands such as vascular cell adhesion molecule 1, VCAM-1. This type of adhesive interaction promotes the activation of pro-survival and proliferative pathways.212, 213 Matsunaga et al. demonstrated that neutralizing antibody targeting VLA-4 in combination with cytarabine significantly prolonged survival in a xenograft model.214 Moreover, monoclonal antibodies targeting the adhesion molecule CD44 efficiently eradicated LSCs as they require interaction with BM niche to maintain their stem cell properties that contribute to their chemoresistance.215
It has been shown that AML cells can impair bulk BM stromal cells via perturbation of osteogenic differentiation as well as a reduction in regulatory molecules, raising the question of which remaining BM niche cell population promotes leukemogenesis and chemoresistance.216 Recently, a specific subset of BMSCs was observed to support the survival of LSCs and chemoresistance. Forte et al. showed that unlike bulk stroma, BMSCs expressing the intermediate filament protein nestin are not reduced in AML patients and their functions change to support LSCs at the expense of normal HSCs. Nestin+ BMSCs not only increase energy production in LSCs through increased TCA cycle and OXPHOS, but also provide LSCs with glutathione-dependent antioxidant defense during chemotherapy to balance reactive oxygen levels and promote survival.217 This study suggests that crosstalk between AML cells and BMSCs is a reciprocal and dynamic process that involves metabolic adaptations in response to therapeutic insults.
As discussed above, many researchers have attempted to mimic the BM microenvironment by adding cytokines and/or growth factors secreted by BMSCs to cell cultures or co-culturing AML cells with BMSCs. However, this in vitro model is still not enough to accurately represent the bona fide BM microenvironment due to its complexity. Even the xenograft mouse model has its own limitation as human AML cells engrafted in a murine environment may not reflect the real interactions present in humans. Even with these limitations, however, an increased understanding of BM-mediated drug resistance will have important implications for future therapies. Importantly, further investigation to determine how aging affects the BMSC-mediated drug resistance would substantially improve outcomes in elderly patients with AML. Novel, less toxic therapies could be developed by either targeting the BM niche itself or effector molecules, such as soluble stromal factors or aberrantly activated signaling pathways, to prevent the survival of AML cells following therapy.
5 TREATING AML IN THE ELDERLY: FUTURE DIRECTIONS
Given the molecular heterogeneity of AML, the ideal therapeutic strategy to treat elderly AML patients could be a proper combination of low-intensity chemotherapy, molecularly targeted therapy, immunotherapy, and/or alloHSCT. Since 2017, the FDA has approved a number of drugs comprising a wide range of targets (detailed in Table 2), enhancing the number of treatment options for specific patient populations to improve clinical outcomes.
| Drug name (approval date) | Description | Indication | Reference |
|---|---|---|---|
| Midostaurin (April 2017) | FLT3 inhibitor (multikinase) | Newly diagnosed AML with FLT3 mutations, in combination with standard 7+3 induction and cytarabine consolidation | 218 |
| CPX-351 (August 2017) | Liposomal cytarabine and daunorubicin | Newly diagnosed therapy-related AML, secondary AML, or AML with myelodysplasia-related changes | 219 |
| Enasidenib (August 2017) | IDH2 inhibitor | Relapsed/refractory AML with IDH2 mutation | 220 |
| Ivosidenib (July 2018 and May 2022) | IDH1 inhibitor | Relapsed/refractory AML with IDH1 mutation | 221 |
| Newly diagnosed AML with IDH1 mutation (>75 years of age or in the presence of comorbidities that preclude intensive chemotherapy), in combination with azacitidine | 113 | ||
| Venetoclax (November 2018) | BCL-2 inhibitor | Newly diagnosed AML (>75 years of age or in the presence of comorbidities that preclude intensive chemotherapy), in combination with azacitidine or decitabine or low-dose cytarabine | 222 |
| Glasdegib (November 2018) | Hedgehog pathway inhibitor | Newly diagnosed AML (>75 years of age or in the presence of comorbidities that preclude intensive chemotherapy), in combination with low-dose cytarabine | 88, 223 |
| Gilteritinib (November 2018) | FLT3 inhibitor | Relapsed/refractory AML with FLT3 mutation | 224 |
| CC-486 (September 2020) | Oral azacitidine | Continued treatment of adult patients with AML who achieved first CR or CR with incomplete blood count recovery following intensive induction chemotherapy and who are not able to complete intensive chemotherapy | 225 |
| Olutasidenib (December 2022) | Mutant IDH1 inhibitor | Relapsed or refractory (R/R) adult AML with a susceptible IDH1 mutation | 109 |
As treatment plans for AML have been moving toward the use of precise and personalized therapy, regimens designed after consideration of a patient's mutational profile and comorbidities would most likely provide several advantages. These include not only enhancing therapeutic response, but also improving patients’ quality of life by reducing the burdens associated with current intensive therapies. With ever-increasing advances in our understanding of AML biology and drug resistance, the future ahead seems much more promising compared to a decade ago when intensive chemotherapy was the only available treatment option. Yet, there is still much room for improvement, particularly for elderly patients. Indeed, a number of clinical trials assessing the therapeutic applicability of different drug regimens that are not currently approved for elderly patients are currently ongoing (Table 3). Successful outcomes of these clinical trials may expand therapeutic options for elderly patients even further.
| Therapy | Rationale | Phase | Trial reference |
|---|---|---|---|
| rhTPO and G-CSF in combination with chemotherapy | To determine if hematologic growth factors rhTPO (recombinant human thrombopoietin) and G-CSF (granulocyte colony-stimulating factor) would enhance overall response rate while reducing chemotherapy-induced cytotoxic effects on hematopoietic cells in elderly patients (>60 years of age) | Phase II | NCT 05258799 |
| Dexamethasone in combination with induction and postremission chemotherapy | To determine if inhibition of inflammation would improve event free survival in elderly patients (>60 years of age) | Phase II | NCT 03609060 |
| Decitabine ± Bortezomib | To determine if addition of proteasome inhibitor to chemotherapy would enhance overall survival in elderly patients (>60 years of age) | Phase II | NCT 01420926 |
| Pembrolizumab in combination with azacitidine | To determine if PD-1 blockade in combination with azacitidine enhances complete remission in elderly patients (>65 years of age) | Phase II | NCT 02845297 |
| Selinexor in combination with induction/consolidation therapy | To determine if selinexor works when given together with induction, consolidation, and maintenance therapy in treating older patients | Phase II | NCT 02835222 |
ACKNOWLEDGMENTS
This work was supported by grants from the Leukemia and Lymphoma Society (7020-19; M.A.G.) and the NCI NRSA F30CA23197 (H.J.P.). We thank Dr. Maria Amaya (University of Colorado Hospital) for critical reading of the manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
No new data was generated.




