Effectiveness and safety of Endostar combined with chemotherapy in treating advanced NSCLC patients with different ages
Wei Jiang and Jing Liang contribute equally to this work.
Abstract
Introduction
This study aims to compare the real-world effectiveness and safety of Endostar in combination with chemotherapy in the treatment of advanced non-small cell lung cancer (NSCLC) in different age groups.
Methods
Electronic medical records of patients with NSCLC who received Endostar combined with chemotherapy from June 2012 to August 2019 in 7 cancer centers were retrospectively collected. Baseline characteristics before and after propensity score matching (PSM), effectiveness evaluation, and safety data of two age groups were analyzed. Tumor response was evaluated according to RECIST v1.1. Adverse events (AEs) were graded according to NCI-CTCAE 5.0.
Results
In this study, 554 and 571 patients were assigned to ≤60 years non-aged group and >60 years aged group, respectively. After propensity score matching (PSM) was introduced, 166 patients in each age group were analyzed. The median PFS for the two groups was 8.9 and 8.0 months, with the overall response rate was 24.7% and 26.5% (p = 0.7060), disease control rate was 64.5% versus 68.7% (p = 0.4600), respectively. Cox regression result showed that advanced age has no significant influence on PFS (hazard ratio = 1.02, 95% CI: 0.98−1.06, p = 0.3034) in contrast with younger patients. The most common AEs in both age groups were myelosuppression, gastrointestinal reactions, and hepatic impairment. The total incidence for the above AEs in the two groups was 18.67% versus 24.10%, 22.89% versus 21.69%, 12.05% versus 7.23%, with no statistically significant difference.
Conclusion
Compared with treating patients with NSCLC younger than 60 years old, the effectiveness of Endostar combined with chemotherapy in treating advanced patients showed no significant differences, with tolerable adverse events.
1 INTRODUCTION
Lung cancer ranks at the leading cause of cancer death in 2020 worldwide1. Nationally, lung cancer is the main cause of cancer-related morbidity and mortality in China and has been increasing over decades. It was estimated that the number of newly diagnosed cases and death cases were about 787,000 and 630,500. The age-standardized incidence and mortality were about 35.92 per 100,000 and 28.02 per 100,000 in 20152. Majority of lung cancer patients are in locally advanced stage or have distant metastatic once upon diagnosis3−5. Approximately 85% of newly diagnosed lung cancers are non-small cell lung cancer (NSCLC)6, 7.
Front-line chemotherapy for the treatment of patients with inoperable locally advanced or/and metastatic NSCLC has been substantially improved8. To the best of our knowledge, platinum combined with a third-generation agent, such as docetaxel, paclitaxel, gemcitabine, vinorelbine, and pemetrexed, is the standard treatment option for advanced NSCLC8−10, but these cytotoxic agents seem to have substantial toxicity and reach a plateau in terms of effectiveness11, 12. Against this background, Endostar, a novel antiangiogenic agent, was developed and approved by State Food and Drug Administration for the treatment of NSCLC in China13. The significant survival benefit and mild toxicity of Endostar combined with platinum-containing chemotherapy have been demonstrated in some prospective clinical trials14, 15. Furtherly, a randomized, multicenter, double-blinded, phase Ш clinical trial had shown that Endostar plus Vinorelbine and Platinum (NP) had a survival benefit in patients older than 60 years. However, the survival and safety benefits of Endostar used in treating patients with different ages are still unclear in real-world clinical settings. Whether efficacy and safety differ between people in different age groups (≤60 years vs. >60 years) remains to be established.
Given these knowledge gaps in the literature, we aimed to assess the effectiveness and safety of Endostar combined with chemotherapy in the treatment of patients with NSCLC in different age groups in a real-world study.
2 METHODS
2.1 Patients
In our study, the electronical medical record (EMR) of patients with NSCLC who received Endostar combined with chemotherapy from June 2012 to August 2019 from seven cancer centers in China was retrospectively collected and analyzed. Inclusion criteria were ≥18 years old, pathologically diagnosed as stage III or IV, with at least one inpatient medical record. Patients who were diagnosed as multiple carcinomas in situ or could not be defined as treatment-naïve or re-treatment patients were excluded. This study was registered on http://www.chictr.org.cn (registration number: ChiCTR2000035129) and approved by Ethic Committees from above cancer centers.
2.2 Data collection
Demographic data including patient characteristics, tumor information, as well as admission record, discharge record, medical orders, and laboratory examinations were collected.
The baseline index date was defined as the first dosage date of Endostar. Natural language processing (NLP) was used to extract information including disease stage, pathological type, family history, smoking history, treatment effects, and adverse events from EMR, such as admission records and discharge records. Disease stage and pathological type were manually reviewed and the final information was confirmed as the ones most close to and within 3 months before or after the baseline index date.
2.3 Efficacy evaluation
Tumor response was evaluated by response evaluation criteria in solid tumors (RECIST) version 1.1. Overall response rate (ORR) was defined as the percentage of patients who had complete response (CR) and/or partial response (PR). Disease control rate (DCR) was defined as the percentage of patients who had CR and/or PR and stable disease (SD).
Progression-free survival (PFS) was defined as the interval (months) between the baseline index date and the earliest date of disease progression or death due to any cause. Disease progression date extracted from picture archiving and communication systems was considered the endpoint date for patients with progressive disease (PD); otherwise, the last tumor evaluation date or last known alive date was considered as endpoint date. PFS was censored at the last follow-up date if no disease progression or death occurred.
2.4 Safety assessment
NLP was used to extract non-hematological adverse events (AEs) and relative grades from EMR and laboratory information management system data; then, AEs were reviewed and classified. Abnormal laboratory test results were also collected and further defined as relevant AEs manually. AEs were assessed according to Common Terminology Criteria for Adverse Events, National Cancer Institute version 5.0 (NCI-CTCAE v5.0).
2.5 Statistical analysis
All statistics comparing the differences of basic clinical characteristics, efficacy and safety between CIV group and IV group were performed with SAS 9.4 (SAS Institute INC., Cary, NC, USA). Categorical data are reported as counts and percentages, whereas continuous data are presented as medians and ranges. The chi-squared test or Fisher exact test was used to compare categorical data, ORRs, DCRs, and incidences of adverse events. PFS was estimated according to the Kaplan−Meier method, and differences between survival curves were compared using the log-rank test. A multivariable Cox proportional hazards regression model was used to calculate hazard ratios and 95% confidence intervals (95% CIs). A two-sided p-value of less than 0.05 was considered statistically significant.
Propensity score matching (PSM) was used to control the selection bias and balance confounding factors. The covariables used to construct PSM model include sex, disease stage, pathological type, smoking history, family history of lung cancer, patient type, and chemotherapy regimen. Standard error was used to compare the differences between the two groups after PSM.
3 RESULTS
3.1 Patient demographics
The basic characteristics of patients in different age groups before and after PSM are summarized in Table 1. The total number of patients was 1125 before PSM, while 554 of them were in the non-aged group and 571 were in the aged group. The median ages for the two groups were 52.0 and 65.9 years old, respectively. The percentages of male, disease stage IV, and treatment-naïve patients were comparatively high in all groups. Statistically significant differences were noticed in the distribution of sex, pathological type, and patient type between the two age groups (p < 0.05). After PSM, 166 patients were matched for each of the age group. The median age is 52.3 for the non-aged group and 65.4 for the aged group. The differences for all characteristic variables became not significant after PSM was applied (p > 0.05).
| Before PSM | After PSM | |||||
|---|---|---|---|---|---|---|
| Variables | Non-aged group (N = 554) | Aged group (N = 571) | p-Value | Non-aged group (N = 166) | Aged group (N = 166) | p-Value |
| Age | ||||||
| Median (Q1−Q3) | 52.0 (47.7–55.5) | 65.9 (62.6–70.3) | 52.3 (47.6–55.4) | 65.4 (62.5–69.0) | ||
| Sex | 0.0066 | 0.6282 | ||||
| Unknown | - | 1 (0.18) | - | - | ||
| Male | 376 (67.87) | 447 (78.28) | 116 (69.88) | 120 (72.29) | ||
| Female | 178 (32.13) | 123 (21.54) | 50 (30.12) | 46 (27.71) | ||
| Disease stage | 0.9446 | 0.4588 | ||||
| III | 159 (28.7) | 190 (33.27) | 48 (28.92) | 42 (25.30) | ||
| IV | 395 (71.3) | 381 (66.73) | 118 (71.08) | 124 (74.70) | ||
| Pathological type | <0.0001 | 0.4478 | ||||
| Unknown | 121 (21.84) | 87 (15.24) | 38 (22.89) | 31 (18.67) | ||
| Squamous cell carcinoma | 130 (23.47) | 231 (40.45) | 51 (30.72) | 47 (28.31) | ||
| Adenocarcinoma | 303 (54.69) | 253 (44.31) | 77 (46.39) | 88 (53.02) | ||
| Family history of lung cancer | 0.2447 | 0.1990 | ||||
| No | 532 (96.03) | 554 (97.02) | 159 (95.78) | 163 (98.19) | ||
| Yes | 22 (3.97) | 17 (2.98) | 7 (4.22) | 3 (1.81) | ||
| Smoking history | 0.0776 | 0.5672 | ||||
| No | 195 (35.2) | 150 (26.27) | 57 (34.34) | 62 (37.35) | ||
| Yes | 359 (64.8) | 421 (73.73) | 109 (65.66) | 104 (62.65) | ||
| Patient type | 0.0206 | 0.5979 | ||||
| Unknown | 152 (27.44) | 182 (31.87) | - | - | ||
| Treatment-naive | 297 (53.61) | 314 (54.99) | 127 (76.51) | 131 (78.92) | ||
| Retreatment | 105 (18.95) | 75 (13.14) | 39 (23.49) | 35 (21.08) | ||
| Chemotherapy regimen | 0.4289 | 0.9116 | ||||
| NVB | 9 (1.62) | 7 (1.23) | 3 (1.81) | 2 (1.20) | ||
| NP | 30 (5.42) | 21 (3.68) | 4 (2.41) | 8 (4.82) | ||
| DXT | 32 (5.78) | 25 (4.38) | 15 (9.05) | 12 (7.23) | ||
| DP | 42 (7.58) | 35 (6.13) | 14 (8.43) | 19 (11.45) | ||
| GEM | 30 (5.42) | 27 (4.73) | 13 (7.83) | 10 (6.02) | ||
| GP | 78 (14.08) | 83 (14.54) | 33 (19.88) | 26 (15.66) | ||
| MTA | 32 (5.78) | 36 (6.30) | 10 (6.02) | 13 (7.83) | ||
| AP | 72 (13.00) | 63 (11.03) | 38 (22.89) | 36 (21.69) | ||
| TAX | 10 (1.81) | 18 (3.15) | 6 (3.61) | 7 (4.22) | ||
| TP | 38 (6.86) | 36 (6.30) | 17 (10.24) | 17 (10.24) | ||
| Unknown | 152 (27.43) | 182 (31.87) | - | - | ||
| Others | 29 (5.22) | 38 (6.66) | 13 (7.83) | 16 (9.64) | ||
- Abbreviations: AP, pemetrexed + platinum; DP, docetaxel + platinum; DXT, docetaxel; GEM, gemcitabine; GP, gemcitabine + platinum; MTA, pemetrexed; NP, vinorelbine + platinum; NVB, vinorelbine; TAX, paclitaxel; TP, paclitaxel + platinum.
3.2 Effectiveness assessment
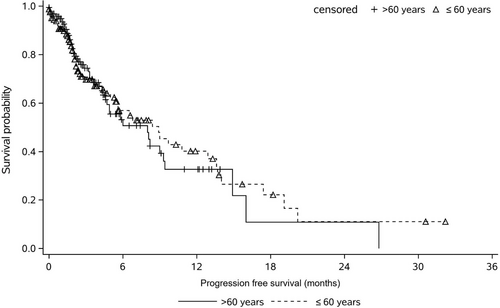
All patients from the two age groups were included in the efficacy analysis set. Table 2 compares the differences in median PFS, DCR, and ORR in both age groups. The median PFS for non-aged group and aged group was 8.9 versus 8.0 months, and the difference was not statistically significant (p > 0.05). The numbers of patients of PR, SD and PD for the non-aged group and aged group were41 versus 44, 66 versus 70, and 59 versus 52, respectively. The DCR and ORR for the non-aged group and aged group were 64.5% versus 68.7%, 24.7%, and 26.5%, respectively, and no statistically significant differences were observed (p > 0.05). Figure 1 presents the Kaplan-Meier curve of PFS for Endostar combined with chemotherapy in different age groups after PSM, no statistically significant difference was detected between the two groups.
| Effectiveness parameters | Non-aged group | Aged group | p-Value |
|---|---|---|---|
| Median PFS (95% CI), month | 8.9 (5.6−13.3) | 8.0 (4.6−9.3) | 0.7433 |
| PR | 41 | 44 | |
| SD | 66 | 70 | |
| PD | 59 | 52 | |
| DCR (%) | 64.5 | 68.7 | 0.4600 |
| ORR (%) | 24.7 | 26.5 | 0.7060 |
- Abbreviations: DCR, disease control rate; ORR, overall response rate; PD, progressive disease; PFS, progression-free survival; PR, partial response.
3.3 Safety assessment
Table 3 displays the most common all AEs and Grade 3 or 4 AEs in the non-aged group and the aged group. The total incidences of all AEs were 68.07% and 62.05%, and Grade 3 or 4 AEs were 7.23% and 6.63%, and the differences were not statistically significant. Myelosuppression, gastrointestinal reactions, and hepatic impairment were the most common AEs. The total incidences for the above AEs in the non-aged group and the aged group were 18.67% versus 24.10%, 22.89% versus 21.69%, and 12.05% versus 7.23%, respectively, and neither of the differences was statistically significant. The most common Grade 3 or 4 AEs was myelosuppression, with the incidence of 6.02% and 4.82% in the non-aged group and aged group, respectively, and there was no significant statistical difference between the two groups.
| AEs | All AEs (%) | Grade 3 or 4 AEs (%) | ||||
|---|---|---|---|---|---|---|
| Non-aged group (N = 166) | Aged group (N = 166) | p-Value | Non-aged group (N = 166) | Aged group (N = 166) | p-Value | |
| All | 113 (68.07) | 103 (62.05) | 0.2497 | 12 (7.23) | 11 (6.63) | 0.8289 |
| Myelosuppression | 31 (18.67) | 40 (24.10) | 0.2283 | 10 (6.02) | 8 (4.82) | 0.6279 |
| Gastrointestinal reactions | 38 (22.89) | 36 (21.69) | 0.7920 | 1 (0.60) | 3 (1.81) | 0.6227 |
| Hepatic impairment | 20 (12.05) | 12 (7.23) | 0.1368 | 1 (0.60) | 0 (0.00) | 1.0000 |
| Renal impairment | 1 (0.60) | 1 (0.60) | 1.0000 | - | - | |
| Rash | 4 (2.41) | 3 (1.81) | 1.0000 | - | - | |
| Others | 19 (11.45) | 11 (6.63) | 0.1257 | - | - | |
4 DISCUSSION
As a recombinant human endostatin, Endostar has been wildly used in combination with some chemotherapy regimens in treating NSCLC in China. A recent review of meta-analyses16 of eight randomized and two prospective single-arm studies representing Endostar combined with chemoradiotherapy is associated with significantly higher efficacy than chemoradiotherapy with similar incidences of main adverse events in NSCLC. Some studies have demonstrated the effectiveness and safety of Endostar combined with different chemotherapy regimens in the treatment of patients with NSCLC. A long-term follow-up study revealed that compared with NP alone, the addition of Endostar to an NP regimen can result in a significant clinical and survival benefit in patients with advanced NSCLC, with a significantly increased median PFS (6.3 and 3.6 months, p < 0.001) and a significantly increased ORR (35.4% and 19.5%, p < 0.001)17. In a randomized, double-blind, controlled, multicenter phase III clinical trial in treating advanced NSCLC, NP plus Endostar was chosen as the treatment group and NP plus placebo was chosen as control group. This clinical trial showed that Endostar in combination with NP could obviously increase the response rate and median time to progress of patients with advanced NSCLC, and with tolerable safety. It was also noticed that median PFS and ORR of patients treated with Endostar in combination with chemotherapy regimen were significantly increased, compared with chemotherapy regimen alone14.
Also in above clinical trial, the subgroup analysis demonstrated that patients above 60 years old in the treatment group had better clinical benefits than the control group14. However, few studies explored and compared the effectiveness and safety of Endostar in treating patients with NSCLC of different ages in real-world clinical settings. To enrich the real-world evidence of the effectiveness and safety of Endostar in combined with chemotherapy regimens, we conducted this study.
In our study, the median PFS for the two age groups was 8.9 versus 8.0 months, respectively, but the difference was not significant. The DCR and ORR for two age groups were 64.5% versus 68.7%, 24.7% and 26.5%, respectively. We also found that DCR and ORR were not significantly different between the two age groups.
In terms of safety, hematologic and gastrointestinal toxicities were the most common adverse events observed in previous studies18, 19. Similar results were also observed in our study, and the most common AEs were myelosuppression, gastrointestinal reactions and hepatic impairment. The most common SAE is myelosuppression for both age groups. However, the differences of incidence of above common AEs and SAE between the two groups were not statistically significant.
Our study has some original innovations and advantages. First, this is a multi-center, retrospective real-world study with a large sample size. Second, for the first time we compared the effectiveness and safety of Endostar combined with chemotherapy in the treatment of advanced NSCLC with different ages, Third, considering that the distribution of characteristic variables was somewhat unequal in baseline, we introduced PSM to decrease the influence of confounding factors, which inevitably reduced the number of patients to be analyzed, but greatly elevated the comparability of patients with different ages and made the analysis result more encouraging. Our study also has a limitation. Given the short follow-up period, the study based on EMR data cannot provide long-term survival data.
To summarize, the retrospective real-world data demonstrated no statistically significant differences in the effectiveness and safety of Endostar in combination with chemotherapy in the treatment of patients with advanced NSCLC with different ages. These data also indicated the great potential of combination of Endostar and chemotherapy in treating older patients with NSCLC in real clinical settings.
AUTHOR CONTRIBUTIONS
All authors have read and approved the manuscript. Wei Jiang contributed to the study conception, assessed the risk of bias of the studies, performed the data extraction and cleaning, conducted the statistical analyses, and drafted the manuscript. Luhua Wang contributed to the study conception, supervised the statistical analyses, and reviewed the manuscript for important intellectual content. Wei Sun, Wenhui Li, Jin Gao, Hui Wang, Wei Zhou, and Jing Liang assisted in performing the search and reviewed the manuscript for important intellectual content. Lixiang Aa assisted in study conception and assessing the risk of bias of the studies.
ACKNOWLEDGMENTS
We would like to express our great thanks to Zhenhai Zhou and Peng Liu from Digital Health China Technologies Co., LTD for their help in data extraction and cleaning.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
FUNDING INFORMATION
No financial support was received from any institution for this research.
ETHICS STATEMENT
This is a retrospective, non-interventional study, which does not interfere with routine diagnosis and treatment, does not affect any medical rights of patients, does not increase the medical risk of patients, and does not increase the medical costs of patients. At the same time, the study did not identify individual patients. In addition, most of the patients who were included in this study have died or lost to follow-up, and their informed consents could not be obtained. For the above reasons, we applied for exempting informed consents of all patients in this study, and this study was approved by ethics committees from all centers.
Open Research
DATA AVAILABILITY STATEMENT
The supporting data and materials could be available on request from the corresponding author.




