The translational potential of the lung microbiome as a biomarker and a therapeutic target for chronic obstructive pulmonary disease
Abstract
Chronic obstructive pulmonary disease (COPD) is a major chronic lung disease with a leading global morbidity and mortality. Emerging evidence suggests that the lung microbiome, the collection of the microorganisms in the lung, plays a crucial role in COPD pathogenesis. The lung microbiome alters in COPD during the acute exacerbations, associates with clinical phenotypes and inflammatory endotypes, and predicts treatment response, lung function decline and mortality, suggesting it could be a potential biomarker for COPD phenotyping, prognosis and personalized therapies. The lung microbiome produces metabolites that interact with host immunity and contribute to COPD pathogenesis, implying that it could be a possible novel target for therapeutic intervention. Despite these potentials, critical gaps exist for translational application of the lung microbiome in COPD clinical practice, starting with the lack of approaches in precisely measuring and manipulating specific members of the lung microbiome, highlighting the need for future collaborative efforts from bench to bedsides. In this perspective, we review existing knowledge and progress on the understanding of the lung microbiome as a possible COPD biomarker or a therapeutic target. We discuss current challenges and share our views on future directions toward fully realize the potential in translational application of the lung microbiome in the promise of COPD precision medicine.
1 INTRODUCTION
The human body harbors a complex microbial ecosystem or microbiome that plays a critical role in human health and diseases.1 Extensive studies have focused on understanding the microbiome in the human gut, while relatively less attention is directed toward investigating the microorganisms residing in the respiratory tract, which has been long thought to be a sterile organ. With the advent of next-generation sequencing technology, it is now well appreciated that there exists a diverse collection of microbes in healthy human lungs, including bacteria, fungi, and viruses that are collectively known as the lung microbiome.2, 3 Overwhelming evidence has further shown that the lung microbiome is altered in an array of respiratory diseases, including chronic respiratory disorders, acute lung injury, and other types of lung diseases such as HIV, tuberculosis and lung cancer,4 suggesting a pivotal role of the lung microbiome in the maintenance of human respiratory health.
| Existing evidence | Current challenges | Future promises | |
|---|---|---|---|
| Lung microbiome as a potential biomarker |
|
|
|
| Lung microbiome as a potential therapeutic target |
|
|
|
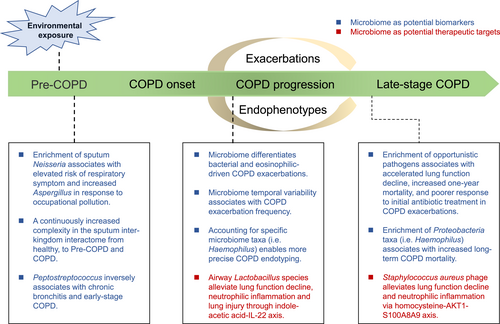
An overview of the existing evidence on the application of the lung microbiome as a potential biomarker or a therapeutic target in the early development, progression, exacerbations, and late stage of chronic obstructive pulmonary disease (COPD).
Chronic obstructive pulmonary disease (COPD) is a major chronic lung disease and is among the global leading causes of morbidity and mortality.5 It is characterized by persistent respiratory symptoms and impaired lung function as a consequence of small airway obliteration, alveolar destruction and airway inflammation.6, 7 Acute exacerbations of COPD are sudden deterioration of symptoms triggered by factors such as bacterial and/or viral infections.8 Emerging evidence suggests that the airway microbiome is altered in COPD compared with healthy individuals,9-12 altered in the early development and during exacerbations of the disease,13-16 associated with specific phenotypes and endotypes of the disease,13, 17 responsive to different types of treatments,13 and predicative of patient mortality.18, 19 Recent studies have further uncovered the mechanistic roles of the lung microbiome in airway inflammation and COPD progression,20, 21 together implicating the potentiality of the lung microbiome as a biomarker or therapeutic target. In this perspective, we discussed the potential applicability of the lung microbiome in the prevention, diagnosis, endophenotyping and therapeutics of COPD, with a translational focus (Table 1, Figure 1). We first introduced the technologies and sample types in studying the lung microbiome. We then described the existing evidence on the role of the lung microbiome as a biomarker for disease risk evaluation, subtyping, and prognosis, and as a possible target for therapeutic intervention. Finally, we discussed current challenges and shared our thoughts on future directions that may facilitate the translational application of the lung microbiome in COPD precision medicine.
2 SAMPLING, SEQUENCING AND ANALYZING THE LUNG MICROBIOME
As it is generally impractical to directly obtain the human lung tissue unless clinically justified (i.e. via lung transplantation), several non-invasive and invasive procedures have been implemented as a surrogate to the lung environment. Sputum is among the most commonly used specimen to studying the lung microbiome due to its non-invasive nature that facilitates large-scale and serial collection. However, the extent of sputum in representing the lung is subject to questioning. Other more invasive sampling approaches, such as bronchoalveolar lavage, bronchial brushing, and tracheal aspirate, have their benefits in better resembling the lower airways and lung microenvironment, but could be particularly challenging for healthy individuals and those with chronic airway diseases. Sampling the lung tissue is the most direct approach in studying the lung microbiome, but is only possible through lung transplantation or surgical resection in patients with such clinical needs.
Most of the omic technologies applied to studying the gut microbiome can be similarly employed in characterizing the lung microbiome.22 As the most widely applied approach for microbiome studies, 16S rRNA gene-based amplicon sequencing allows the characterization of the taxonomic composition of the lung microbiome. The conventional, short read-based approach, by sequencing a proportion of the hypervariable region of 16S rRNA gene, has allowed the taxonomic characterization of the microbiome generally up to the genus level. The full-length 16S rRNA gene sequencing leveraging the third-generation long-read sequencing (i.e. PacBio) has uncovered additional diversity of the lung microbiome at the species or even strain level.23 Metagenomic sequencing, using shotgun sequencing the DNA fragment in the microbial community, enables the characterization of the functional capacity of the lung microbiome, but is subject to unique challenges in achieving sufficient microbial coverage due to the low microbial biomass and notoriously high host-to-microorganism ratio in the lung. Metatranscriptomics, metaproteomics, and metabolomics can be further applied to characterizing the transcriptionally active microbiome, and the set of proteins and metabolites that they produce, toward depicting a comprehensive multi-omic landscape of the lung microbiome.24 Nevertheless, notable technical challenges exist for these more advanced types of omic technologies.25 Finally, culturomics can be leveraged to systematically obtain pure culture of microbial strains from the lung samples,26, 27 providing valuable resources for experimental and mechanistic investigation of the microbiome.
Due to the low microbial biomass and high host-to-microbe ratio, additional care needs to be taken to analyze the lung microbiome. For instance, blank control samples during sampling, DNA extraction, and PCR amplification are required to be processed and sequenced along with the actual samples to assess potential contaminations during the procedure. Potential contaminants can be identified using tools such as decontam,28 which implements a statistical algorithm to flag microbial taxa whose frequencies are inversely correlated with sample DNA concentration or are highly prevalent in blank samples. Paired specimens from the upper respiratory tract and oral cavity can also be collected and analyzed,29 to identify microbial taxa that are more likely originated from the lower respiratory tract or the lung. Finally, an adequate reference database is a prerequisite for accurate metagenomic functional profiling. Compared with the gut microbiome, where reference gene catalogs are well established,30 there remains a lack of comprehensive microbial gene catalog specific for the lung microbiome.
3 LUNG MICROBIOME AS A BIOMARKER FOR COPD RISK EVALUATION, ENDOPHENOTYPING, AND PROGNOSIS
COPD is the subject of extensive focus for the lung microbiome studies.31, 32 One of the most prominent features for COPD is the heterogeneity, where differential clinical traits or phenotypes are manifested among different individuals.33 Underlying the phenotypes are the biological endotypes, which are defined by distinct functional and pathobiological mechanisms of the disease.34 Identifying biomarkers in relation to phenotypes and endotypes are paramount for patient stratification and tailored therapies toward the goal of COPD precision medicine, where the lung microbiome could be applicable. In a seminal study by Bafadhel et al.,35 COPD exacerbations are classified into distinct phenotypes namely bacterial, viral, eosinophilic, and pauci-inflammatory exacerbations. Our previous study has shown that the lung microbiome is distinct among COPD exacerbation phenotypes, in particular between bacterial and eosinophilic exacerbations.13 In terms of inflammatory endotypes, COPD can be broadly categorized into neutrophilic and eosinophilic inflammation.34 Through an integrative analysis of the lung microbiome involving COPD participants in four UK centers, we found that neutrophilic COPD can be further classified into dysbiotic and non-dysbiotic states based on the lung microbiome, which were further manifested by distinct inflammatory profiles, temporal variability, microbiome-host cytokine associations, as well as antimicrobial resistant gene patterns, and therefore may warrant different types of therapies.17, 36 Based on these findings, a ‘host-microbial’ endotyping strategy is proposed toward a more precise COPD patient stratification.37
Upon hospitalization, COPD exacerbation patients with evidence of bacterial infection are often subject to initial empirical antibiotic treatment, which may or may not be effective in practice. In a pilot study of 41 COPD exacerbation patients, we found that the lung microbiome is distinct between individuals responsive or non-responsive to initial antibiotic treatment, with the enrichment of pathogenic bacterial taxa such as Pseudomonas, Achromobacter, and Stenotrophomonas, in non-responders.38 Together with C-reactive protein, procalcitonin, and blood neutrophil count, the microbiome showed a sound predictability for antibiotic treatment outcome. Exacerbations are an important cause of COPD mortality.39 Through a sputum microbiome survey in 102 hospitalized COPD patients, Leitao Filho et al. showed that the microbiome was associated with one-year mortality, with higher abundance of Veillonella and Staphylococcus found in survivors and non-survivors, respectively.18 In a four-year longitudinal study on 253 COPD patients, Dicker et al. found that the predominance of Proteobacteria, in particular Haemophilus, was associated with elevated mortality compared with Firmicutes-dominated or balanced microbiome profiles, suggesting that the lung microbiome may be a marker for long-term COPD prognosis.19 Progressive lung function decline is a hallmark in some COPD patients and is associated with increased mortality.40 In a multi-cohort study spanning across the UK and China, we showed that baseline airway microbial dysbiosis with the co-presence of multiple opportunistic pathogenic taxa such as Staphylococcus, Stenotrophomonas, Achromobacter, and Actinobacillus was associated with accelerated lung function decline in COPD patients, suggesting monitoring the lung microbiome dynamics may assist in predicting lung function trajectories in COPD patients.21 Environmental exposure alters the lung microbiome, the disruption of which could trigger the aberrant host response toward the development of COPD. Leveraging a provincial-wide COPD epidemiological survey in Guangdong, China, we surveyed the sputum microbiome in 1651 individuals in relation to environmental exposure and respiratory health.15 We found that the lung microbiome mediates the influence of environmental exposure on lung function and respiratory symptoms. Specifically, the enrichment of Neisseria in the microbiome is associated with elevated respiratory symptom burden in response to occupational pollution, suggesting the potential application of the lung microbiome in environmental risk stratification. A markedly enhanced lung inter-kingdom microbial interaction was observed from healthy individuals toward early-stage COPD, suggesting the respiratory interactome could be a potential marker for early COPD development. Another recent study based on a provincial-wide COPD cohort in China (COMPASS) has further identified Peptostreptococcus to be continuously decreased from health, to chronic bronchitis and early-stage COPD, implying it could indicate the early development of the disease.16 Together, these studies demonstrated the potential applicability of the lung microbiome in COPD risk evaluation, subtyping, identification of patient subpopulations responsive to treatment, and short- and long-term prognosis.
4 LUNG MICROBIOME AS A TARGET FOR COPD INTERVENTION
One of the ultimate goals of the COPD lung microbiome studies is to manipulate the microbiota as a therapeutic option for disease prevention and treatment. To achieve this goal, it is critical to understand the functional and mechanistic roles of the lung microbiome in COPD. By establishing an emphysema murine model that mimics key features of COPD, Yadava et al. showed that the microbiota mechanistically contributed to host IL-17A inflammation and autoantibodies.41 Microbiome interacts with host largely through metabolites that interact with host ligands to regulate host immune and inflammatory responses. Through a multi-omic analysis on a multi-center cohort in Guangzhou, China, together with in vivo murine model and in vitro cellular assays, we have shown that airway lactobacilli could produce indole-acetic acid that ameliorates neutrophilic inflammation and tissue apoptosis through IL-22 signaling, the decrease in which was implicated in COPD pathogenesis.20 This study suggests the potential for novel therapeutic interventions by administering anti-inflammatory bacterial strains and metabolites. Leveraging a combination of cross-continental multi-cohort analysis, multi-omic characterization, and mechanistic studies, we showed that chronic airway colonization of Staphylococcus aureus accelerated lung function decline by producing homo-cysteine that promoted neutrophil activation and NETosis via AKT1-S100A8/A9 axis.21 To seek for potential translational application, we isolated a bacteriophage specifically targeting S. aureus and showed that intranasal administration of the phage slowed lung function decline and neutrophilic inflammation in emphysema mice, suggesting an intriguing possibility of bacteriophage therapy in halting COPD progression through precise manipulation of the lung microbiome. Other than the microbiome in the local respiratory tract, recent studies suggest that the gut microbiome is also altered in COPD individuals and that manipulation of the gut microbiome (i,e, through fecal microbiota transplantation) can alter lung inflammation, airway remodeling, and mucus hypersecretion in a COPD murine model,42, 43 suggesting a role of gut-lung axis in COPD pathogenesis. Despite interest, whether there exists a sustainable gut-to-lung flow of microbes and metabolites in stable state COPD remains unclear.
It is of relevance to note that current COPD therapies could also modify the lung microbiome. For instance, azithromycin, a macrolide antibiotic, is commonly used to prevent exacerbations in COPD individuals, those who frequently exacerbate. In a randomized, double-blind, placebo-controlled trial, Segal et al. found that azithromycin altered both the lung microbiota and metabolome, in particular with the increase in certain anti-inflammatory bacterial metabolites such as indole-3-acetate.44 Inhaled corticosteroids (ICS) are the routinely used anti-inflammatory therapy for COPD patients. However, its use has been reported to be associated with increased risk of pneumonia in some patients.45 Our previous study has shown that steroid treatment during COPD exacerbations decreased alpha diversity and increased the representation of Proteobacteria. In support of this, a recent randomized controlled trial by Leitao Filho et al. showed that ICS treatment leads to reduced alpha diversity and alteration of microbial composition, with decreased relative abundance of taxa such as Alloprevotella.46 In a mechanistic study, Singanayagam et al. showed that ICS leads to lung microbiota disruption with impaired capability in the clearance of Streptococcus pneumoniae by suppressing the production of the antimicrobial peptide cathelicidin.47 Although these medications are not designed to specifically target the microbiota, whether microbiome alteration indirectly contributes to their therapeutic efficacy is an intriguing question to be further elucidated.
5 CHALLENGES AND FUTURE OUTLOOK
Despite all these progresses in understanding the lung microbiome in COPD, critical gaps and hurdles remain in the translational application of the microbiome in COPD prevention, phenotyping, treatment and prognosis. In terms of biomarker discovery, the first and foremost question would be to identify which specific microbial taxa need to be measured to identify which subset of COPD patients who may be amenable for specific types of treatment. For instance, Haemophilus influenzae colonization is a persistent phenotype for COPD patients who would most likely benefit from a therapy to eradicate the colonization of the bacterium.17 The identification of other lung microbial components that have similar values for disease phenotyping would be instrumental to design a point-of-care test panel to be implemented clinically. Second, a quick and reliable measurement of specific taxa in the lung microbiome independent of next-generation sequencing is a prerequisite for clinical application. Due to the complexity and sparsity nature of the microbiome data, the relative abundance of specific taxa in the microbiome may not directly translate to the clinical microbiology results as assessed by culture or qPCR. Of note, our study shows generally consistent measurements between the sequencing and qPCR results for Haemophilus in sputum, based on which we derived a log10 H. influenzae copy number threshold of 7.2 with optimal capacity in identifying the Haemophilus-predominant patient subgroup, demonstrating the promise in clinical measurement of H. influenzae for detecting patients with persistent colonization.17 Other more complex measurements of microbiome features, such as microbial-microbial interactions or metabolites, would require substantial additional efforts to be interpreted and translated to clinically meaningful readouts. Third, the heterogeneity, geographic, spatial and temporal variability of the lung microbiome need to be considered. Similar as the gut microbiome, the lung microbiome can be influenced by changes in external environments and could have its circadian rhythm and seasonal variability.15, 48 Our recent study suggests that the lung microbiome varied greatly according to geographic locations,15 which needs to be carefully assessed in the establishment of disease subtyping models utilizing the microbiome. Limited data are available on how stable an individual's lung microbiome is over different periods of time within a year, a month, or even a day, rendering difficulty in optimizing the time point for microbiome sampling and measurement to adequately reflect the disease state. Topographic variability of the lung microbiome is another important aspect to be considered as different airway sample types are known to yield markedly different microbiome profiles.49 In this regard, it would be of interest to explore other more non-invasive approaches for sampling the airway microbiome (i.e. nasopharyngeal swabs, exhaled breath) and assess their resemblance with the lower airways or the lung in terms of the microbiome.50 Furthermore, considerable efforts would be required for the education of clinicians so that they are able to access, comprehend, interpret and utilize the microbiome readouts for subtyping patients and guiding therapeutics.
In terms of therapeutic modulation of the lung microbiome, a causative relationship between the lung microbiome and COPD needs to be firmly established with precise knowledge on which microorganisms, which microbial metabolites or effector proteins, and the corresponding host genes and pathways, that are involved in COPD pathogenesis, and how the biological links of microbiome-metabolite-host genes differ across different phenotypes and endotypes of COPD patients. Second, compared to the gut microbiome in which multiple microbiome manipulation approaches such as probiotics, prebiotics, and fecal microbiota transplantation are available, there is currently a lack of approach for precise manipulation of the lung microbiome in preclinical animal models. Given the lack of proof-of-concept, no study has ever been performed in attempting to directly modulate the lung microbiome in humans. Although antibiotic and other treatments in COPD can alter the microbiome, their targets are broad with unpredictable changes of the microbial community. Phage therapy is a promising approach in achieving precise microbiome manipulation and has seen increasing clinical application in treating patients with severe lung infections including COPD exacerbations.51 However, there remain practical and ethical concerns for applying phage therapy to patients with stable state of COPD or any chronic lung diseases. Another serious concern is the unknown safety and the adverse effect of instilling bacteria or phages into the lower respiratory tract. Extensive investigations are needed using preclinical animal models for lung microbiome manipulation to ensure safety and efficacy, before it can be possibly considered in human trials. In this regard, it is however worthwhile to mention that, in comparison to directly manipulating the microbiota, it could be more clinical tractable to consider the administration of beneficial metabolites from the lung microbiome as a potential source of new drugs. It would be even more promising to explore if there exists clinically approved compounds with similar chemical properties and biological effects on the beneficial microbial metabolites, and if they can be repurposed for COPD therapeutics.52
In light of these challenges, we propose several broad aims on which future efforts on clinical translation of the COPD lung microbiome should focus. First, efforts should be made toward establishing a comprehensive landscape of the lung microbiome, its functional capacity and metabolic activity throughout the early development, progression, exacerbations, and end stage of COPD, and across different endophenotypes and in populations with different ethnicities. Second, a precise, mechanistic understanding on the role of specific components of the lung microbiome and its metabolic products in COPD pathogenesis needs to be achieved, utilizing preclinical animal models and state-of-the-art in vitro systems such as organoids.53 Third, a rapid, accessible and accurate approach in sampling and measuring specific members of the lung microbiome should be developed, and abundance thresholds should be accurately defined to allow clinicians to interpret the microbiome readouts and make informed decisions on diagnosis, treatment, and prognosis. Fourth, a clinically viable and ethically acceptable approach in modulating specific members of the lung microbiome or administering beneficial microbial metabolites needs to be established and rigorously tested in preclinical animal models for efficacy and safety. We call for collaborative efforts across countries and from bench to bedsides to achieve these specific aims, toward realizing the genuine potential of the lung microbiome in preventing, diagnosing, phenotyping and treating COPD patients.
ACKNOWLEDGMENTS
This work was supported by the National Key R&D Program of China (2022YFA1304300), and the National Natural Science Foundation of China (31970112, 32170109, 41907211).
CONFLICT OF INTEREST STATEMENT
The authors declare no competing interests.
Biographies

Lei Yang received his master's degree at South China Normal University in 2022. He is studying for his Ph.D. degree in Microbiology at South China Normal University. His current research focuses on host-microbiota interactions and the gut-lung axis.

Naijian Li is a Postdoctoral Research Fellow at Johns Hopkins University School of Medicine in Maryland, USA, and an attending Physician at the National Center for Respiratory Medicine in Guangzhou, China. He obtained his Ph.D. degree from Guangzhou Medical University in 2018. His current research primarily focuses on COPD, atmospheric particulate matter, and gut microbiota.

Xinzhu Yi is currently an associate professor in School of Life Sciences, South China Normal University, Guangzhou, China. She obtained her Ph.D. degree from National University of Singapore in 2015. Her main research interest is about antimicrobial resistance in environmental and human microbiome.

Zhang Wang is a professor in School of Life Sciences, South China Normal University, Guangzhou, China. He obtained his doctoral degree from University of Virginia, USA and worked as a post-doctoral fellow and an investigator in GlaxoSmithKline, Pennsylvania, USA, before joining South China Normal University. His lab is interested in the airway microbiome and its roles in chronic respiratory diseases. Much of his recent work involves the utilization of the multi-omic approaches, together with in vivo and in vitro models, to understand how the airway microbes interact with host in chronic obstructive pulmonary disease.




