Recent advances of liquid biopsy: Interdisciplinary strategies toward clinical decision-making
Abstract
Liquid biopsy has emerged as a promising avenue for non-invasive and rapid retrieval of pathological information from patient body fluids. Over the years, liquid biopsy has garnered significant attention for clinically treating cancer by selecting appropriate biomarkers such as circulating tumor cells (CTCs) and extracellular vesicles (EVs). Further integration of advanced technologies has facilitated the efficient capture of biomarkers for liquid biopsy, revolutionizing clinical decision-making in the multiple processes and stages of cancer patients. Underscoring the intersection of different disciplines, this review provides a holistic summary of recent breakthroughs specifically designed for the capture and application of distinctive biomarkers to blend real-world clinical decision-making with material science. Firstly, we focus on the main principles of liquid biopsy and recent technologies that facilitate the capture and release of biomarkers (e.g., CTCs and EVs), leveraging their physicochemical properties. Then, the clinical applications of biomarkers are summarized, highlighting their potential for providing comprehensive clinical information. Later, the incorporation of machine learning is also emphasized for enhancing clinical applications and enabling deeper insights in the design of next-generation platforms for specific biomarker isolation. Finally, future opportunities for clinical decision-making are explored by combining advanced nanotechnologies and artificial intelligence, thereby offering inconceivable possibilities for improving patient care.
1 INTRODUCTION
Liquid biopsy has emerged as a promising and comprehensive method in clinical healthcare for obtaining valuable biological information about diseases directly from human body fluids, such as blood,1 urine,2 saliva,3 and cerebrospinal fluid.4 This non-invasive and rapid technique enables real-time decision-making5, 6 in various clinical scenarios by isolating, detecting, and analyzing biomarkers presenting in these fluids, which circumvents the limitations of traditional tissue biopsy, including low-specific blood tests, invasive sampling, and inaccessible metastatic tissues.7, 8 The potential of liquid biopsy for frequent cancer testing has garnered significant attention by revealing tumor-related biomarkers such as circulating tumor cells (CTCs),9, 10 extracellular vesicles (EVs),11, 12 and cell-free DNA (cfDNA)13, 14 to guide clinical judgments.
Circulating tumor cells and EVs are promising biomarker in liquid biopsy for various cancers. Circulating tumor cells, which are cells detaching from solid tumor tissues and entering the lymphatic system or bloodstream, have been identified as potential triggers for tumor metastasis.10, 15, 16 They can be detected even at early tumor stages, being associated with a more aggressive biological behavior. Despite their scarcity in body fluids, numerous studies have established a correlation between CTCs and cancer clinical outcomes, underscoring the potential in guiding cancer decision-making.7, 17, 18 Furthermore, CTCs carry the complete information of the tumor tissue, including its genome, transcriptome, proteome, and metabolome. As a result, CTCs offer tremendous potential by providing comprehensive and dynamic information about cancers through the analysis of easily accessible body fluids to improve cancer diagnostics, treatment monitoring, and personalized therapies.7, 19, 20 EVs are small vesicles surrounded by a lipid-bilayer membrane that are derived from various types of cells,11, 21 which are produced by outward budding and fission, cell fusion, or cell apoptosis.22 They play a crucial role in intercellular communication, facilitating processes such as membrane remodeling, removal of cellular component.21 In the context of tumors, EVs derived from tumor cells have been shown to contribute to tumorigenesis by promoting metastasis by initiating stromal support for tumor angiogenesis,23, 24 suppressing the antitumor immune response,25, 26 and enhancing the proliferation of tumor cells.27 With diameters ranging from 50 to 4000 nm, they encapsulate various cargos such as nucleic acids, proteins, metabolomics, and lipids, thus providing a snapshot of their parent cells.21, 28 In contrast to the rarity of CTCs, EVs present higher concentrations in body fluids positioning them as potential biomarkers for unveiling insights into the status of tumor tissue and facilitating clinical decision-making in cancer diagnosis, prognosis, and monitoring.29, 30
cfDNA refers to DNA fragments that are released into body fluids during various processes, including necrosis, apoptosis, secretion, and EV communication by cancer cells.14, 31 Due to their convinces in obtaining information of disease by gathering body fluids and standard PCR analysis, cfDNA has gained commercial availability for guiding parts of clinical decision-making processes like cancer immune-therapy monitoring and early diagnosis.32, 33 Several excellent aspects have been summarized to inspire the clinical application of cfDNA. However, one major challenge that needs to be addressed is the interference caused by non-specific cfDNA originating from normal tissues. Compared to cfDNA, the CTCs and EVs contain more information but face challenges in implanting into real clinical decision-making process due to their disequilibrium return on investment from isolation, analysis, and clinical application, which still requires timely summary to bridge the gap between detection of these two biomarkers and clinical scenarios.
In detecting biomarkers, numerous efforts have been devoted to striking a balance among multiple parameters such as sensitivity, specificity, accuracy, efficiency, and cost-effectiveness, which necessitates careful consideration in clinical trial.7, 34-36 Recently, several comprehensive reviews have provided valuable and deep insights into the technologies associated with CTCs or EVs as biomarkers, and their roles in cancer management from individual materials to clinical perspectives.11, 12, 37, 38 To further enhance our understanding of advanced biomarker detection technologies and their practical application in clinical scenarios, this overview aims to explore the emerging technologies and their potential for liquid biopsy, considering the demands of real-world clinical scenarios. Firstly, the main principles behind liquid biopsy are emphasized, covering isolation, analysis, and clinical applications. Next, we summarize several advanced technologies for isolating CTCs and EVs, respectively. Additionally, we introduce clinical application based on CTCs and EVs. Then, the potential benefits of machine learning-assisted integration of isolation technologies and clinical decision-making are highlighted. Finally, we discuss how these approaches can better bridge the gap between research and clinical practice utilizing interdisciplinary strategies, ultimately leading to more effective and individualized cancer treatments based on liquid biopsy.
2 MAIN PRINCIPLES BEHIND LIQUID BIOPSY
To overcome the limitations of tissue biopsy in malignant cancers such as brain cancer, lung cancer (LC), pancreatic cancer, colorectal cancer, prostate cancer (PCa), and melanoma, researchers are actively optimizing liquid biopsy techniques to address the shortages of traditional cancer examinations, including low-specificity blood tests, low-sensitivity of imaging tests for early-stage cancers, and the potential risks associated with tissue biopsies.8 The key to liquid biopsy involves isolating biomarkers that contain CTCs and EVs from various body fluids, employing both physical and chemical principles (Figure 1A).
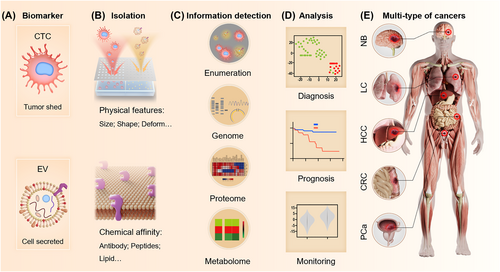
Liquid biopsy based on isolation and detection of circulating tumor cells (CTCs) and extracellular vesicles (EVs) toward clinical analysis for guiding clinical decision-making. The liquid biopsy can assist clinical practices by isolating (A) biomarkers such as CTC (circulation tumor cell) and EV (extracellular vesicle) via their (B) physical features or chemical affinities. After detecting (C) information encapsulated in biomarkers like enumeration, genome, proteome, and metabolome, clinicians can make decisions on (D) diagnosis, prognosis, and monitoring of (E) multi-type of cancers, including NB (neuroblastoma), lung cancer (LC), HCC (hepatocellular carcinoma), CRC (colorectal cancer), and prostate cancer (PCa).
On one hand, CTCs and EVs, being integral components of the biological system, possess unique physical properties such as characteristic dimensions, deformation abilities, hydrodynamic properties, thermodynamic properties, electric properties, and mechanical properties. By manipulating these physical properties, CTCs and EVs can be effectively isolated from complex mixtures of biological particles.37, 38 These isolation strategies offer advantages including rapid speed (less than 10 min), scalability for large-population testing, preservation of biological activity, and convenience for subsequent analysis. However, the physical properties of biomarkers in specific individuals may deviate slightly from the ideal models, potentially leading to negative consequences for clinical decision-making, such as poor isolation performance and low specificity. Additionally, the cost-efficiency for large-scale and robust examinations may be limited due to the requirements of laborious fabrication of specialized facilities or precise operational processes.39
On the other hand, both CTCs and EVs possess specific molecules embedded on their membranes. These molecules can specifically bind with antibodies,40 peptides,41 and aptamers,42 or non-specifically bind with molecules such as TiO2 43 and lipids.44 These mentioned interactions facilitate the isolation and enrichment of CTCs and EVs, enabling more precise analysis and characterization. Besides, the structured interface can topologically match with the biomarker at multiple dimensions,7, 45, 46 such as the nano-scale structures matching the pseudopodia of CTCs and the micro-scale structures matching the body of CTCs. As a promising strategy, the combination of structural matching and molecular recognition greatly enhances the efficiency and expedites of isolation processes (Figure 1B). However, several limitations still hinder this strategy into clinical scenarios, including the costs of specific molecular, cumbersome and time-consuming procedures, and inconvenient downstream analysis.46
To obtain a comprehensive understanding of their original tissues, it is essential to analyze the information encapsulated within CTCs and EVs. The enumeration of CTCs and the measurement of tumor-derived EV concentration directly reflect the cancer state. Tumors with higher malignancy tend to release a larger number of CTCs, promoting metastasis,19 and secrete more tumor-derived EVs, contributing to microenvironment reconstruction.24 Furthermore, the cargo encapsulated within CTCs and EVs provides valuable information regarding cancer stage,29 genetic mutations,47 the stage of epithelial-mesenchymal transition (EMT),41 and specific sites for immunotherapy48 by analyzing the genome,47 proteome,49 transcriptome,29, 50 and metabolome51 of these biomarkers (Figure 1C). The integration of this complex information derived from CTCs and EVs by interdisciplinary strategies provide comprehensive description of the tumor, ultimately assisting in analysis such as accurate diagnosis,7, 52 reliable prognosis assessment,53 ongoing cancer monitoring,54 and personalized therapy selection (Figure 1D).55 Thus, the clinicians can make precise decisions toward multiple cancers, like neuroblastoma, LC, hepatocellular carcinoma, colorectal cancer, and PCa (Figure 1E).
3 ADVANCED TECHNOLOGIES FOR ISOLATING AND RELEASING CTCS
Liquid biopsy begins with the crucial step of isolating CTCs from body fluids. Significant advancements have been made for advanced technologies with satisfactory performance in isolating CTCs. Manipulating the physical properties of CTCs enables label-free isolation, leading to easy downstream analysis with minimal interference to their biological integrity.39, 56 Moreover, specific isolation methods utilized molecules with a high affinity for tumor cells. By integrating with topological structure matching, these affinity-based approaches significantly enhance the sensitivity and specificity of CTC isolation,7, 11, 40, 57 but the CTCs are stationary on the interface. To address this, CTC release strategies based on multiple stimulation have been developed to remove CTCs from the isolation materials for further analysis.58, 59 By leveraging physical and chemical signals, the challenges can be overcame from isolating and releasing CTCs, ultimately enhancing the accuracy and reliability of liquid biopsy analyses(Table 1).
| Biomarker | Method | Separation criteria | Target marker | Throughput & time | Efficiency & recovery | Purity | Detection methods | Advantage | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| CTC | Physical features | Size | NA | 1 min | 95.80% | 99.7% | Fluorescence |
|
60 |
| WBC removal | |||||||||
| 2.5 mL/h | >80% | NA | Fluorescence | 61 | |||||
| Deformation | 1 mL/h | >90% | 90% | Fluorescence |
|
62 | |||
| 100 μL/min | 97.10% | 92.5% | Optical microscope | 63 | |||||
| Hydrodynamics | 400 μL/min | 75% | 93.9% | Optical microscope | 64 | ||||
| Viscoelastic | 21 mL/h | 93% | NA | Fluorescence |
|
65 | |||
| 500 μL/min | 94.80% | 98% | Fluorescence | 66 | |||||
| Light manipulation | 100 μL/h | >90% | 92% | Fluorescence |
|
67 | |||
| 100 μL/h | >90% | >90% | Fluorescence | 68 | |||||
| Dielectrophoresis | 126 μL/min | 99.24% and 94.23% for blood cell replication | >97% | Fluorescence | 69 | ||||
| 2 μL/min | 75% | >98% | Fluorescence | 70 | |||||
| WBC removal | |||||||||
| Acoustophoresis | 400 μL/h | 91.5 ± 4.5% | 97.1 ± 1.0% | Fluorescence | 71 | ||||
| 300 μL/min | 42.10% | 99.75% | Fluorescence | 72 | |||||
| WBC removal | |||||||||
| Magnetophoretic | 100 μL/min | >95% | 99% | Fluorescence | 73 | ||||
| Chemical interactions | Antibody | EpCAM/PSMA | 45 min | 90.3 ± 2.1% | <20% | Fluorescence |
|
7 | |
| WBC captured | |||||||||
| EpCAM | 0.5 mL/h | 89.90 ± 1.43% | NA | Fluorescence | 40 | ||||
| EpCAM/N-cadherin | 40 min | >90% | <20% | Fluorescence | 74 | ||||
| WBC captured | |||||||||
| EpCAM | 10 μL/min | 86.7 ± 10.0%/84.7 ± 12.2% | 2.1 ± 0.7% Hela captured | Fluorescence | 58 |
| Biomarker | Method | Separation criteria | Target marker | Throughput & time | Efficiency & recovery | Purity | Detection methods | Advantage | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| CTC | Chemical interactions | Peptide | EpCAM | 1 h | >90% | >90% | Fluorescence |
|
94 |
| N-cadherin | 30 min | About 85% | NA | Fluorescence | 41 | ||||
| Aptamer | EpCAM/HER2/EGFP | 0.2 mL/h | 90.23%/80% | <0.04% | Fluorescence |
|
120 | ||
| WBC captured | |||||||||
| EpCAM/N-cadherin | 0.5 mL/h | 91%/95% | 13.21 ± 1.90% WBC captured | Fluorescence | 89 | ||||
| NA | 40 min | 90.2%/90.2% | 0.522% | SERS | 98 | ||||
| WBC captured | |||||||||
| Biological | Metastatic carcinoma | 30 min | 72.40% | 8.8% | Fluorescence |
|
90 | ||
| WBC captured | |||||||||
| EV | Physical features | Filtration | NA | 30 min | >95% | 25 times higher than UC | NTA/PCR |
|
134 |
| 30 min | 91.70% | 20 times higher than UC | NTA/WB | 135 | |||||
| Viscoelastic | 200 μL/h | >80% | 94% | NTA |
|
136 | |||
| 200 μL/min | 5 times higher than UC | NA | NTA | 242 | |||||
| AF4 | 250 μL/min | 2 times higher than UC | 2 times higher than UC | NTA/dsELISA |
|
137 | |||
| 200 μL/min | NA | 98% | NTA/WB/MS | 143 | |||||
| Pinched flow | 200 μL/min | 75% | NA | Optical microscope | 139 | ||||
| DLD | 0.1–0.2 nL/min | NA | NA | NTA | 138 | ||||
| 1.3 μL/h | NA | NA | NTA | 144 | |||||
| Light manipulation | 10 min | 1400-fold enrichment | NA | NTA | 34 |
| Biomarker | Method | Separation criteria | Target marker | Throughput & time | Efficiency & recovery | Purity | Detection methods | Advantage | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| EV | Physical features | Dielectrophoresis | NA | 0.16 mL/h | 81 ± 6.2% | 80% | NTA |
|
140 |
| 25 μL/min | 3 times higher than UC | NA | Electro sensor | 146 | |||||
| Acoustophoresis | 1.5 mm/s | >90% | >90% | NTA/WB/Fluorescence | 147 | ||||
| 0.45 μL/min | NA | NA | Fluorescence | 148 | |||||
| 2.5 μL/min (10 min) | NA | >96% | NTA/WB | 141 | |||||
| Magnetophoretic | 150 μL/h | 86% | 98.02% | Fluorescence/NTA/WB | 142 | ||||
| Specific chemical affinity | Antibody | EpCAM | 0.2 mL/h | 82 ± 8% | NA | ddPCR |
|
150 | |
| EpCAM/ASGPR1/CD147 | 1 mL/h | 82.7 ± 1.34% | 90.2 ± 6.2% | ddPCR | 29 | ||||
| EGFRvIII/EGFR/hPDGFR/Podoplanin/Cetuximab | 1 mL/h | 93 ± 2.4% | NA | Fluorescence/dPCR | 157 | ||||
| Aptamer | CD63 | 45 min | NA | NA | NTA/Metabolic Profiles |
|
151 | ||
| CD30 | 30 min | NA | NA | NTA | 158 | ||||
| PD-L1 | 30 min | NA | NA | NTA | 159 | ||||
| Peptide | Canonical heat shock proteins | Overnight | Comparable to UC | NA | NTA/WB/nanoLC-MS | 160 | |||
| Specific chemical affinity | Peptide | CD9 | 50 μL/min (20 min) | 70.0 ± 4.5% | NA | NTA | 152 | ||
| TiO2-Lipid affinity | Lipid | 20 min | Slightly < UC | Equal to UC | NTA/Metabolic Profiles |
|
153 | ||
| 5 min | 93.40% | NA | NTA/WB | 43 | |||||
| DSPE inserting | DSPE | 10 min/30 min | 77.6%/84 ± 3% | 89.3% | MS |
|
44 | ||
| Non-specific chemical affinity | 60 min/10 min | 90%/95% | NA | NTA/RT-PCR | 154 | ||||
| PS affinity | Annexin A5 | 0.3–1.2 mL/h | 90.19 ± 5.70% | ∼90% | NTA |
|
155 | ||
| TIM-4 | 20 μL/min (10 min) | 90.43 ± 2.12%/78.36 ± 11.73% | 75.32 ± 1.18% | NTA | 163 | ||||
| 0.2 μL/min | Appr. 68.5% | NTA | Electrochemical detection | 164 | |||||
| Zwitterionic coordination | Phosphatidylcholine | <30 min | 95%/92% | >90% | NTA/WB |
|
156 |
3.1 CTC isolation based on physical features
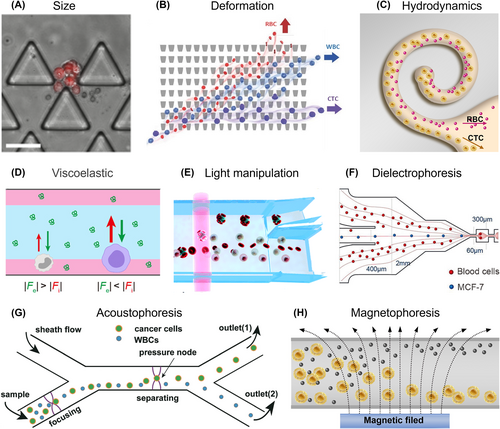
The physical strategies for CTC isolation can be categorized into two main approaches: active isolation and passive isolation. Passive isolation techniques rely on specialized channel designs to separate CTCs based on their intrinsic properties, such as size,60 deformability,62 hydrodynamic features,64 and viscoelastic migration.65 On the other hand, active isolation involves the use of external fields to manipulate cancer cells, such as light,67 dielectric fields,69 acoustic fields,71 and magnetic force.73 These techniques rely on applying forces to selectively move CTCs and separate them from blood cells.
3.1.1 Size based CTC isolation
Traditionally, the primary principle for CTC isolation is based on the size difference between CTCs and blood cells. Consequently, various filtration-based strategies have been developed and continuously improved (Figure 2A).61 These strategies offer simple fabrication processes and easy operation procedures, accelerating their clinical implementation. However, challenges such as low CTC recovery and clogging of the porous filter arise due to high filtration pressure and elevated hematocrit levels.60 It is worth noting that the future of size-based isolation can serve as an accessible and preliminary method for CTC isolation. By sidestepping complex channel designs, they may enable rapid CTC isolation and enumeration within 10 min, while maintaining clinically acceptable sensitivity and specificity. Besides, further categorization based on cancer types is also required to achieve commercial and public acceptance.
Circulating tumor cell (CTC) isolation based on physical features. Passive isolation strategies without external stimulation: (A) Size difference between cancer cells and blood cells. Reproduced with permission.61 Copyright 2015, Springer Nature. (B) Deformation variety. Reproduced with permission.62 Copyright 2016, Wiley-VCH. (C) Hydrodynamic variation. Reproduced with permission.64 Copyright 2019, American Chemical Society. (D) Viscoelastic feature. Reproduced with permission.65 Copyright 2023, American Chemical Society. Active isolation strategies utilizing external stimulation: (E) Light manipulation. Reproduced with permission.67 Copyright 2019, The Royal Society of Chemistry. (F) Dielectrophoresis. Reproduced with permission.69 Copyright 2011, The Royal Society of Chemistry. (G) Acoustic force manipulation. Reproduced with permission.71 Copyright 2019, The Royal Society of Chemistry. (H) Magnetophoresis.
3.1.2 Deformability based CTC isolation
In addition to the disparity in size, the less deformability of CTCs compared to red blood cells (RBCs) is another distinctive feature. Leveraging these differences, the deterministic lateral displacement (DLD) method has emerged as a powerful tool for the separation, purification, and enrichment of cells, encompassing aspects ranging from channel design to clinical applications (Figure 2B).62 By modifying pillar shapes, DLD chip with topology-optimized pillar shapes and a wider DLD channel can be developed to enhance the lateral displacement of cells with different sizes, achieving separation recovery rate of 97.1% for ECA cells with purity of 92.5%.63
3.1.3 Hydrodynamics based CTC isolation
Microfluidic-based techniques leverage CTC hydrodynamics properties within the microchannels to drive the migration of cells, enabling the isolation of CTCs.64 Cell experiences two internal forces, pushing them either toward or away from the channel walls.75, 76 The disparity in these forces anchored CTCs in the center of the microchannel while pushing blood cells toward the walls. Building upon this principle, inertial spiral combining with DLD sorting has been proposed for high-accuracy CTC separation from diluted blood samples (Figure 2C).64 This microchannel achieved a high throughput of 400 μL/min and demonstrated the removal of 99% of blood cells while capturing 75% of tumor cells by adjusting the diameter, flow rate, and cell concentration. However, it is crucial to acknowledge as channel structures become more complex to enable more efficient CTC isolation, the operational complexity increases, posing challenges to their clinical applicability. Therefore, striking a balance between complexity and usability remains an ongoing challenge in the development of microfluidic devices for CTC isolation.
3.1.4 Viscoelastic based CTC isolation
To eliminate complex channel structures, the intrinsic properties of viscoelastic fluids have sparked considerable interest in viscoelastic non-Newtonian microfluidics offering rapid and label-free CTC isolation. By utilizing the combined forces of inertial lift and Dean drag inside simple microchannel, these devices enable efficient size-dependent CTC isolation.77, 78 By modulating the rheological properties of these viscoelastic fluid (mainly biological-friendly polymer solution), cell isolation can be achieved over a wide range of flow rates, which facilitates streamlined and effective CTC isolation. A microfluidic method establishes a stable flow interface between blood and viscoelastic fluid within a simple microchannel (Figure 2D),65 by employing a concentration gradient of polyethylene oxide (PEG) for size-based and label-free CTC isolation. This microfluidic chip processed 1 mL of blood sample in just 30 min, achieving isolation efficiency exceeding 90%. The flow characteristics of viscoelastic fluids were also investigated by altering fluid concentrations for evaluating the behavior of particles with different sizes.66 In a 0.2% hyaluronic acid solution, small particles were precisely focused at the center of the microchannel, while large particles were patterned into two distinct fluorescent streams resulting an impressive isolation efficiency of 94.8% and a purity of 98% for MCF-7 cells.
3.1.5 Light manipulation based CTC isolation
In addition to passive methods that rely on fluid design, external forces can be applied to selectively manipulate and separate CTCs from blood cells. Unlike passive isolation techniques that rely on fixed parameters of microfluidic structures, external forces can be customized and adjusted in real-time for individual patients with heterogeneous CTCs. Optical force is an appealing approach for CTC isolation based on their unique optical constants, but it faces challenges due to the similarity in optical constants between tumor cells and white blood cells (WBCs). To overcome this, Yang et al. introduced an innovative method that binds CTCs with RBCs, creating a noticeable difference in optical constants and achieving precise isolation without compromising specificity (Figure 2E).67 By utilizing lasers in an optofluidic system, they attained high purity (>92%) and cell recovery rates (>90%) in CTC isolation. Other techniques like optically-induced dielectrophoresis and channel design have also demonstrated potential for utilizing optical forces in CTC isolation.68, 79 However, the small disparity in optical constants between CTCs and WBCs adds complexity to the pre-treatment process and device fabrication, despite the high purity and recovery rates.
3.1.6 Dielectrophoresis based CTC isolation
The dielectrophoresis (DEP) technique has been proven as an effective method in isolating CTCs due to the distinct dielectric properties of CTCs compared to blood cells. When exposed to a non-uniform electric field, cells become polarized, generating a dipole moment across their structure. The polarized cells move based on the region of maximum field intensity, depending on factors such as conductivity, permeability, electric field magnitude and frequency, and liquid polarizability.80 DEP have been integrated with flow fractionation to achieve high-rate and highly efficient CTC isolation (Figure 2F),69 enabling continuous flow-through separation at a flow rate of 126 μL/min, removing RBCs and WBCs with efficiencies of 99.24% and 94.23%, respectively. Another study introduced a DEP-driven microfluidic device with a V-shape electrode, enabling the continuous isolation of CTCs from 2 mL peripheral blood sample within 1 h, resulting in a 96.5% depletion of blood cells and an 18.6-fold enrichment of cancer cells.70
3.1.7 Acoustophoresis based CTC isolation
Acoustophoresis, a gentle and label-free method for trapping, separating, and concentrating particles or cells, has proven to be successful due to its ease of implementation and biocompatibility. This technique utilizes acoustic streaming and acoustic radiation force (ARF), based on acoustic waves generated by a piezoelectric actuator, to manipulate particles across a wide range of sizes.72, 81 By designing the channel width to create an optimized acoustic standing resonant cavity, the acoustofluidic-based particle separation technique achieves precise control over fluids and particles by efficiently coupling acoustic energy to the liquid.82 A simple and transparent acoustofluidic device have been demonstrated for effective sorting of particles and cancer cell lines under the standing acoustic field with separation efficiencies of approximately 97 ± 1.0% and 91.5 ± 4.5%, respectively (Figure 2G).71 A two-step acoustophoresis method uses an initial acoustofluidic to pre-separate cells based on their acoustic mobility for removing the RBCs.72 Subsequent purging step was employed to remove contaminating WBCs through negative selection acoustophoresis with anti-CD45-functionalized negative acoustic contrast particles. This method provides extensive elimination of WBCs combined with the gentle recovery of viable cancer cells suitable for downstream functional analyses and in vitro culture. The multi-stage acoustofluidic devices was integrated with different wavelengths driven by modulated signals to address the challenge of separating more than two different sizes with high efficiency and accuracy.81 The slanted angle, acoustic pressure, and resonant frequency of the device were systematically investigated, revealing that the multistage acoustofluidic devices achieved a separation efficiency of 99% for the three different size particles in theorical analyses.
3.1.8 Magnetophoresis based CTC isolation
Magnetophoresis-based CTC isolation techniques can be categorized into positive magnetophoresis and negative magnetophoresis. Positive magnetophoresis involves attracting CTCs toward a magnetic source, requiring the labeling of CTCs with magnetic particles.83 Conversely, negative magnetophoresis repels CTCs away from the magnetic source, necessitating a higher susceptibility magnetic fluid.56 Typically, a permanent magnet generates a magnetic field gradient that pushes the CTCs (without magnetic properties) away by attracting the magnetic medium. By adjusting the sample flow rate, negative magnetophoresis based CTC isolation was proposed by manipulated volumetric concentration of magnetic materials in the ferrofluid, and the magnetic intensity gradient (Figure 2H).73 These optimized parameters resulted in high CTC recovery and separation efficacy in both spiked samples and clinical samples.
While label-free CTC isolation techniques preserve cell integrity and facilitate downstream analysis, active methods for CTC isolation have been limited by their low throughput, as they require a substantial amount of time for the samples to be influenced by external force fields. In contrast, passive methods do not rely on external force fields and can achieve relatively higher throughput. However, passive methods often involve intricate channel structures or complex fabrication processes that may largely elevate the threshold of large-scale clinical application. It is important to note that relying solely on physical features for CTC isolation may encounter problems like cellular heterogeneity in clinical trials, leading to reduced sensitivity and specificity for a specific group of patients. Addressing these challenges through future research by integrating several strategies of passive and active isolation will provide valuable insights for clinicians in their decision-making processes.
3.2 CTC isolation based on chemical features
Aside from their physical features, CTCs originate from tumor tissue carrying diverse information distinct from blood cells.1, 6 A fundamental principle of CTC isolation leverages the biological molecules present in the cell membrane; especially specific proteins expressed uniquely by tissue cells. These include EpCAM,84 Epithelial Growth Factor Receptor (EGFR),85 Human Epidermal Growth Factor Receptor 2 (HER2 or EGFR/ERBB2),86 human epidermal folate binding protein receptor,87 and mesenchymal stem cell antigens such as CD318, N-cadherin,74 and c-MET.88 By identifying and utilizing specific binding agents, such as antibodies,40 peptides,41 aptamers,89 or biological membranes90 that can selectively interact with these proteins, CTC isolation can be achieved by modifying these molecules on substrates by chemical links. For the attainment of highly efficient and specific CTC isolation in real-world applications, the topological match between cells and substrate provides additional advantages for chemical-based CTC isolation.7, 40, 57, 91, 92 Firstly, the higher specific surface area of the substrate increases the density of specific molecules, thereby enhancing the chances of specific bonding. Additionally, through the design of appropriate structures, topological matches can be created with the structures on CTCs, especially the cell body and pseudopodia. This combination of molecular recognition and topological matching offers promising avenues for efficient and specific CTC isolation in real-world applications (Figure 3).
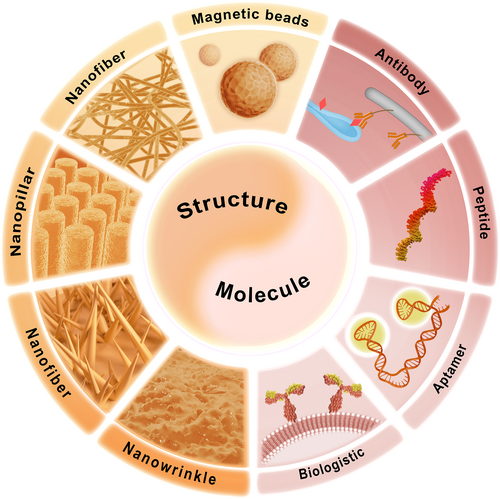
Circulating tumor cell (CTC) isolation based on chemical features. These strategies integrate specific molecular recognition with topological structure match to obtain specific and efficient isolation of CTCs. The specific molecules mainly target the surface proteins only appear on the membrane of CTCs, which commonly include antibodies, peptides, aptamers and recently reported hybrid cell membrane. The structures are mostly nanoscale referring to magnetic beads (MBs), nanofiber, nanopillar, nanowire, nanowrinkle, etc. to provide both high specific surface area and topological match for increasing the volume of specific molecular and interacting with cell body and/or pseudopodia.
3.2.1 Specific molecules
After the successful utilization of antibody-labeled MNPs for CTC isolation,15 researchers have explored various specific antibodies to establish chemical bonds with CTCs. EpCAM protein has been widely targeted for CTC isolation due to its high expression in CTCs derived from epithelial tissues. For instance, Tseng et al. and other researchers have demonstrated the feasibility and high efficiency of anti-EpCAM-based CTC isolation across different cancer types.40, 45 However, the dynamic nature of cancer cells, particularly during EMT, can lead to changes in surface protein. During EMT, cancer cells exhibit reduced EpCAM but increased expression of other antigens such as N-cadherin.93 This provides opportunities to use co-antibodies targeting both EpCAM and N-cadherin for CTC capture. Pei et al. developed a uniform poly-(lactic-co-glycolic acid) (PLGA) nanofiber substrate modified with co-antibodies.74 Compared to single antibody approaches, the co-antibodies demonstrated improved capture efficiency for both epithelial (e.g., MCF-7, up to 66.5%) and mesenchymal (e.g., GIST882, up to 76.4%) cells. In addition to EMT, CTCs originating from different tissues may exhibit different proteins. Wang et al. addressed this challenge by demonstrating a reduced graphene oxide (rGO)-based CTC isolation platform modified with a mixture of anti-EpCAM and anti-PSMA (anti-prostate-specific membrane antigen).7 By investigating the ratio of anti-PSMA, the roughness of the rGO, and the capture time, they achieved a capture efficiency of up to 90.3% for a PCa cell line within 45 min.
Peptides have also emerged as promising alternatives to antibodies in CTC isolation due to their advantages over antibodies, such as lower cost, improved preservation, stable consistency, and ease of chemical synthesis production. Hu et al. have been actively researching CTC enrichment platforms using peptides and have developed the TumorFisher® platform for highly efficient CTC isolation.94 They have designed a variety of peptides for isolating CTCs from different types of cancers and have successfully translated these strategies into clinical practice. For CTCs undergoing EMT, Hu et al. screened and identified a novel peptide targeting N-cadherin,41 achieving high-efficiency capture of mesenchymal CTCs, with a capture rate of approximate 85%. In addition, López and colleagues formulated peptide-modified nano-emulsions specifically targeting cell membrane proteins EpCAM and EGFR on breast cancer CTCs.95
Aptamers, synthetic oligonucleotides with specific affinity for proteins on the cancer cell membrane, have gained attention as another interesting molecule for CTC isolation.96 Similar to peptides, aptamers are easily synthesized and modified, thereby enhancing their performance in CTC isolation. Song et al. developed a microfluidic chip modified with three-dimensional DNA structures (TDNs) as frameworks,97 enhancing capture efficiency to around 87% due to the highly precise dimensions and rigid framework of TDNs. By functionalizing double-tetrahedral DNA probes on nanorough Ag nano-biointerfaces, the synergetic effects of molecules and topographies improved the capture efficiency to 90.2% for SGC-7901 cells in PBS.98 Pei et al. utilized dual aptamers targeting EpCAM and N-cadherin proteins to enhance the capture sensitivity of both epithelial and mesenchymal CTCs,89 achieving capture efficiencies of 91% for A2780 cells (high N-cadherin and low EpCAM) and 89% for OVCAR-3 cells (low N-cadherin and high EpCAM).
The basic principles underlying CTC isolation methods are similar, involving the identification of highly expressed proteins, screening specific molecules, modifying them on substrates, and isolating CTCs. However, an intriguing alternative approach utilizes a substrate covered with biological membrane, which might arouse broad interests. This method aims to address the limitations associated with recognizing EpCAM-negative CTCs and the non-specific adsorption of leukocytes. Wang et al. developed biomimetic magnetic beads (MBs) by cloaking them with a hybrid membrane derived from cancer cells and leukocytes.90 These biomimetic MBs retained the CTC binding capability of cancer cell membranes while showing reduced affinity for background cells due to the leukocyte membrane.
3.2.2 Topological structure
The specific isolation also requires a substrate to load specific recognition molecules. Generally, three strategies have been frequently proposed as the substrates of CTC isolation, which are MBs,99 microfluids,40 and capture chip.91 Usually, the larger the specific surface area will load more recognizing molecules and generate topological match with cells, increasing the affinity between cells and substrate and thereby achieving higher capture efficiency.
Among the three strategies mentioned, nano-/micro-scale MBs are the most extensively utilized for CTC isolation.100, 101 By incorporating specific recognition molecules onto the surface of MBs, they can rapidly bind to target cells and be easily separated from other cells using magnetic fields. Depending on the choice of recognition molecules, MBs can be employed for positive CTC capture99 or negative isolation of blood cells.102 Numerous reviews have been published focusing on MBs, covering aspects such as synthesis,103 specific molecules,104 CTC isolation,105 and clinical applications.106 However, most MBs currently in use have smooth surfaces without distinct structures, which can lead to challenges of low sensitivity, low specificity, and poor reproducibility, despite their widespread clinical utilization. Recently, Wang et al. have introduced a novel approach to synthesize nanofractal microparticles using electrostatic interaction-regulated emulsion interfacial polymerization.107 These nanofractals exhibit exceptional capabilities for rapid protein capture, release, and separation. The topological features can well-matched with pseudopodia on cancer cells, suggesting a promising and versatile method for the synthesis of microparticles that enable efficient and rapid CTC capture. In addition to spherical MBs, Shi et al. developed DNA aptamer-functionalized magnetic short nanofibers for highly efficient capture and release of CTCs.108 Through a blended electrospinning process, magnetic nanoparticles (MNPs) were encapsulated within nanofibers, resulting in structures with a mean diameter of 350 nm and an average length of 9.6 μm. These nanofibers exhibited specific capture capability for cancer cells, achieving an efficiency of 87%, presenting an alternative pathway for fabricating high-performance CTC isolation units, expanding the possibilities in this field.
Apart from MBs, structured substrates offer unique surfaces for interacting with cells and which have been already intensively summarized by excellent researchers. We aim to categorize perspectives based on the principles of designing structured substrates. Along with the development of photolithography, structures like nanowires demonstrated their potential in providing higher specific surface area. Tseng et al. have optimized this technique and expanded its application to isolate not only individual CTC but also CTC clusters, broadening the scope of cancer monitoring beyond simply CTC enumeration.40, 55, 109 Wang et al. have provided insights into bio-inspired interfaces such as nanosheets, nanofractals, and nanoflowers.7, 46, 57, 91, 92 These nanostructured interfaces can achieve topological matching with cancer cells and their pseudopodia, and specifically interact with molecule on the cell membrane. Additionally, PLGA nanofibers have also been utilized to entangle and trap cells with the help of specific recognition molecules.110
To enhance CTC capture efficiency, the principles can be summarized as follows: (i) increasing the specific surface area to accommodate more specific recognition molecules, (ii) providing a topological match with the cell structure by exhibiting similar sizes (80–100 nm for cell pseudopodia and 5–15 μm for the cell body) or complementary structures (nanowires for pseudopodia and micro-bowls for the cell body), and (iii) trapping cells based on their size differences. In addition, traditional specific isolation remains reliant on passive interaction with cells, signifying that the capture substrate waits for the cells' actions, instead of actively applying structure onto cells, as immune cells do. Future developments in active CTC capture could significantly reduce the time and process, making it easier for clinicians to access cancer information and facilitating large-scale physical examinations for early cancer screening.
In conjunction with CTC isolation methods based on physical characteristics, superior capture efficiency and specificity demand increasingly intricate substrate architectures and more potent, selective chemical bonds. These tactics, despite showcasing exceptional capture performances and offering novel CTC isolation methods, often rigidly confine cells to the capture substrates.7, 91, 92 This can pose challenges when trying to extract the wealth of data contained within the CTCs. Contemporary advancements in chemically based isolation strategies have paved the way for multi-analytical capabilities, incorporating techniques such as photoelectrochemical biosensors,111 surface plasmon resonance (SPR),112 and sensors employing quenching effects.113 These strategies, however, continue to grapple with obstacles related to expensive reagents, laborious procedures, and non-intuitive readouts - all key considerations for clinical implementation in cancer patients.
3.3 Advanced technologies for CTC release
Following the isolation of CTCs, it becomes imperative to detach the CTCs from the affinity-bound substrates to facilitate downstream analyses. Factors significantly influencing these analyses include cell viability, release efficiency, and specificity. Except potentially harmful methodologies such as the application of physical forces114 and trypsinization,115 suitable techniques for liberating CTCs from capture apparatus can also be organized into physical signal response (heat, light, and electric) and chemical signal response (pH, enzyme, and substrate breakdown).
3.3.1 Thermo-response
A prevalent strategy for the release of CTCs from capture substrates employs thermo-responsive materials, which primarily invalidate the link between recognition molecules and the substrate in response to temperature changes. Among thermo-responsive polymers, poly(N-isopropylacrylamide) is frequently used for this purpose.116 This polymer undergoes structural conformation changes below the lower critical solution temperature (around 20°C), transitioning from hydrophobicity to hydrophilicity thereby eliminating the hydrophobic interaction with recognition molecules. When engineering switchable SA-recognition sites into thermo-responsive hydrogel, the bingeing can by reversed from strong binding at 37°C and weak binding at 25°C, thus capturing cancer cells and non-invasively releasing them by reducing the temperature (Figure 4A).117
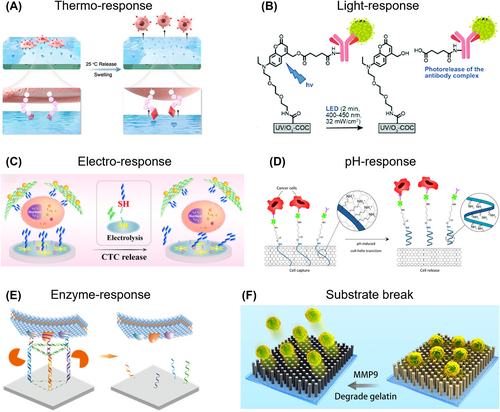
Circulating tumor cell (CTC) release based on different external stimuli. (A) Thermo-response. Reproduced with permission.117 Copyright 2020, Elsevier. (B) Light-response. Reproduced with permission.118 Copyright 2020, The Royal Society of Chemistry. (C) Electrochemical-response. Reproduced with permission.119 Copyright 2021, Springer Nature. (D) pH-response. Reproduced with permission.58 Copyright 2022, American Chemical Society. (E) Enzyme-response. Reproduced with permission.120 Copyright 2022, American Chemical Society. (F) Substrate break. Reproduced with permission.121 Copyright 2019, IOP Publishing.
In addition to thermo-response bond invalidation, Xu et al. developed a near-infrared (NIR) light-responsive microfluidic chip to invalidate the micro-area of a gelatin substrate, facilitating biocompatible single-cell manipulation.122 The captured single CTC can be recovered in large quantities or individually released at a specific location using NIR light, thereby precisely acquiring information about CTC heterogeneity at the single-cell level. For the advancement of thermo-response CTC release, it is important to balance the temperature change range and the thermo-response time to preserve cell integrity and viability.123
3.3.2 Light-response
Light-responsive CTC release can be achieved by designing bonds that are sensitive to light, like those commonly used in drug release applications. For example, Soper et al. synthesized a 7-(diethylamino)coumaryl-4-methyl photo-release agent that undergoes rapid cleavage upon exposure to visible light (400–450 nm) (Figure 4B).118 This light-responsive bond minimizes nucleic acid damage and exhibits minimal side reactions. In their study, SKBR3 cells can be rapid and efficient released, with 94% release efficiency and 94% cell viability after only 2 min of visible light LED exposure. The use of visible light for release also prevents damage to nucleic acids caused by photo-absorption and oxidation typically associated with UV irradiation. Thus, the overall design of light-responsive CTC release aims to identify specific bonds that are sensitive to visible light and easily modifiable for efficient release.
3.3.3 Electro-response
By leveraging electrochemical manipulation, the conductive substrate delivers the electro-signal to the location of electrically-responsive units, consequently instigating chemical reactions. Electrochemical reductive desorption presents several advantages, encompassing the preservation of cell viability, strong release potential, and time efficiency. A cyto-sensor was designed using gold nanoparticles-loaded two-dimensional bimetallic PdMo (2D Au@PdMo) nanozymes, coupled with thiol-anti-EpCAM for the CTC isolation (Figure 4C).119 Subsequently, the captured CTCs could be efficiently and non-destructively released from the modified electrodes through the reductive desorption of the Au-S bond with release rate between 93.7% and 97.4% and high cell viability. By incorporating antibody-modified with electrochemically cleavable semiconducting silicon surface, specific individual cells can be released of interest,124 when both light and electrochemical potential were simultaneously applied.
3.3.4 pH-response
The release of captured CTCs can be facilitated by manipulating the pH value, primarily to invalidate the link between the recognition molecule and the substrate. Han and colleagues integrated pH-responsive poly-L-lysine functionalized carbon nanotube into a microfluidic chip for the proficient capture and release of cancer cells (Figure 4D).58 Using anti-EpCAM, they achieved a capture efficiency of 86.7 ± 9.3%. Subsequently, by raising the pH value to 11.0 to induce a conformational change, they accomplished a release efficiency of 84.7 ± 12.2%. In addition, with a biological viability of 84.6 ± 5.5%, the liberated cells could be cultured for up to 7 days without alterations in morphology and EpCAM expression. However, a pH-responsive approach demands either a pH alteration beyond the standard cell culture environment range, or a prolonged treatment duration that might impair cell viability and integrity.
3.3.5 Enzyme-response
The detachment of CTCs from substrates through enzyme responses is a common approach in cell culture, typically involving trypsin to invalidate the membrane-coherent molecules. While this method has been utilized for CTC release,125 the enzyme's action can harm the surface markers and membrane structures, potentially disrupting subsequent CTC analysis. To mitigate this issue, enzymes designed specifically to invalidate the links between specific molecules and substrates have been developed to retain the information encapsulated in cancer cells. A multi-marker microfluidic chip modified with aptamer leverages the synergistic effect of multivalent aptamers and DLD arrays (Figure 4E).120 The aptamer nanostructures enhance the accessibility of nucleases, enabling cell release with an efficiency of nearly 80% and a viability of 90.42%, while also maintaining molecular integrity. Similarly, Wang and colleagues utilized DNA probes for capture and nondestructive release of CTCs.98 After capturing 90.2% of SGC-7901 cells in PBS, 93.4% of cells were released via Zn2+-assisted DNAzyme cleavage, with the viability of post-released CTCs reaching approximately 98.0%. These specialized aptamers, specifically targeting the designed site, offer a unique perspective on efficient CTC isolation and low-damage release, by simultaneously constructing the structure and composition of the aptamer.
3.3.6 Substrate breakdown
One of the most straightforward strategies for detaching CTCs from the capturing substrate involves simply degrading the substrate itself. This can be achieved through various methods, each tailored to the intrinsic properties of the substrate.126 For instance, Guo and colleagues utilized TiO2 nanopillar arrays coated with a gelatin film for the efficient capture and damage-free release of CTCs (Figure 4F).121 The interaction between the cell membrane and the nanostructure substrate contributed to an impressive CTC capture rate (94.98%). The gelatin layer, with outstanding biocompatibility, can be swiftly digested by matrix metalloproteinase. This allows for the non-destructive release of CTCs, with nearly 100% release efficiency and 100% cell activity.
In general, there are multiple strategies for efficient CTC release based on the properties of recognition molecules, which should be chosen based on the materials and cells with various capture substrates or strategies with the principles to ensure the viability of cells.
4 ADVANCED TECHNOLOGIES FOR ISOLATING EVS
Extracellular vesicles are minuscule biological entities, encapsulated within a lipid bilayer. Based on their size and origin, there are mainly three categories of EVs: (i)endosomal-originated exosomes formed within the endosomal and released upon fusion of multi-vesicular bodies with the plasm membrane (50–150 nm), (ii) microvesicles produced by outward budding and fission of the plasma membrane (100–1000 nm), and (iii) apoptotic bodies released as blebs of cells undergoing apoptosis (100–5000 nm).21, 127 Other names (e.g., prostasomes, oncosomes, and dexosomes) have also be applied to exhibited their origins. Here, in order to focus on the isolation, EVs have been adopted as the only nomenclature generic term, irrespective of the origin and other features.
Extracellular vesicles are secreted by a variety of mammalian cells carrying proteins and nucleic acids from their parent cells, positioning them as crucial mediators in intercellular communication.11, 21 First identified in human plasma in 1967,128 EVs have since been discovered in numerous body fluids, and they offer a window into the health or pathological condition of their originating tissues. This makes them potent markers for a wide spectrum of conditions, such as cancer, infectious diseases, and neurodegenerative disorders.25, 26 Blood-derived EVs are especially promising as holistic health markers, given their high concentration in blood about 107 to 1013 particles/mL. The standard isolation methods for EVs are rooted in physical attributes like size, density, and solubility, prompting the creation of technologies such as ultracentrifugation (UC),129 density gradient centrifugation (DGC),130 polymer-based precipitation (PBP),131 and size exclusion chromatography (SEC)132 for efficient EV separation and isolation.
UC and DGC are widely adopted methods for extracting EVs clinically and commercially, leveraging their size and density differentials. These approaches require high-speed centrifugation (usually around 100,000 × g) to separate EVs from other components. Although these methods are considered the gold standard, they are quite laborious and lengthy, necessitating multiple steps and specialized machinery.11 Moreover, they often lead to low EV yield and purity. Methods based on PBP involve introducing water-soluble polymers, like PEG, to provoke EV precipitation.131 However, this method can cause protein aggregation and potentially compromise the integrity of the information carried within the EVs. Size exclusion chromatography operates on the principle of segregating EVs based on their size using a molecular sieve effect. Yet, the purity of EVs obtained via SEC needs further optimization, especially when compared to UC and DGC.
Recently, some EV isolation platforms are commercially available, such as ExoFACS® Kit using MBs as platform to isolate EVs with different origins by various specific molecules, 3D FloTrix® adopting multi-filtration system realizing isolation efficiency higher than UC, and Exolator® utilizing flow cytometry technology. These recent commercial platforms demonstrate rapid, automated and high-efficient EV isolation with small machine volume, promisingly enabling point-of-care EV testing. However, their basic principles still rely on traditional EV isolation technologies, unable to offer a universal solution for EV isolation. In order to fully interpret the information carried by EVs, there is a pressing need for improved methods to isolate and purify EVs from the body fluids.
4.1 EV isolation based on physical features
Similar to CTC isolation, the physical features-based EV isolation can also be grouped into active isolation and passive isolation. The passive isolation strategies focus on the inner features of EVs for separation by designing and optimizing the special structures of separation channels. The active isolation strategies put concentration on the external manipulation to separate EVs from body fluids (Table 1).
4.1.1 Size based EV isolation
Size-selective filtration has been a classical method for differentiating between blood cells and EVs. Previous approaches utilized porous membranes with specific pore sizes to block blood cells (200–600 nm) while enriching EVs (30–50 nm).133 However, these methods faced challenges such as channel blockage, integrity damage, and impurities, limiting their broader application. Recently, a centrifugal microfluidic device with two nanofilters enabled automated enrichment of EVs smaller than 600 nm from urine within 30 min 134 Despite its efficiency, this method exerted a high shear force that could potentially damage the EVs. In a novel approach, Sun et al. leveraged the concept of electric-hydraulic analogy to design cascaded microfluidic circuits for pulsatile filtration of EVs (Figure 5A).135 This design incorporated a cell-removal circuit and an EV-isolation circuit. At the core of this system was the fluidic capacitor, which comprised an elastomeric membrane sandwiched between two microchambers. The membrane could deform under applied pressure, storing and releasing fluid accordingly. Upon applying pressure, the membrane deformed, allowing particles to flow, while reducing the pressure caused the membrane to recover, collecting the particles in another channel. This microfluidic device enabled clog-free, gentle, high-yield, and high-purity isolation of EVs directly from blood within a short timeframe of 30 min.
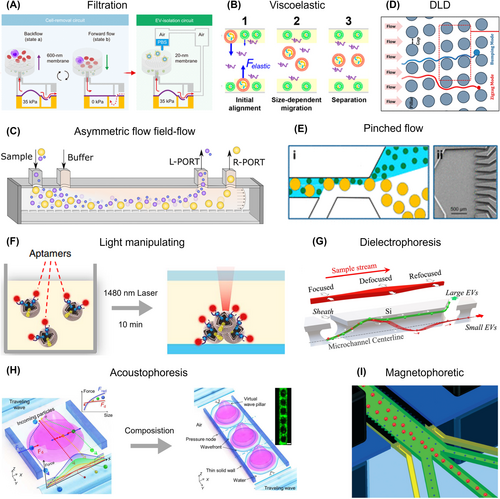
Extracellular vesicle (EV) isolation based on physical features. (A) Filtration. Reproduced with permission.135 Copyright 2023, American Association for the Advancement of Science. (B) Viscoelastic flow-based isolation. Reproduced with permission.136 Copyright 2017, American Chemical Society. (C) Asymmetric flow field-flow fractionation. Reproduced with permission.137 Copyright 2023, MDPI. (D) Deterministic lateral displacement (DLD) based isolation. Reproduced with permission.138 Copyright 2016, Springer Nature. (E) Pinched Flow Fractionation (PFF). Reproduced with permission.139 Copyright 2017, Springer Nature. (F) Light manipulation-based isolation. Reproduced with permission.34 Copyright 2019, Springer Nature. (G) Dielectrophoresis fractionation. Reproduced with permission.140 Copyright 2023, The Royal Society of Chemistry. (H) Acoustophoresis based isolation. Reproduced with permission.141 Copyright 2022, American Association for the Advancement of Science. (I) Magnetophoretic flow-based isolation. Reproduced with permission.142 Copyright 2022, The Royal Society of Chemistry.
4.1.2 Viscoelastic based EV isolation
The burgeoning field of viscoelastic non-Newtonian microfluidics offers an avenue for the rapid, label-free isolation of EVs from various body fluids. Using a similar principle to the viscoelastic-based isolation of CTCs, this methodology harnesses the power of elastic forces to guide larger EVs toward the center of the microfluidic channel. Sun et al. presented a microfluidic system leveraging viscoelasticity for the direct, size-dependent, and label-free separation of EVs from either cell culture media or serum (Figure 5B).136 The system adeptly isolated EVs from intricate media housing a multitude of EVs of varying sizes. By introducing a minor quantity of biocompatible polymer as an additive in the media, they were able to manipulate the viscoelastic forces acting on EVs. This approach demonstrated high separation purity (>90%) and recovery (>80%) rates, promisingly supporting the streamline of EVs analysis of biochemical applications.
4.1.3 Hydrodynamics based EV isolation
Microfluidic channels offer an innovative approach to EV isolation, allowing for the separation of particles based on their hydrodynamic properties. One of these techniques is asymmetric flow field-flow fractionation (AF4), which uses particle density and hydrodynamic properties to separate nanoscale soluble particles, potentially as small as a few nanometers.143 AF4-based devices generate an orthogonal force to a porous membrane within the microfluidic channels by using a combination of field flow and Poiseuille flow. This force effectively separates particles based on their different lateral positions in the channel, driven by their respective diffusion rates (Figure 5C).137 DLD-based isolation can also effectively separate EVs. In this method, particles smaller than a critical diameter follow the streamline through a pillar array without lateral displacement (depicted by the red line). In contrast, particles larger than the critical diameter flow away from a pillar and are laterally shifted to the next row (depicted by the blue line). Astier et al. scaled DLD to the nanoscale (nano-DLD) by using arrays of uniform gap sizes ranging from 25 to 235 nm (Figure 5D).138 This nano-DLD array effectively separates particles ranging from 20 to 110 nm based on size, with excellent resolution, resulting in further size-based displacement of EVs. Morgan et al. applied an alternating current electric field perpendicular to the fluid flow, which led to an approximate ten-fold reduction in the intrinsic critical diameter of the device.144 Pinched Flow Fractionation (PFF) is another promising strategy for particle separation based on size. In this method, particles are directed along the sidewall of a microchannel under a strong sheath fluid flow. Due to this flow, smaller particles tend to position closer to the sidewall, while larger particles are located slightly further away. This subtle position variation is then amplified by streamline divergence in a downstream section with sudden branching, resulting in effective size-based separation. Hong and colleagues demonstrated the use of PFF with multiple outlets with sudden bunching for separating particles of different sizes (Figure 5E) with potential in the isolation of specific EV subpopulations.139
4.1.4 Light manipulation based EV isolation
Light manipulation has recently demonstrated their potential of manipulating the separation of EVs. Unlike the optical force-based CTCs isolation, manipulating smaller entities like EVs could prove more effective and straightforward. Sun et al. have explored the potential of light manipulation in EVs separation, demonstrating an innovative laser-induced thermophoretic aptasensor (Figure 5F).34 They enabled enrichment of EVs conjugated with aptamers through size-dependent accumulation, relying on the interaction of thermophoresis, diffusion, and convection, all induced by localized laser heating. Their approach enabled a 1400-fold enrichment within just 10 min, with no thermal degradation of EV surface proteins and molecular cargos occurred. Furthermore, the team combined this technique with PEG to enhance the EVs' accumulation.145 This integration allowed for the sensitive, selective, and in situ detection of mutated RNA within EVs, proving a significant stride in EVs research and potential diagnostic applications.
4.1.5 Dielectrophoresis based EV isolation
DEP is an electrokinetic phenomenon that allows for the manipulation of suspended particles within an electric field based on their size and electrical properties. Yobas et al. demonstrated the use of DEP in a microfluidic device for continuous-flow, label-free size fractionation of EVs (Figure 5G).140 By combining electrothermal fluid rolls and DEP, they achieved the separation of larger EVs from smaller ones. By optimizing voltage activation and flow rate, the smaller EVs were concentrated around the channel centerline and exited through the main branch, effectively separated from the large EVs. Another approach involved combining alternating current electroosmosis and DEP to attract EVs toward a sensor chip surface, specifically concentrating them on plasmonic sensing areas. This method enabled the sensitive collection of tumor-derived EVs in a short time, revealing significant differences in yield signals between cancer and healthy control groups, demonstrating the potential of DEP-based techniques for both EV isolation and disease diagnostics.146
4.1.6 Acoustophoresis based EV isolation
Acoustophoresis centrifugation have revealed its potential in separating particles based on size differences and become one of the popular high-precision and low-damage isolation strategies. Lee and colleagues developed an acoustic nanofilter system that separates EVs from biological samples,147 employing surface acoustic waves to generate ARF. Larger EVs are pulled toward the pressure nodes near the channel sidewalls, while smaller EVs remain at the central flow as the acoustic force exerted on them is weaker. A unique approach was developed called Acoustic Nanoscale Separation via Wave-pillar Excitation Resonance for the fractionation of nanoscale bioparticles (Figure 5H).141 They used excitation resonance to form an array of virtual acoustic wave pillars in a microfluidic channel, where the pillars act as filters, exerting stronger influence on larger particles to separate EVs. Their strategy provides a rapid and efficient method for the high-purity fractionation EV subpopulations from biofluids in less than 10 min. Collins et al. expanded on these methodologies by developing a device that leverages focused traveling acoustic waves to manipulate micro and nanoparticles.148 They observed different focus points for nanoparticles of 100 nm, 300 nm, and 500 nm diameters, regardless of their initial positions, demonstrating the flexibility and precision of this approach.
4.1.7 Magnetophoresis based EV isolation
Magnetophoresis-based methods for EV isolation employ magnetic fields to segregate EVs. Typically, this process utilizes a magnetic field gradient from a permanent magnet source to displace EVs by attracting a magnetic medium such as an aqueous suspension of MNPs. These nanoparticles have a magnetic susceptibility much greater than that of a paramagnetic solution, enabling the manipulation of bioparticles within a microchannel using regular permanent magnets. Yang's group proposed an improved separation platform that is both label-free and biocompatible, with a higher sample throughput (Figure 5I).142 To enhance the magnetic force and achieve nanoscale resolution, while also addressing the biocompatibility issues of the ferrofluid, they used arrays of magnetic poles. This approach successfully produced an ultra-high magnetic field gradient of 105 T/m within the microchannel, enabling the isolation of EVs from cell culture media with a recovery rate of 86% and a purity of 80% at a sample flow rate of 150 μL/h. This methodology demonstrates the potential of magnetophoretic techniques in the efficient and high-throughput isolation of EVs.
Without preselection of EV subpopulations, most physical feature-based isolation nanotechnologies retain the primary bulk properties and biological activity of EVs. However, their clinical applications may be largely restricted because of the laborious fabrication of the facilities and the robustness of these complex systems. Thus, the simplification of the design and process technology should be considered in the subsequent development of these EV capture devices.
4.2 EV isolation based on chemical affinity
Antibodies, aptamers, and peptides that can specifically bond with the proteins in the membrane of tumor cells, are also capable of isolating EVs secreted from these cells, carrying similar surface proteins. Rather than EV isolation based on their physical features, specific isolation can focus on EVs secreted from targeting tissues and reveal more purity information (Table 1). However, these strategies still can hardly sidestep issues for isolating EVs from complex biological samples, like long interaction time, low amount of yielded EVs, and lack of automatic processes. Except from specific isolation, EV isolation based on chemical affinity can also apply non-specific molecules that have a strong affinity to the composition of EV membrane, mainly lipid.
4.2.1 Antibody based EV isolation
Antibody-based EV isolation employs a method similar to antibody-based CTC isolation. This technique utilizes surface proteins found in EV membranes, such as CD63, CD9, CD81, EpCAM, EGFR, and GPC-1, to bind and isolate specific EVs from various fluids.149 For example, microvilli-like silicon nanowire arrays featured an increased surface area and high-efficiency EV isolation utilizing modification of anti-EpCAM (Figure 6A).150 The combination of antibodies targeted EpCAM, ASGPR1, and CD147 on the EV membrane, to create a hepatocellular carcinoma (HCC)-specific EV purification system.29 The antibodies were modified with click-on reaction agents trans-cyclooctene (TCO), and the capture substrate (silicon nanowires) was modified with tetrazine (Tz). After the slow interaction reaction between TCO-antibodies and EVs in fluids, the rapid click-on reaction of TCO with Tz will bond EVs to the capture substrate soon after TCO-EVs injection. Similarly, Stott et al. designed a sensitive microfluidic platform for tumor-specific EV-RNA isolation within 3 h157 which was equipped with a nanostructured substrate functionalized with streptavidin-coated nanoparticles to bind with antibodies.
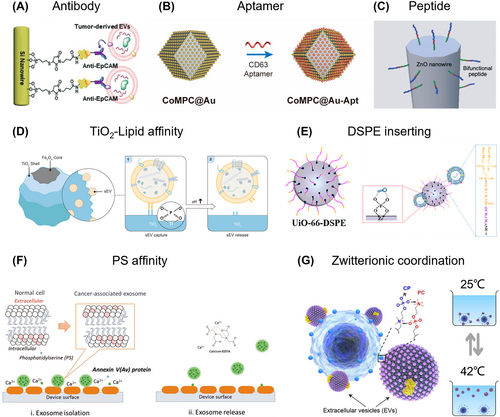
Extracellular vesicle (EV) isolation based on chemical affinity. Specific isolation: (A) Antibody. Reproduced with permission.150 Copyright 2019, American Chemical Society. (B) Aptamer. Reproduced with permission.151 Copyright 2022, American Chemical Society. (C) Peptide. Reproduced with permission.152 Copyright 2021, The Royal Society of Chemistry. Non-specific isolation: (D) TiO2-lipid affinity Reproduced with permission.153 Copyright 2022, American Chemical Society. (E) DSPE inserting. Reproduced with permission.154 Copyright 2022, American Chemical Society. (F) PS affinity. Reproduced with permission.155 Copyright 2019, Wiley-VCH. (G) Zwitterionic coordination. Reproduced with permission.156 Copyright 2023, American Association for the Advancement of Science.
4.2.2 Aptamer based EV isolation
Aptamers, due to their high specificity and affinity like antibodies, are capable of binding to specific targets. This characteristic makes them valuable for developing affinity-based isolation strategies. For example, aptamer-coupled Au-decorated polymorphic carbon (CoMPC@Au-Apt) exhibited EV capture from urinary of gastric cancer patients. By utilizing a CD63-specific aptamer, they achieved efficient capture within a short period of 45 min (Figure 6B).151 In another study, Anastasia Malek and colleges developed an AuNP aptasensor for the analysis of CD30-positive EVs.158 This approach facilitated the isolation of CD30-positive EVs from Hodgkin and Reed-Sternberg cells, providing insights into the activity of classical forms of Hodgkin lymphoma. Chen et al. utilized PD-L1-targeting-aptamer-anchored magnetic microspheres for the specific capture of PD-L1-positive EVs.159 Their research revealed that PD-L1-positive EVs can directly bind and inhibit T cell proliferation, like their parent cells. Additionally, these PD-L1-positive EVs can transfer immunosuppressive proteins related to the “synapse” to antigen-presenting cells, inducing a T-cell-inhibitory phenotype. Compared to antibody-based capture techniques, aptamer-based methods hold great potential for EVs separation due to their high binding affinity for protein biomarkers present on the surface of tumor-derived EVs.
4.2.3 Peptides based EV isolation
Peptides with a specific affinity for EV surface proteins are used for EV isolation. Ouellette's group developed a unique class of peptides, exhibiting a specific affinity for canonical heat shock proteins, have been validated to capture these protein-containing EVs from sources like cell culture growth media, plasma, and urine.160 Okochi et al. identified a peptide based on the amino-acid sequence of EWI-2 protein (Figure 6C),152 which specifically bonded with CD9 protein on cancer EVs. By combining this peptide with a ZnO-binding sequence, they realized reversible capture of EVs under a neutral salt condition.
4.2.4 TiO2-phosphate affinity based EV isolation
Non-specific EV isolation strategies based on chemical affinity typically leverage the affinities between lipid and the capture substrate. Among these methods, the TiO2-phosphate affinity demonstrated simple fabrication processes, straightforward isolation protocols, and compatibility with downstream analyses. Hu et al. employed this user-friendly strategy selectively isolating EVs and detecting their metabolic alternations (Figure 6D).153 Zhang and co-workers reported a similar TiO2-mediated EV isolation strategy from a small volume of plasma, where they simultaneously profiled proteins and metabolites.161 Another research group utilized this strategy for reversible EV isolation, achieving high recovery rates (93.4%) using an alkaline solution.43 In addition, Deng et al. utilized dual-affinities MBs, integrating TiO2 with CD63 specific aptamer, achieving high-efficient EV isolation up to 92.6% within 10 min49 However, this strategy can isolate all particles with a phosphate group, thereby requiring a preliminary treatment to first remove cells.
4.2.5 Lipid based EV isolation
Except form the head of phospholipid, the lipid molecules on the surfaces of EVs significantly influence EV isolation. In 2017, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine (DSPE) emerged as a promising probe for non-specific EV isolation, since it can insert into the phospholipid membrane of EVs through its two hydrophobic fatty acid tails.44 Wang et al. prepared DSPE-functionalized UiO-66 metal-organic framework,154 by leveraging the synergistic affinity interaction of Zr4+ ion and DSPE with EVs for efficiently EV enrichment from urinary samples, achieving release efficiency up to 99% through alkaline elution (Figure 6E). Chen et al. intravenously injected DSPE-PEG-Biotin to directly label circulating EVs in vivo, aiming to minimize alterations in their behavior.162 Using this strategy, they revealed the distinct in vivo fate of circulating EV subpopulations originating from different cell sources. Notably, they discovered that erythrocyte-derived EVs exhibited the longest lifespan. Similar to pretreatment with antibodies, this approach allows for an interval between interaction and isolation, facilitating the enrichment of EVs.
4.2.6 Phosphatidylserine affinity based EV isolation
Due to the nano-size of EVs, the EV membrane contains a larger proportion of cone-shaped lipids such as phosphatidylserine (PS), which typically isn't present on the outer layer of normal cells. By exploiting specific affinities toward PS, EVs can be efficiently isolated. Annexin A5 and T-cell membrane protein 4 (TIM-4) have been proposed as efficient probes for EV isolation due to their high affinity for PS molecules. For example, Nagrath et al. conjugated Annexin A5 with a microfluidic chip to isolate cancer-associated EVs and release immobilized EVs using Ca2+ ion chelation (Figure 6F).155 Deng et al. modified a herringbone microfluidic chip with TIM-4 to isolate tumor-derived PS-positive EVs,163 demonstrating that cancer patients have a higher number of PS-positive EVs circulating in the bloodstream compared to healthy donors. Ye and colleagues presented a combination of TIM-4 based specific isolation and a signal transduction strategy for the high-sensitive detection of CD63 positive EVs, achieving an extremely low limit of detection around 4.39 × 103 particles/mL, which underscore the potential of PS-specific approaches in enhancing the efficiency of EV isolation and detection.164
4.2.7 Zwitterionic coordination based EV isolation
Zwitterionic coordination leverages the specific polyvalent interactions between the phosphatidylcholine (PC), a prevalent headgroup on EV membranes, and choline phosphate, an “inverse” of PC.165 A method was introduced based on zwitterionic coordination that enabled the extraction of EVs from a variety of biofluids such as blood serum, urine, and saliva, in 30 min, yielding over 90% purity and recovery (Figure 6G).156 Furthermore, the PC-CP coordination could be reversibly regulated by merely altering the surrounding temperature, facilitating the instant release of EVs. In a related study, zwitterionic polymeric coacervates were designed, composing sulfabetaine methacrylate and [2-(methacryloyloxy)ethyl]trimethylammonium.166 The researchers used these coacervates to recruit and release EVs involving one-minute incubation followed by the separation through centrifugation and release approximately 86% of EVs after NaCl treatment.
Based on above-mentioned strategies, EVs can be rapidly and high-efficiently isolated from body fluids. While, EVs exist in near all body fluids making them promising biomarkers for various disease (Table 2). Other than blood samples, urine with large volume and easy sampling processes attracted large attention for guiding clinical decision-making in diseases associated with urinary system, such as focal segmental glomerulosclerosis,171 systemic lupus erythematosus,183 PCa,172, 173 and bladder cancer.184 Likewise, tear and saliva have also been applied for decision-making of diseases related to their origin, such as dry eye syndrome175 for tear or periodontitis178 and glioblastoma179 for saliva. As another kinds of body fluids accumulated in body cavity, cerebrospinal fluid and ascites contain hormones, and other actively or passively secreted metabolites from cells and cell apoptosis. Extracellular vesicles isolated from cerebrospinal fluid can reflect the situation of diseases related to brain and central nervous system like aneurysmal subarachnoid hemorrhage177 and cancer leptomeningeal metastasis.176 EVs in ascites also demonstrated great performance in cancer diagnosis181 and therapy monitoring.182
| Specimen | Pretreatment | Isolation technologies | Clinical significance | Ref. |
|---|---|---|---|---|
| Blood | Centrifugation | Precipitation | EV piRNAs can be diagnostic signatures for NSCLC | 167 |
| Ultracentrifugation | EV proteome enhances the diagnosis and prognosis prediction of NSCLC | 168 | ||
| Ultracentrifugation & filtration | EV are promising predictors of the efficacy of thermoradiation therapy | 169 | ||
| Precipitation | EV miRNAs enable diagnosis of prostate cancer. | 170 | ||
| Urine | Centrifugation | Precipitation | Urine EV miR-193a levels help diagnosis of focal segmental glomerulosclerosis in children. | 171 |
| Filtration | Ultracentrifugation & Precipitation | Urine extracellular vesicle circRNAs help the detection prostate cancer for patients with prostate-specific antigen 2–10 ng/mL at initial biopsy | 172 | |
| Centrifugation | DSPE modified MBs | EV phosphoproteomic analysis enables noninvasive biomarker screening and early cancer diagnosis. | 173 | |
| Tear | NA | Antibody-conjugated size-based signaling nanocavities | Tears can be used for detection of cancer-related EVs for breast cancer diagnosis | 174 |
| Filtration | Tear EV have the capability of distinguishing patients with dry eye syndrome and providing potential biomarkers | 175 | ||
| Cerebrospinal fluid | Centrifugation | NA | The size distribution and concentration of nanoparticles indicate leptomeningeal metastasis. | 176 |
| Filtration | Interleukin-6 (IL-6) in cerebrospinal fluid EVs are potential biomarkers for prognosis of aneurysmal subarachnoid hemorrhage | 177 | ||
| Saliva | Centrifugation | SEC | miRNAs in EVs can be diagnostic signatures for periodontitis patients | 178 |
| Differential centrifugation and/or ultracentrifugation | Prognosis monitoring for glioblastoma patients | 179 | ||
| Ascites | NA | Sequential centrifugation & SEC | EV with arginase-1 suppress T-cell responses and promote tumor growth | 180 |
| Centrifugation | Precipitation | miRNA in EVs can be diagnostic signature for ovarian cancer | 181 | |
| Ultracentrifugation | Ascites-derived CDCP1+extracellular vesicles are novel therapeutic target | 182 |
Even though EVs from various body fluids can reflect multi-dimensional situation of diseases, it can be noticed most of novel strategies focused on the universal-available and high-stable fluids for feasibility verification, due to several challenges caused by their heterogeneous physical and chemical characteristics. For example, the EV concentration of urine and saliva is easily affected by different physiological conditions, and the timing of sample collection largely affects the results of EV detection. In addition, the sampling processes are damageable for cerebrospinal fluid, or require precision operation for tears. This gap between advanced isolation strategies and real-world clinical applications hinders the implantation of EV related liquid biopsy into clinical scenarios, leading to the consequences that clinician trends to rely on commercial-available, mature but inefficient technologies (Table 2).
In summary, the next generation of EV isolation strategies should retain the primary bulk and properties biological activity of EVs, while simplifying laborious fabrication and operation processes in the meantime to broaden their clinical applications for various diseases using different body fluids.
5 DETECTION OF BIOMARKERS
The urgent need for highly sensitive detection technologies arises from the low concentration of CTCs and EVs. Advanced sensors, such as optical sensors, electrochemical sensors, and others, have significantly enhanced the detection ability of CTCs and EVs. Additionally, single biomarker detection methods have been developed to comprehensively address the heterogeneity of biomarkers.
Fluorescent signal-based techniques utilize fluorophores conjugated to specific antibodies, aptamers, or probes for the detection of CTCs and EVs.7, 34, 185 These techniques enable real-time monitoring, imaging, and high-throughput analysis using flow cytometers, microscopes, and microfluidic devices. Immunofluorescence staining, fluorescence in situ hybridization (FISH),17 and proximity ligation assays186 enhance sensitivity by allowing visualization of biomarkers. Advancements in nanotechnology introduce bright and photostable fluorescent nanostructures, such as quantum dots,187, 188 for multiplexing capabilities. Despite challenges in improving signal-to-noise ratios and addressing autofluorescence and non-specific binding, fluorescence-based methods offer high sensitivity and compatibility.
In addition to fluorescence, spectral signal sensing serves as an alternative for CTC and EV detection, including near-infrared (NIR) spectroscopy,189 SPR,112, 190 and surface-enhanced Raman spectroscopy.191, 192 These methods leverage the unique spectral characteristics of CTCs and EVs, which arise from their intrinsic properties or specific labeling. By analyzing the emission spectra of these particles upon excitation, spectral signatures can be obtained, enabling their identification and quantification offering advantages such as multiplexing capabilities, increased specificity, and improved signal-to-noise ratios. However, challenges remain in optimizing spectral resolution, minimizing background noise, and developing standardized protocols for spectral data interpretation.
Electrochemical sensors utilizing electrochemical principles to detect and quantify biomarkers, offering advantages of high sensitivity, rapid response, and wide compatibility. By linking specific signal molecule with biomarkers, CTCs and EVs can be selectively recognized and quantified using electrochemical sensors, allowing for the sensitive and accurate analysis. Enzymatic cascade reaction193, 194 electrochemical impedance spectroscopy,195 and electrochemical luminescence (ECL)196 are employed for electrochemical detection. Moreover, ultrasensitive nanoprobes, including Ti3C2 MXene,197-199 ferrocene (Fc),200, 201 and metal ions, have been utilized to further improve the sensitivity. Additionally, dual signal detection systems such as G-quadruplex/Hemin202 and nanointerdigitated electrodes203 have been explored.
Various sensors, including field-effect transistors,204 giant magnetoresistance signals,205 electrokinetic signals,206 and surface acoustic wave sensors,207 have also been applied for biomarker detection. Besides, information includes by analyzing the genome,47 proteome,49 transcriptome,29, 50 and metabolome51 of these biomarkers can also be analyzed by PCR, ELISA, and mass spectrum. These studies have demonstrated the performance of different sensors and reported promising sensitivity for many new approaches. Nevertheless, the further application of these sensors is limited by the complexity and cost of signal-processing devices. Moreover, since these studies were conducted using diverse body fluids and different isolation technologies, conducting parallel comparisons under strictly controlled conditions is necessary to provide a fair assessment.
6 CLINICAL APPLICATION BASED ON CTCS AND EVS
The ultimate aim of efficiently and specifically isolating biomarkers, such as CTCs and EVs, is to harness the information they contain about the primary tumor. This rich data source can empower clinicians to guide patient treatment more effectively. Both CTCs and EVs have shown considerable potential in clinical applications across various tumor types. They can significantly aid in clinical decision-making, spanning diagnosis, prognosis, disease monitoring, and selection of the most appropriate therapeutic strategy (Table 3).
| Cancer | Biomarker | Clinical requirements | Clinical decision-making | Detection | Participants enrolled | Ref. |
|---|---|---|---|---|---|---|
| NB | CTC | Poor OS for high-risk patients and lack of instrumentation for monitoring | Metastasis monitoring and prognosis | i-FISH to CEP8 | 28 patients | 208 |
| Less-considered MRD leading to metastases and relapse | MRD detection for metastasis and relapse monitoring | Genome profiles | 17 patients with 11 post-chemotherapy and 3 relapse | 209 | ||
| EV | Unclear mechanism between hypoxic EVs and NB dissemination | Metastasis monitoring | Transcriptome profiles | NA | 210 | |
| Patients fail to respond or develop resistance to immunotherapy | Immunotherapy optimization | Transcriptome profiles | NA | 211 | ||
| LC | CTC | Poor OS requiring early diagnosis | Cancer diagnosis | Immunofluorescence | 39 patients and 11 controls | 212 |
| Lack of methods for metastasis detection with heterogeneous malignant cells | Metastasis monitoring | Immunofluorescence and Fish | 25 NSCLC patients | 18 | ||
| Whether proteins on CTCs can be markers for prognosis | Prognosis | Proteome profiles | 45 NSCLC patients | 213 | ||
| EV | Most patients diagnosed at an advanced stage | Early diagnosis | Transcriptome profiles | 115 patients (95 in stage I) and 47 controls | 167 | |
| Only 20%–40% of patients positively respond to anti-PD-1/PD-L1 immunotherapy | Immunotherapy optimization | Transcriptome profiles | 27 non-responding and 27 responding to immunotherapy | 214 | ||
| HCC | CTC | Relationship of CTCs to microvascular invasion (MVI) and prognosis | Prognosis | Microscopic images | 137 patients | 218 |
| Whether CTC-WBC clusters suggesting poor prognosis | Prognosis | RNA-ISH | 214 patients | 17 | ||
| Relationship of genomic alterations in CTC and cancer monitoring | Cancer monitoring | Genome profiles | 29 patients | 220 | ||
| EV | Earlier detection reducing high HCC mortality rates | Early diagnosis | Transcriptome profiles | 158 patients | 29 | |
| Cancer-associated fibroblasts (CAFs)-derived EVs promote tumor progression and chemoresistance | Therapy optimization | Transcriptome profiles | NA | 221 | ||
| CRC | CTC | ARB101 antibody as a tool for CRC detection, progression, and therapy response | Cancer diagnosis | Microscopic images | 171 patients stage I–IV: 22, 32, 45, and 72 | 222 |
| Clinical value of CTC enumeration in prognosis of metastasis CRC | Prognosis | Immunofluorescence | 218 patients | 223 | ||
| EV | New markers for CRC diagnosis | Cancer diagnosis | Proteome profiles | 100 patients | 227 | |
| EV surface marker for monitoring metabolic status and tumor angiogenesis | Cancer monitoring | Proteome profiles | NA | 169 | ||
| PCa | CTC | Overdiagnosis and overtreatment for patients in PSA gray zone (4–10 ng/mL) | Early diagnosis | Immunofluorescence | 48 patients | 7 |
| Stratify patients with clinical outcomes | Prognosis & therapy optimization | Transcriptome profiles | 40 patients | 228 | ||
| PCa | EV | Explore suitable EVs for cancer monitoikring from heterogeneous populatioikn | Cancer diagnosis and monitoring | Transcriptome profiles | 36 patients and 18 healthy controls | 170 |
| AR-V7 related to resistance of hormonal therapy, but lack of analysis methods | Therapy optimization | Transcriptome profiles | 36 patients | 230 |
6.1 Neuroblastoma
Neuroblastoma (NB), the most prevalent tumor found outside the cranium in children, is responsible for 10%–20% of pediatric cancer-related deaths. The disease's poor prognosis and substantial biological diversity have resulted in little improvement in patient survival over the past several decades.
Circulating tumor cells from brain tumors can be isolated from either blood or cerebrospinal fluid (CSF). Patients with a count of three or more CTCs per 4 mL of peripheral blood are at a higher risk of metastasis compared to those with fewer CTCs (sensitivity at 88.89% and specificity at 78.59%).208 Increased expression of pro-metastatic genes in CTCs led to bone marrow metastases and relapse, by isolating CTCs from the peripheral blood of 17 neuroblastoma patients at three sequential treatment stages. Moreover, consistent monitoring identified sustained overexpression of certain genes in CTCs, which could potentially act as new predictive markers for hematogenous minimal residual disease in neuroblastoma patients who later relapse.209 Single-cell next-generation sequencing of CTCs have been adopted for acquiring information encapsulated in CTCs. NB patients with advanced stages expressed ALK mutation (p.F1174L) in both tumor tissue and CTC as well as more angiogenesis-related, cell cycle-related genes, CCND1 and TUBA1A genes,215 which showcasing the importance for guiding clinical decision-making.
Extracellular vesicles isolated from CSF offer potential advantages over serum-derived due to lower interference from leukocyte-derived EVs. Normoxic EVs overexpressing miR-210-3p exhibited enhanced pro-metastatic characteristics, whereas silencing miR-210-3p in hypoxic EVs suppressed their metastatic abilities both in vitro and in vivo.210 Additionally, Wang et al. discovered that EVs derived from neuroblastomas significantly reduced the efficacy of the drug dinutuximab in vivo.211 These EVs were also found to manipulate the immune cells, creating an immunosuppressive micro-environment populated with increased numbers of tumor-associated macrophages and fewer tumor-infiltrating NK cells. Such insights could offer valuable clinical interpretations for understanding therapy-induced senescence.
6.2 Lung cancer
Primary LC is one of the most prevalent malignant tumors. At the time of initial diagnosis, approximately 60% of patients already have distant metastasis, which serves as the leading cause of mortality among these individuals. The prognosis of LC remains grim, with a 5-year survival rate varying from 10% to 20% depending on the stage at diagnosis.
Several studies have shown a correlation between the number of CTCs and the diagnosis of LC. A significant increase in the number of CTCs in LC patients can be observed, with the sensitivity (89.7%), specificity (90.9%), and an area under the curve (AUC) value of 0.966 for diagnosis.212 Moreover, CTCs can also be detected in patients with non-small cell lung cancer (NSCLC),18 suggesting that fluctuation in CTC counts following surgery and/or chemotherapy might be indicative of cancer progression. The large data using 550 patients in a recent European multicenter study demonstrated that the number of CTCs ≥2 and ≥5 per 7.5 mL blood could be regarded as signs for the judgments of a diminished PFS and OS, respectively.216 The count of CTCs presents a real-time portrayal of disease progression during treatment and may, under specific conditions, offer valuable predictive insights into potential relapse ahead of manifest clinical symptoms. To identify lung adenocarcinoma and lung squamous cell carcinoma, genome analysis revealed NSCLC-specific DNA methylation enable accurate distinguishment between these two with 100% diagnostic accuracy.217 In the progression of cancer, CTCs may express the CD47 protein, responsible for the inhibition of phagocytosis. In a cohort of 45 NSCLC patients, the presence of CD47-positive CTMs resulted in poor PFS.213
Extracellular vesicles also contribute to early diagnosis of NSCLC. By investigating biocargos encapsulated in EVs, such as Piwi-interacting RNA (piRNA), the level of piR-hsa-164586 in EVs from 115 NSCLC patients (including 95 cases in stage I) was significantly upregulated compared with EVs from healthy individuals, benefiting the early diagnosis of NSCLC.167 The elevation of EGFR and C-X-C chemokine receptor 4 (CXCR4) in serum EVs from advanced stage NSCLC benefits the diagnosis and prognosis prediction of NSCLC.168 EMT-induced protein changes were also detected in the EVs from NSCLC patients, which demonstrated prognostic significance for monitoring at risk metastatic prediction.53 Reátegui et al. indicated the capability of PD-1/PD-L1 messenger RNA in EVs in differentiating responders from non-responders for immunotherapy with an accuracy of 72.2% from a cohort of 27 non-responding and 27 responding NSCLC patients.214
6.3 Hepatocellular carcinoma
Hepatocellular Carcinoma (HCC) ranks as the fifth most prevalent cancer and a leading cause of cancer-related fatalities worldwide with high mortality rate and poor prognosis. The existing diagnostic methods—serum markers, imaging tests, and tissue biopsies—frequently fall short in delivering accurate and prompt diagnosis and surveillance of HCC.
In the realm of prognosis, a prospective study involving 135 HCC patients discovered that surgical intervention resulted in a reduced CTC count.218 CTCs and CTC clusters from 214 HCC patients was analyzed revealing the presence of CTC clusters in peripheral blood to be indicative of poor HCC prognosis.17 As for treatment outcomes, Zhou et al. collected peripheral blood and pathological samples from 117 patients who underwent hepatectomy, observing CTC-positive patients displayed a higher incidence of microvascular invasions (mVI) and a larger spread distance of mVI.219 As cancer progression, CTCs tend to experience EMT process and invade into circulation system and tissues, thus enabling the monitoring of cancers by profiling mesenchymal genomic alterations in CTCs and CTC-WBC clusters.220
The bio-cargos contained within EVs also show potential as biomarkers for clinical decision-making in HCC. For instance, Tseng et al. utilized EVs expressing three specific proteins for the early detection of HCC.29 After purifying these HCC EVs, they used reverse transcription droplet digital PCR to quantify 10 HCC-specific mRNA markers. Testing this system on 158 plasma samples from five cohorts demonstrated that mRNA encapsulated in HCC EVs can effectively detect early-stage HCC in high-risk cirrhotic patients. This non-invasive approach had an impressive area under curve of 0.93 (95% CI, 0.86–1.00; sensitivity = 94.4%, specificity = 88.5%). Furthermore, Wu et al. illustrated that during hepatic fibrosis, TGF-β stimulates the palmitoylation of hexokinase 1 (HK1) in hepatic stellate cells.30 This reaction promotes the TSG101-dependent secretion of HK1 through large EVs. HCC cells take up these vesicles, resulting in increased glycolysis and HCC progression. Shao et al., meanwhile, discovered that miR-1228-3p, encapsulated in EVs derived from cancer-associated fibroblasts, promotes proliferation, migration, invasion, and resistance to the drug sorafenib in HCC cells.221
6.4 Colorectal cancer
Colorectal Cancer (CRC) is the third most prevalent form of cancer globally and a leading contributor to cancer-related fatalities. The elevated mortality associated with CRC primarily stems from metastasis, wherein the disease spreads to remote organs, severely impairing their function.
In a prospective study, Wong and colleagues employed the ARB101 antibody to isolate CTCs from CRC patients.222 They identified ARB101-positive CTCs in 64 out of 83 (77%) CRC patients across all TNM stages (I to IV) and observed a significant variation in CTC levels between pre- and post-surgical patients. In another study, CRC CTCs were utilized to predict patient prognosis,223 demonstrating the best prognosis for no evidence of CTCs at any of the measured time points, while lower PFS and OS for patients showing positive CTCs on two separate checks time points. Further, 288 distinct metabolites were identified, suggesting that CTCs undergo metabolic pathway alterations during liver metastasis, involving pathways such as the folate biosynthesis, one carbon pool by folate, and histidine metabolism, and others.224 Proteome of CTCs-derived organoids revealed the increased expression of stemness-associated markers as cancer progression, leading to the EMT state and poor treatment outcome of chemotherapy, with potential for investigating the cancer metastasis and prognosis.225 Single-cell level RNA sequencing technology was also applied for transcriptome profiles, revealing the phenotype of CTCs during the EMT process, showcasing patients with higher EMT gene express had a significantly shorter PFS and OS.226
Participating in cell communication, Yan et al. discovered EVs secreted by colorectal cancer cells exhibited an elevated level of TNF-α, which is linked to the aggressive features of CRC.227 Additionally, research conducted by Tamkovich et al. uncovered noticeable disparities in the subtypes of EVs derived from adipocytes when comparing CRC patients with those colorectal polyps patients. They pinpointed that the circulating EV subpopulations marked as CD9+MMP9+MMP2-TIMP1- and MMP9+MMP2-TIMP1+ could potentially serve as effective predictors for the success of thermal radiation therapy.169 This determination was based on the substantial variations in their baseline levels observed in CRCPs with different tumor responses.
6.5 Prostate cancer
Prostate cancer ranks as the second most prevalent cancer among men in the United States. Although the use of prostate-specific antigen (PSA) has enhanced early screening and diagnosis of PCa, it remains imperfect due to a high false-positive rate, especially for patients within the PSA gray zone (PSA levels between 4 and 10 ng/mL).
In an effort to reduce excessive testing for patients within the PSA gray zone, a study by Wang et al. utilized a rGO platform, which was modified with dual-antibodies, to facilitate efficient and precise CTC isolation.7 By integrating the levels of CTCs, PSA, PSAD, and T/P-PSA, they achieved a high sensitivity of 91.7% and a high AUC value of 99.1% for the early diagnosis of PCa patients, thus reducing the potential for overdiagnosis and overtreatment. Additional studies have also shown that CTCs exhibit impressive performance in the diagnosis of PCa patients. Beyond early diagnosis, CTCs also offer the potential to predict therapy response. Schalken et al. employed transcriptome analysis using HyCEADTM technology with a tailored PCa gene panel to stratify metastatic castration-resistant PCa patients based on their clinical outcomes.228
Regarding the early detection of PCa, Malek et al. applied MNPs fortified with five kinds of DNA-aptamers for isolating EVs from the plasma of 36 PCa patients and 18 healthy donors. Utilizing three sets of miRNAs (miR-205/miR-375, miR-26b/miR375, and miR-20a/miR-375), this method differentiated PCa patients with a sensitivity of 94%, specificity of 76%, and accuracy of 87%.170 In an effort to uncover the mechanism of bone metastases in PCa, Ochiya et al. highlighted that the receptor activator of NF-κB ligand. Extracellular vesicles from metastatic PCa cells have the potential to encourage osteoclast formation due to the presence of CUB-domain.229 Androgen receptor splice variant 7 (AR-V7) RNA in EVs can be regarded as prediction of the resistance to hormonal therapy.230 14 out of 36 CRPC patients positive for AR-V7 and received either enzalutamide or abiraterone exhibited both a decreased median PFS of 3 months and a median OS of 8 months.
7 MACHINE LEARNING ASSISTED OPTIMIZATION OF LIQUID BIOPSY
Recently, the rapid development of machine learning techniques has revolutionized data analysis by enabling the integration of multidimensional data.231 These sophisticated algorithms offer a unique perspective enabling identification of data trends potentially overlooked by traditional experimental approaches, thus deriving relations without explicit programming or human intervention.232 In the field of liquid biopsy, machine learning participated in a wide variety of biomedical applications especially in optimizing biomarker isolation technologies and clinical decision-making processes. Traditionally, experimental methods can hardly snapshot all trends of a phenomenon. Additionally, single biomarker may hardly provide sufficient sensitivity, specificity, and accuracy for making clinical decisions. To address these challenges, machine learning algorithms have been explored, enabling the classification of samples, and deciphering the complex information. However, as the complexity of machine learning algorithms increases, they may produce more accurate results but become less interpretable.11 This trade-off means that highly complex algorithms can become a black box that are difficult to interpret. Furthermore, the risk of overfitting arises as algorithms become more intricate, necessitating careful examination within the machine learning framework.
7.1 Machine learning assisted optimization for biomarker isolation
Machine learning algorithms play a critical role in enhancing the optimization of liquid biopsy isolation by extracting parameters and training models to output results integrated several parameters (Figure 7D). The conventional way of optimizing an isolation process usually entails the laborious task of manually investigating various factors associated with the performance of isolation, often involving repetitive and tedious experiments. To surmount these hurdles, machine learning can help construct microfluidic design models, considering performance metrics such as sorting efficiency and yield, and channel characteristics. The incorporation of machine learning not only elevates design performance but also facilitates the prediction of new samples' behavior, thus providing a more streamlined and effective approach for isolation optimization (Figure 7A).233 Beyond isolation based on physical features, machine learning can significantly improve the specific identification of CTCs by determining the best combination of recognition molecules for particular tumor types or even individual patients. This method allows clinicians to make evidence-based decisions on the selection of recognition molecules for CTC isolation, utilizing machine learning models integrating initial blood tests and patient data, rather than depending on pre-set combinations of mixed antibodies.
Machine learning assisted optimization of liquid biopsy. The optimization of isolating and detecting biomarkers: (A) The model of machine learning. (B) Optimization of isolation methods. (C) Cell recognition. (D) Extracellular vesicle (EV) classification. The optimization of clinical decision-making: (E) Early diagnosis. (F) Metastasis monitoring. Reproduced with permission.234 Copyright 2023, Springer Nature. (G) Prognostic prediction. Reproduced with permission.168 Copyright 2022, MPDI.
Machine learning facilitates effective categorization based on data derived from CTCs, such as their morphology or enclosed biomolecules. Yang and co-workers proposed a label-free technique named bright-field image cytometry assisted by a multi-frame image correlation algorithm, for detecting CTCs in peripheral blood.235 They accomplished commendable average precision rates of 99.40%, 99.52%, and 99.47% for HT29, A549, and KYSE30 cells, respectively. However, merely detecting and counting CTCs might not offer clues about the origin of tumors or permit the analysis of rare information captured in CTCs at extremely low frequencies. To tackle this, Song and colleagues incorporated single-cell RNA sequencing (scRNA-seq) into CTCs (Figure 7B).236 Their algorithm demonstrated the ability to classify CTCs based on their originating lesions and EMT process with high accuracy (varying between 83.33% and 100%).
In addition to leveraging machine learning to enhance the performance in CTC analysis, EVs with a wealth of information from various analyses can also greatly benefit from machine learning for disease diagnosis, classification, and deep mining of EV data.11, 237 Basile et al. evaluated the potential of Fourier Transform Infrared Spectroscopy (FTIR) to differentiate between EVs obtained from the sera of HCC patients and control subjects based on their mid-IR spectral response (Figure 7C).238
7.2 Machine learning assisted clinical analysis
Machine learning models have proven their ability to process unstructured data and autonomously generate high-quality features, enhancing the precision of diagnoses and the optimization of treatment plans. In clinical settings, pathologists frequently utilize machine learning tools to address the limitations of subjective visual assessments and to incorporate computational techniques for improved accuracy in tumor management.239 Practically, once information from biomarkers such as CTCs and EVs is procured, these data can be merged with clinical information garnered from blood tests, imaging examinations, and patient demographics aiding clinical decision-making in terms of early diagnosis, metastasis monitoring, and prognosis.240
For early diagnosis, Wang et al. utilized machine learning to enhance the early diagnosis of PCa by integrating CTCs with clinical parameters, such as PSA, F/T-PSA, and PSA (Figure 7E).7 The model, integrating 4 parameters, showed a significantly higher AUC value of 99.1% and a higher sensitivity of 91.7%, markedly improving the diagnostic sensitivity for PCa patient within the PSA gray zone (PSA levels from 4 to 10 ng/mL). Machine learning can also aid in monitoring metastasis. Chen et al. designed a molecular typing system to predict the potential for metastasis in colorectal cancer based on the metabolic fingerprints of individual CTCs (Figure 7F).234 By combining CTCs with potentially metastasis-related metabolites using a machine learning model, patients can be segmented into two subgroups. Regarding EVs in prognosis prediction, Yang et al. employed machine learning to analyze the expression of EGFR and CXCR4 in EVs for diagnostic and prognostic prediction of NSCLC. Patients with both higher level of EGFR and CXCR4 were predicted as malignancy for NSCLC with accuracy of 97.4% in the training cohort and 91.7% in the validation cohort (Figure 7G).168
Machine learning offers promise in developing automated and precise isolation techniques for clinical decision-making. These capabilities could be instrumental in shaping clinical expertise into standardized guidelines applicable across all medical organizations.11, 231 However, machine learning models require large, multidimensional datasets that encompass various patient information including gender, age, type and stage of cancer, proteome, metabolome, level of biomarkers, and information contained within biomarkers, to avoid overfitting-induced impressive accuracy but potentially misleading results. To ensure more reliable outputs from machine learning models, it's vital to: (i) Increase the number of participants; (ii) Establish training, validation and test groups to limit overfitting.241
8 CONCLUSION AND OUTLOOK
In this review, we summarize the latest advancements in technology that facilitate the proficient isolation of biomarkers for liquid biopsy. We also discuss how these technologies intersect with clinical applications to provide real-world clinical decision-making based on the information contained in these biomarkers.
Years of progress have refined the isolation processes, enabling the highly efficient, specific, and pure isolation from a variety of bodily fluids. Thorough analysis allows us to extract and interpret the information carried by these biomarkers. The meticulous design of microchannels, accurate manipulations, and the synergy of topological match and molecular interactions enhance the performance of biomarker isolation. Real-world clinical requirements prioritize high sensitivity and specificity while minimizing manual processes and maximizing information acquisition (Figure 8). Recent research has demonstrated the potential of automated machines to simplify labor-intensive operations. However, there is still untapped potential for creating even more efficient biomarker isolation processes.
The future of liquid biopsy. Current strategies have limitations, making them hardly fulfill the clinical requirements of high sensitivity, high specificity, high efficiency (samples needing manual processes per hour), and more information provided by single test. To address these issues, the future of liquid biopsy needs the interdisciplinary integration of advanced isolation technologies, artificial intelligent and automation. In the field of material science, on-demand isolation can be achieved by meticulous design of microchannels, accurate control of manipulations, and the synergy of superior topological match and high-affinity molecular interactions. As for artificial intelligent, the big data from multiple centers can accelerate the iteration of algorithms to provide more accurate prediction. Besides, the automation enables auto-isolation, auto-detection, and auto-analysis, thus reducing manual operations and minimizing disruptions. These strategies assist clinical decision-making containing diagnosis, prognosis, cancer monitoring, and therapy selection.
Despite decades of advancements, the implementation of biomarkers such as CTCs and EVs in clinical practice remain limited, which can be improved by the interdisciplinary of material science, clinical trials, machine learning algorithms, and automation. Future research for material science could initiated from clinical requirements, such as the diagnostic challenges in the PSA gray zone for PCa patients or drug resistance after chemotherapy, which need on-demand isolation strategies. Regarding clinical trials, an effective way to accelerate the adoption of liquid biopsy could involve multi-center collaborations to expand patient cohorts for validating high-performance isolation strategies. Simultaneously, embracing innovative strategies that offer highly sensitive and specific isolation of biomarkers is crucial for accurate information acquisition in cancer patients, guiding clinical decision-making based on consistent enumeration, genome, metabolome, etc.
Interdisciplinary should extend beyond material fabrication and clinical trials. Machine learning and mechanical automation can significantly enhance the development of liquid biopsy in clinical decision-making processes. Artificial intelligent enable the classification and integration of multi-dimensional complex data, offering insightful and unique predictions to optimize isolation strategies and improve the understanding of patients' conditions. Mechanical automation standardizes processes, reducing disruptions caused by manual operations. Furthermore, cost-efficient automated machines can enable the implementation of these standard, easy-to-operate, and full-procedures for biomarker isolation, recognition, and analysis to broaden these precise cancer detection strategies for the healthcare organizations worldwide.
In conclusion, the amalgamation of advanced technologies will pave the way for the creation of next-generation techniques applicable in clinical practice. We are confident that the close interdisciplinary collaboration between materials scientists and clinicians in the field of liquid biopsy will significantly enhance the optimization of clinical decision-making procedures in the future.
ACKNOWLEDGMENTS
This work was supported by grants from the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB 0470201), the National Key R&D Program of China (2019YFA0709300), National Natural Science Foundation of China (22035008), and Beijing Natural Science Foundation (JQ23008).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interests.
Biographies

Han Bao received his master's degree at Beijing University of Chemical Technology in 2020. He is now studying for his Ph.D. degree at Technical Institute of Physics and Chemistry of the Chinese Academy of Sciences (TIPC-CAS). His current research major includes bio-inspired interface and liquid biopsy.

Li Min is currently an associate professor at Beijing Friendship Hospital, Capital Medical University and National Clinical Research Center of Digestive Diseases (NCRCDD). He received his Ph.D. (2011–2016) in molecular biology from Peking University, and then worked at Beijing Friendship Hospital as an instructor and assistant professor (2016–2019). His current research focuses on the microenvironment change of early tumorigenesis and the development of EV-based biomarkers to detect cancer at an early stage.

Fanqin Bu is currently a Ph.D. candidate (2021-) at the Beijing Friendship Hospital, Capital Medical University. His current research focuses on separations of EV with nanostructures and EV-based liquid biopsy for early screening of digestive carcinogenesis.

Shutao Wang is currently a full professor at TIPCCAS. He received his B.S. degree (2000) and M.S. degree (2003) from Northeast Normal University and his Ph.D. degree in 2007 from ICCAS under the supervision of Prof. Lei Jiang. He then worked in the Department of Molecular and Medical Pharmacology and California NanoSystem Institute at the University of California at Los Angeles as a postdoctoral researcher (2007–2010). He was appointed as a full Professor of Chemistry in 2010–2014 at ICCAS. His research interests include the design and synthesis of bio-inspired interfacial materials with special adhesion and their applications at the nano-biointerface.

Jingxin Meng is currently a professor at the Technical Institute of Physics and Chemistry, Chinese Academy of Sciences (TIPCCAS). He received his Ph.D. degree from Northeast Normal University in 2011. He then worked as a postdoctoral researcher at the Institute of Chemistry, Chinese Academy of Sciences (ICCAS) (2011–2014). His research interests focus on bioinspired anti-adhesive materials and their applications in disease diagnosis, environmental protection, and energy saving.




