Nanozymes: Applications in clinical biomarker detection
Abstract
Enzymes are highly efficient catalysts employed in the biomedical field. However, their use is limited by poor stability and high cost. To address this, there is an imperative need for research on nanozymes. In the last decade, the introduction of nanozymes has led to exponential growth in research on nanozyme-based biosensors. The rapid and precise detection of clinical biomarkers is crucial for various disease diagnostics. In recent years, researchers have developed different catalytic active nanomaterials and combined them with colorimetric, fluorescent, and electrochemical methods to detect clinical biomarkers. This review critically overviews the progress, future prospects, and challenges of nanozyme-based clinical biomarker assays in the past few years.
1 INTRODUCTION
Enzymes are efficient biological catalysts mainly used in catalyzing the conversion between biomolecules.1 Due to their high specificity and efficient catalytic activity, enzymes have been extensively studied in the fields of biology, chemistry, and medicine.2, 3 However, natural enzymes have shortcomings, such as poor stability, strict storage conditions, and high production costs, limiting their widespread use in complicated conditions. Thus, it is urgent to explore emerging artificial enzymes as substitutes.4 Since Fe3O4 was proposed as a peroxidase (POD) mimic in 2007,5 nanozymes have received significant attention. Nanozymes are nanomaterials that exhibit excellent catalytic activity and good stability, participating in catalytic reactions with biological molecules. Due to their superiority over natural enzymes, nanozymes are extensively used in clinical disease diagnosis and treatment.6, 7
Biomolecule detection is crucial for clinical disease diagnosis. Traditional detection methods are categorized by their signal output mode as colorimetry,8 fluorescence,9 and electrochemical assay, etc. Moreover, enzyme-linked immunosorbent assay (ELISA) is a common sandwich immunoassay technique used to detect large quantities of protein-based biomolecules.10 Nanozymes can effectively replace enzymes in ELISA. For instance, POD mimic nanozymes can catalyze the production of colorimetric or fluorescent substrates, reducing the reaction time and cost. Therefore, nanoplatforms based on nanozymes continue to be widely applied in the detection of biomolecules.
A large number of reviews related to nanozymes have been reported; however, few focused on the nanozyme-based assays for clinical biomarker detection. Herein, we systematically summarized the catalytic activity of nanozymes and their applications in clinical biomarker assays based on colorimetric, fluorescent, electrochemical and point of care (POC) assays. Finally, we discussed the shortcomings and prospective applications of nanozymes in clinical biomarker assays. It is expected that more researchers will dedicate to develop novel nanomaterials as nanozymes and further enhance their practical applications for clinical biomarker assays in the future (Scheme 1).
A schematic overview of nanozyme-based biosensors combined with various techniques for the detection of different clinical biomarkers. Reproduced with permission.11 Copyright 2021, Elsevier B.V.
2 ENZYME-LIKE ACTIVITY OF NANOMATERIALS
Nanozymes are artificial enzyme mimics combining the physicochemical properties of nanomaterials with the catalytic activity of natural enzymes. In comparison with natural enzymes, nanozymes present excellent catalytic efficiency and superior stability under extreme conditions. Since the inorganic nanomaterial Fe3O4 with the POD-like activity5 was discovered in 2007, numerous nanomaterials, including noble metals12 and their oxides,13 metal-organic frameworks (MOFs),14 and carbon materials,15 have been demonstrated as nanozymes and widely applied in bioanalysis.
Most metal-based nanozymes exhibit better catalytic ability as the metal active center can mimic electronic redox processes of natural enzymes. For example, MOFs16 consist of metal ions and organic ligands, providing uniform cavities that can imitate the catalytic activity sites of natural enzymes.17 Carbon-based materials with the large specific surface area provide more catalytic active sites,18 and the electronic transfer efficiency can be improved by heteroatom doping and surface functionalization.19 For these nanomaterials, researchers have discovered their different catalytic capability as mimic enzymes,20 including POD, oxidase (OXD), superoxide dismutase (SOD),21, 22 catalase (CAT),23 and others (Figure 1).
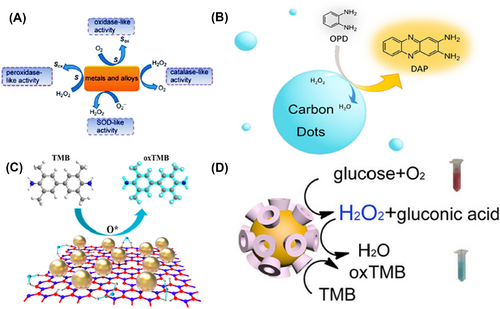
Catalytic activity of nanozymes. (A) Schematic illustration of the catalytic activity of metal-based nanozymes. Reproduced with permission.24 Copyright 2015, American Chemical Society. (B) Catalytic activity of carbon dots as hydrogen peroxidase mimics. Reproduced with permission.25 Copyright 2020, American Chemical Society. (C) Catalytic activity of Cu2+-modified boron nitride nanosheets loaded with Au nanoparticles (Cu2+-BNNS-AuNPs) as oxidase mimics. Reproduced with permission.26 Copyright 2019, American Chemical Society. (D) Catalytic activity of cyclodextrin-modified Au nanoparticles (Au-CDs) as glucose oxidase and hydrogen peroxidase mimics. Reproduced with permission.27 Copyright 2016, American Chemical Society.
In the case of POD-like nanozymes, nanomaterials can oxidize substrates to specific products in the presence of H2O2 as an electron acceptor. Currently, the most popular methods to evaluate the catalytic activity of POD rely on the color changes from chromogenic substrates, such as 3, 3', 5, 5'-tetramethylbenzidine (TMB) or 2, 2'-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS).28 In the case of OXD-like nanozymes, nanomaterials can oxidize substrates to specific products in the presence of O2 as an electron acceptor. In contrast with POD, OXD-like nanozymes can be used to detect some substances directly, such as glucose and uric acid, without the need of H2O2 as the co-substrate. As for CAT-like nanozymes, they oxidize H2O2 to O2 to remove the active oxygen in the body. By contrast, SOD-like nanozymes can oxidize O2- to H2O2, demonstrating the ability to scavenge free radicals. In clinical disease studies, the catalytic activity of POD and OXD is primarily utilized in biosensors and the detection of clinical biomarkers,29 while CAT and SOD activities are mainly applied in cancer therapy.30, 31 Therefore, it is of great interest in the development of various nanomaterials with POD mimic activity and OXD mimic activity for clinical biomolecule detection.
3 NANOZYMES APPLICATION: THE DETECTION OF CLINICAL BIOMARKERS
Clinical biomarkers are important biomolecules to reveal the physiological state of the organism. Traditionally, clinical biomarkers, including nucleic acids, proteins, saccharides, lipids, and metabolites, are known as crucial indicators of disease diagnosis. In contrast to the gene detection and conventional immunoassay, nanozyme-based methods for clinical biomarker analysis are cheap, rapid and sensitive. At present, combining with different signal readout techniques, nanozyme-based assays are mainly divided into optical and electrochemical assays. In particular, optical assays consist of colorimetric, fluorescent, and chemiluminescent assays. The specific applications based on nanozymes for clinical biomarker detection are as follows.
3.1 Colorimetric assay
The colorimetric assay is a simple, rapid, and cheap method, using naked eye or ultraviolet and visible spectrum (UV-Vis) spectrophotometer to discriminate the color change or measure the absorption variation. Traditionally, enzyme-based colorimetric assay is a method that utilizes natural enzymes to catalyze a reaction with a colored substrate, resulting in a color change. Based on the intensity of light absorbed by the colored solution, the concentration of the analyte can be quantitatively determined. However, natural enzymes are expensive, and the production, catalytic reaction, and storage conditions are complex, which limits the wide application of enzyme-based colorimetric assays. Moreover, nanozyme-based colorimetric assays use nanomaterials to mimic the catalytic ability of enzymes to oxidize a chromogenic substrate to produce changes in color and absorbance to quantitatively analyze substances. Since nanozymes have the catalytic activity of natural enzymes along with the advantages of low cost, simple synthesis, and good stability, nanozymes-based colorimetric assays have been widely investigated. Currently, colorimetric assays based on nanozymes can be divided into direct and indirect methods.
3.1.1 Direct methods
The direct method involves the analyte directly interaction with the nanozyme or nanozyme substrates, leading to the variation of catalytic products. For instance, the direct colorimetric detection of H2O2 has been extensively used in cancers and certain nervous system diseases diagnosis. Therefore, it is of great importance to measure H2O2 concentration in the clinical disease diagnosis. Recently, Xia and co-workers successfully prepared Au-CDs with extraordinary POD and glucose oxidase (GOD)-like properties.27 On the one hand, Au-CDs catalyzed glucose to form gluconic acid and H2O2. On the other hand, the produced H2O2 further oxidized TMB to form blue oxTMB in the presence of the Au-CDs. Since blue oxTMB exhibited a signal absorption peak at 652 nm (A652 nm), Au-CDs could be used for the direct detection of glucose and H2O2 by monitoring the absorbance variation.
Similarly, due to the excellent POD-like property of Fe3O4,32 Zhou et al. successfully synthesized a novel ternary magnetic nanocomposite hemin-Fe3O4@polypyrrole (h-Fe3O4@ppy) using one pot method to detect H2O2 and glutathione (GSH)33 (Figure 2A). In this paper, hemin as an active cofactor of many enzymes has been used to improve the catalytic activity of Fe3O4. The ppy modified on Fe3O4 also brings good stability and catalytic activity. Since h-Fe3O4@ppy could catalyze H2O2 and TMB to produce a color change, H2O2 was successfully detected by the changes of absorption signal with the detection limit (LOD) of 0.18 μM. Significantly, GSH was also detected by reducing yellow oxTMB to colorless TMB and the LOD of 0.15 μM was obtained in biological samples from acute coronary syndrome patients.
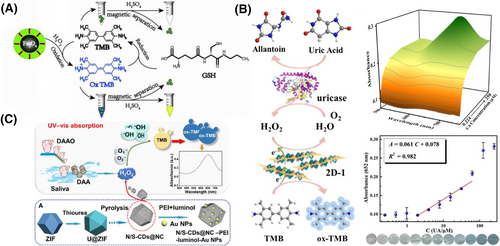
Nanozyme-based colorimetric assays for biomarker analysis. (A) h-Fe3O4@ppy based colorimetric platform for the detection of H2O2 and GSH. Reproduced with permission.33 Copyright 2021, Elsevier B.V. (B) 2D-1 and uricase-based colorimetric sensor for the detection of UA. Reproduced with permission.40 Copyright 2022, American Chemical Society. (C) N/S-CDs@NC and DAAO as catalysts for the detection of DAA by cascade catalytic reaction. Reproduced with permission.42 Copyright 2022, American Chemical Society.
As an important indicator for the diagnosis of kidney and liver, urea participates in many metabolic processes in the kidney and liver. While for p-aminophenol (p-Ap), a primary metabolite of paracetamol, leads to acute liver failure. To quantitatively detect urea and p-Ap, Yan and colleagues devised a single colorimetric biosensor for dual-component analysis based on the inherent OXD-like property of Rh stabilized 2D Co3O4 nanocomposite (2D Co3O4@Rh NC). 2D Co3O4@Rh NC could catalyze TMB to blue oxTMB with obvious absorption peak at 652 and 371 nm. However, the addition of p-Ap decreased the absorbance of 652 and 371 nm as the color changed from blue to colorless. This phenomenon might be attributed to the strong reducibility of -OH and -NH2 groups on the surface of p-Ap, which reduced blue oxTMB to form TMB. With the addition of urea, the absorption peak at 652 nm gradually decreased and the absorption peak at 436 nm rapidly increased as the color changed from blue to yellow. For the appearance of the absorption peak at 436 nm, Yan et al. considered that a possible imine product was generated during the reaction between the -NH2 in the blue oxTMB and the C = O in urea under the acidic condition. This nanozyme-based platform showed good selectivity with the LOD of 0.68 μM for p-Ap and 1.1 μM for urea, which could be used as a biosensor for the dual-detection of p-Ap and urea in urine samples.34
It is widely acknowledged that trimethylamine N-oxide (TMAO) is a uremic toxin associated with heart, kidney, and gut diseases.35 However, the common detection methods for TMAO, such as LC-MS or NMR, require complex processes, long detection time, and professional operators. Thus, a rapid and simple detection method is required, such as the TMB colorimetric method. Recently, Yang et al. developed a polyallylamine hydrochloride-modified manganese dioxide (PAH@MnO2) nanozyme as a POD-like catalyst,36 which facilitated the reaction of TMB in the presence of H2O2. Due to the depletion of HCl in the presence of TMAO, A650 nm increased while A450 nm decreased. The nanozyme-based detection method displayed a wide linear range from 15.6 to 500 μM, with a LOD of 6.7 μM. The method was further applied to detect TMAO in the plasma of chronic kidney disease rats and the results were consistent with those of LC-MS and colorimetric methods. In addition to the above clinical biomarkers, nanozymes based on direct colorimetric methods can be used to detect other molecules, such as heparin, protamine,37 protease,38 pyrophosphate,25 etc.
3.1.2 Indirect methods
For the indirect methods, the analytes could be detected by the assistance of enzyme or antibody, without the need of direct participation in the nanozyme-catalyzed reaction. Based on the enzyme-assisted method, the analytes can also be detected indirectly by detecting the produced H2O2 during catalysis reaction between analytes and the specific enzymes. For example, in the presence of glucose hydrolase, glucose can be transformed into gluconic acid and H2O2. Gong's group reported a novel histidine-functionalized graphene quantum dot (His-GQD)/hemin complex with POD-like activity to detect H2O2 and glucose.39 Histidine and hemin modification could mimic the active site microenvironment of the enzyme to improve the catalytic rate. The linear ranges for H2O2 and glucose detection were 5–240 μM and 2.5–200 μM, respectively. This nanocomplex would be used as a biosensor for the detection of H2O2 and glucose in blood samples. Uric acid (UA) can be transformed to allantoin and H2O2 with the assistance of uricase. Song et al. developed a pure polyoxometalate 2D materials (PP2DMs)-based colorimetric assay to indirectly detect UA40 (Figure 2B). PP2DMs served as POD mimics of oxidized TMB to oxTMB in the presence of H2O2. In the ultrathin 2D materials, Fe3+ centers and picolinic acid ligands donated or trapped electrons more easily in the reaction, so that PP2DMs presented excellent POD-mimicking catalytic activities. This platform showed superior sensitivity with a linear range of 1.953–62.5 μM and LOD of 295 nM, respectively. Thus, it was used to analyze the UA content in actual human serum samples. Similarly, D-amino acid (DAA) could be catalyzed to generate H2O2 under D-amino acid oxidase (DAAO). For example, the content of DAA in saliva was positively correlated with early-stage gastric cancer.41 Zeng et al. proposed an N-rich porous carbon doped carbon dots nanoplatform to detect D-proline (D-Pro) and D-alanine (D-Ala) in the human saliva samples. This nanoplatform performed the LOD of 0.14 μM for D-Pro and 0.35 μM for D-Ala, showing good sensitivity in the early diagnosis of gastric cancer42 (Figure 2C).
Based on the antibody-assisted method, protein biomarkers can be detected indirectly via ELISA.43 However, various nanozymes replaced natural enzymes, exhibiting high stability and sensitivity. For instance, carcinoembryonic antigen (CEA), as one of the significant cancer biomarkers, is associated with many cancers, such as colon and breast cancers.44 Lin et al. successfully synthesized a novel OXD-like nanozyme, Cu2+-BNNS-AuNPs. As an excellent OXD-like nanozyme, Cu2+-BNNS-AuNPs could catalyze chromogenic substrates, such as TMB and 3, 3'-dimethylbiphenyl-4, 4'-diamine (OT), to produce color changes without the presence of H2O2. Furthermore, based on the nano-ELISA, the capture antibody (Ab1) supported on a microplate combined with CEA could be captured by the detection antibody (Ab2) modified on the surface of Cu2+-BNNS-AuNPs. With this sandwich immunoassay, the concentration of CEA could be measured by monitoring the catalysis process between Cu2+-BNNS-AuNPs and TMB. This nanozyme immunoassay system exhibited a wide linear range from 1 to 16 ng mL−1 for CEA, which was potentially used for the detection of CEA in real samples.26
Similarly, the proximity hybridization technique (PHA) is a novel label-free method that uses single-stranded DNA (ssDNA) and antibody-assistance in the recognition of analytes. Specifically, Lin and colleagues synthesized MoS2 nanosheets (MoS2 NSs) with an excellent POD-like property to detect a significant ovarian cancer biomarker, human epididymis-specific protein 4 (HE4). MoS2 NSs catalyzed 2, 2'-azinobis (3-ethylbenzothiazoline) -6-sulfonic acid (ABTS) in the presence of H2O2 to form green oxABTS, with clear absorbance bands at 415 nm, 650 nm, 732 nm, and 820 nm. The principle of PHA method mainly relied on ssDNA (DNA3) modified microplate and the specific binding of a pair of ssDNA-antibody probes (DNA1, DNA2) to HE4, which induced a proximity hybridization between DNA3, DNA1-Ab1, and DNA2-Ab2. The strong interaction between unhybridized DNA3 and MoS2 NSs indirectly measured the concentration of HE4 by monitoring the absorbance changes of oxABTs. Compared with nano-ELISA, the method in combination of DNA signal amplification and dual-antibodies recognition techniques improved the sensitivity and specificity, with a broad linear range of 10-4−10 ng mL−1 and LOD of 3 × 10−5 ng mL−1, respectively. Therefore, this PHA nanoplatform was utilized to analyze HE4 in human serum samples.45 Colorimetric assays based on nanozymes without the need of expensive detection instruments have the advantages of visualisation, simple operation and fast response, which has attracted much attention from researchers. However, it needs to be further developed and pioneered due to its poor tolerance to interference and insufficient sensitivity.
3.2 Fluorescence assay
Based on the principle of fluorescence enhancement or quenching mediated by analytes,46 fluorescence assays with high sensitivity and specificity have been expected to become an effective platform for clinical biomarker detection. The fluorescence signal generation mainly depends on two ways: (i) fluorophores, such as o-phenylenediamine (OPD), amplex red (AR), and cyanine; (ii) fluorescent materials, such as silver nanoclusters (AgNCs), QDs, and MOFs. Therefore, the principle of nanozyme-based fluorescence assay was the catalytic interaction between nanozyme and fluorophores or fluorescent nanomaterials affected by analytes, leading to the changes of fluorescence signals. Currently, fluorescent detection probes based on nanozymes are mainly classified into single fluorescent probes and ratio fluorescent probes.47
3.2.1 Single fluorescent probe
The single fluorescent probe generated only a single fluorescent signal from either the fluorophore or fluorescent material. For instance, Huang et al. successfully developed OXD-like nanozymes in the form of MnOx/MnFe2O4 nanopopcorns (MFNPs) to detect fructose,48 a sugar associated with liver diseases and obesity.49 On the one hand, the obtained MFNPs could selectively catalyze fructose to produce H2O2. On the other hand, MFNPs could also catalyze AR to yield fluorescent resorufin, which generated obvious fluorescence intensity at 585 nm (IF585) under an excitation wavelength of 530 nm. It is demonstrated that H2O2 could oxidize MnOx causing the decomposition of MFNPs. As a result, H2O2 inhibited the catalytic interaction between MFNPs and AR, leading to a decrease in fluorescence signals with the addition of fructose. This phenomenon inspired the development of a nanozyme-based fluorescent biosensor for fructose detection with a LOD of 32 μM. Based on the above fluorescence response of AR, Han and co-workers also engineered nanozyme (AuNPs) to achieve the fluorescent detection of different kinds of amyloids for Alzheimer's disease diagnosis.50
In addition to fluorophores, the nanomaterials with fluorescence properties have attracted wide attention. For instance, Yao et al. synthesized near-infrared fluorescent AgNCs based on cupric oxide nanoparticles (CuO NPs) and methacrylic acid (MAA) for the detection of GSH.51 The prepared CuO NPs with POD-like and glutathione-like (GPx-like) activities could catalyze GSH to form ·OH. Then, polymethacrylic acid (PMAA) was formed by the interaction between ·OH and MAA. In the presence of GSH, -NH2 of GSH anchored on the surface of AgNCs connected to the -COOH of PMAA, forming Ag nanoparticles (AgNPs) with no fluorescent signals to quench the fluorescence signal. On the basis of this principle, a strong linear correlation between the fluorescence quenching rate and the GSH concentration was observed within the range of 0.01–40 μM, and a LOD of 8.0 nM was obtained. This nanoplatform could be used as a fluorescent biosensor for the detection of GSH in serum samples. In addition to the above-mentioned analytes, fluorescent materials based on nanozyme could be used for the detection of other clinical biomarkers, such as H2O2,52 glucose, lactate, phosphatase,53 tyrosinase,54 creatine,55 etc.
3.2.2 Ratio fluorescent probe
Noteworthy, the results of a single fluorescent probe can be disturbed by several factors such as the probe concentration, light scattering from the sample, and environmental fluctuations. To avoid these disturbances, the development of ratio fluorescent probes has been regarded as ideal candidates for clinical biomarker detection. Compared to single fluorescent probes, ratio fluorescence probes provide two or multi-emission wavelengths, which ensures the accuracy in analysis by incorporating self-calibration mechanisms to correct disturbances from the environment and sample. For instance, Lin et al. designed fluorescent switchable carbon dots (o-CDs) as fluorescent signal output and amino-functionalized dendritic mesoporous SiO2-coped gold nanoparticles (SiO2@AuNPs) as GOD-like nanozyme, respectively.56 Using the nano-ELISA method, the authors successfully detected CEA using o-CDs and SiO2@AuNPs (Figure 3A). The interaction between -NH2 of o-CDs and Fe2+ quenched the yellow fluorescence of o-CDs to green. When glucose was catalyzed by SiO2@AuNPs to generate H2O2, a redox reaction between H2O2 and Fe2+ was triggered, resulting in the dissolution of the o-CDs-Fe2+ system and recovery of yellow fluorescence of o-CDs. Hence, this ON-OFF-ON type of ratio fluorescent probe reduced interference between samples and environment, showing good sensitivity with a LOD of 74.5 pg mL−1 and a linear range of 0.1–80 ng mL−1. In addition to CEA, nanozyme-based proportional fluorescence sensors are expected to be used for the discovery and detection of other clinical biomarkers. Using the same nano-ELISA method, Li et al. synthesized POD-like Pd-Ir nanocubes as ratio fluorescent probe for the detection of cardiac troponin I (CTnI),57 a clinical biomarker for myocardial infarction. The method showed a good linear relationship between the concentration of CTnI and the ratio of fluorescence intensity at 570 and 450 nm within the range of 1 pg mL−1 to 1 ng mL−1, which was consistent with the results obtained from commercial chemiluminescence assay kits. In conclusion, the use of the nano-ELISA fluorescence detection platform held great potential for protein-based clinical biomarker diagnosis.
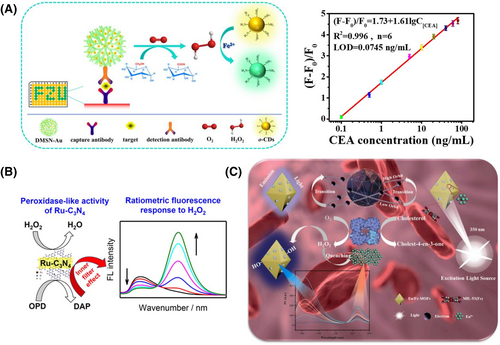
Nanozyme-based fluorescence sensors for clinical biomarker detection. (A) Switchable fluorescent CDs combined with Au@SiO2 for the ratio fluorescence detection of CEA. Reproduced with permission.56 Copyright 2020, American Chemical Society. (B) Ru-C3N4 based single fluorescent probe for the detection of H2O2. Reproduced with permission.58 Copyright 2019, American Chemical Society. (C) The detection mechanism of Eu/Fe-MOFs as ratio fluorescent probes for cholesterol detection. Reproduced with permission.59 Copyright 2021, Elsevier B.V.
Furthermore, the detection of H2O2 and glucose was also widely used in ratio fluorimetry. In 2019, Chen et al. constructed ruthenium ion-doped carbon nitride (Ru-C3N4) as a kind of fluorescent POD-like nanozyme (Figure 3B). In the presence of H2O2, the obtained Ru-C3N4 could catalyze OPD to produce DAP, which had a fluorescent band at 565 nm. Due to the inner filter effect, the inherent fluorescence was quenched at 455 nm. By using glucose oxidase, the glucose and H2O2 were detected by ratio fluorimetry in the serum samples with LODs of 0.1 μM and 50 nM, respectively. Moreover, cholesterol, as an important indicator of human health, can also be detected through ratio fluorescent probes.58 For instance, Yue and co-workers59 designed MIL-53(Fe) doped with europium (Eu/Fe-MOFs) as a kind of POD-like nanozyme (Figure 3C). Using cholesterol oxidase, cholesterol could be catalyzed to produce H2O2. On the one hand, the Eu/Fe-MOFs broke down the H2O2 to produce ·OH, which could be attached to the benzene ring of the TPA ligand on the Eu/Fe-MOFs. In the process, the electron density of the TPA ligand increased and enhanced its fluorescence intensity at 458 nm. On the other hand, due to the photocatalytic activity of europium, the H2O2 adsorbed on the surface of europium was decomposed by consuming phototropic electrons. The energy disturbance transferred to europium, resulting in its fluorescence quenched at 615 nm. On the basis of the above mechanisms, the ratio fluorescent probes had good sensitivity with an LOD of 10.5 μM, promising to be a nanozyme-based platform for cholesterol detection. Nanozyme-based fluorescence assays have the advantages of good sensitivity, high selectivity, simple operation and real-time detection. However, the high selectivity limits the versatility of the fluorescence assay and there is still a need to improve its sensitivity, stability, and accuracy by eliminating potential interferences from effects such as background fluorescence and quenching effects, which requires further development and exploitation by researchers.
3.3 Electrochemical assay
Currently, clinical biomarkers are low abundant in humans, making it difficult to develop sensitive analytical techniques. Electrochemical assay relies on the redox reaction occurring between the target analyte and an electrolyte on the surface of electrodes, which subsequently converts chemical signals to electrochemical signals. Furthermore, the current intensity is directly proportional to the concentration of the analyte. It is a sensitive, rapid, and simple method that has been utilized to detect many clinical biomarkers. The superiority of electrochemical assay primarily stems from the characteristics of the electrode materials.60 In recent years, various nanomaterials with catalytic activities, such as metals, MOFs, and QDs, have been extensively utilized. In this section, we will discuss the utilization of nanozyme-based electrochemical detection for the detection of clinical biomarkers. According to different techniques, electrochemical assay-based clinical biomarker analysis mainly includes direct sensors, antibody-based sensors, and aptamer-based electrochemical sensors.
3.3.1 Direct sensor
To date, nanozymes have been discovered as effective catalysts for electrochemical signal amplification. For instance, Li et al. designed a novel ZIF-67 coped Au@Pt nanoflower (MOF-Au@Pt) with POD-like properties to modify glassy carbon electrode (GCE) for the electrochemical detection of H2O2 (Figure 4A). The porous structure of MOF provided large specific surface area, allowing for loading more Au@Pt to increase the catalytic activity. Moreover, the bimetallic core-shell structure of Au@Pt increased the overall negative charge area of the catalyst, which facilitated the electrochemical catalysis of H2O2. On the basis of the above mechanism, this platform showed a wide linear range of 0.8 μM–3 mM with an LOD of 86 nM for the detection of H2O2.61 In addition to nanomaterials mentioned above, electrochemical sensors for H2O2 with the aid of nanozymes with POD-like activity have been extensively studied. For example, Huang and co-workers synthesized defect-rich cobalt hydroxide (D-Co(OH)2) with POD-like activity for the direct detection of H2O2 in Hela cells.62 Xiao and colleagues successfully constructed gold nanoparticles wrapped MnO2 nanowires (MnO2@Au) to modify graphene fiber (GF) as the electrode. The obtained dual nanozyme microelectrode could detect H2O2 in liver cancer cells.63 Another work by Xiao et al. synthesized AuPt alloy modified graphene QDs (AuPt-GQDs) with the above properties and were successfully applied to the electrochemical detection of H2O2 in breast cancer samples.64 Notably, in addition to the direct detection of H2O2, the indirect detection of different clinical biomarkers is also possible based on the principle that enzymes and analytes catalyze the production of H2O2. For instance, Song et al. synthesized Fe3O4 polymers by molecular imprinting technology for indirect electrochemical detection of choline with the aid of choline oxidase.65 Xu et al. self-assembled mesoporous carbon/silica coped gold nanoparticles (OMC@AuNPs) using the solvent evaporation method for indirect electrochemical detection of xanthine with the aid of xanthine oxidase.66
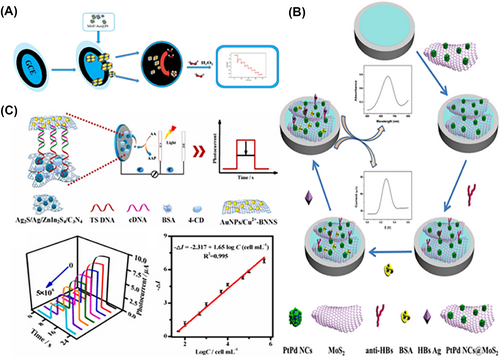
Nanozyme-based electrochemical sensors for biomarker assay. (A) MOF/Au@Pt as POD-like nanozyme-modified GCE for the electrochemical detection of H2O2. Reproduced with permission.61 Copyright 2021, Elsevier B.V. (B) Combining the POD-like property of PtPd NCs@MoS2 with antibody for the electrochemical detection of HBs Ag. Reproduced with permission.67 Copyright 2019, Elsevier B.V. (C) The POD-like property of AuNPs/Cu2+-BNNS with aptamer for the electrochemical detection of telomerase. Reproduced with permission.68 Copyright 2022, Elsevier B.V.
3.3.2 Antibody-based sensor
Furthermore, it is well known that antibody-based ELISA has good potential for the detection of protein-based clinical biomarkers. Dong and co-workers constructed PtPd nanocubes@MoS2 (PtPd@MoS2) as a POD mimic to detect Hepatitis B surface antigen (HBs Ag).67 PtPd@MoS2 catalyzed the production of OPD in the presence of H2O2, leading to the generation of electrochemical signals. By differential pulse voltammetry (DPV) and sandwich method, the concentration of HBsAg was detected with a wide linear range of 32 fg mL−1 to 100 ng mL−1 and a LOD of 10.2 fg mL−1 (Figure 4B). Similarly, Jia et al. designed PbS nanocrystals69 (PbS NCs), which had an excellent electrocatalytic activity to H2O2, to detect the Alpha fetoprotein (AFP) by the antibody-based ELISA. Due to the use of chitosan to immobilize nanozyme and the combination of anti-AFP, this antibody-based sensor showed good sensitivity and selectivity with a LOD of 3.0 fg mL−1 and a linear range of 1 × 10−5 to 800 ng mL−1. Recently, Gao and co-workers designed Ru nanozyme combined polystyrene sulfonate (PSS) ligand, which displayed a 2-fold higher catalytic activity than horseradish peroxidase (HRP) (1305 U mg-1), to detect AFP by the antibody-based ELISA similarly. It was worth noting that the sensitivity of this nanozyme-based platform was improved 140-fold higher than HRP.70 Thus, antibody-based ELISA could be widely introduced in the detection of clinical biomarkers.
3.3.3 Aptamer-based sensor
Although antibody-based methods have many advantages, they still suffer from difficulties in chemical modification and poor antibody stability. Recently, aptamer-based electrochemical detection has been widely studied. Feng et al. designed boron nitride nanosheet-coped gold nanoparticles and Cu2+ (Au/Cu2+@BNNs) as a POD mimic and used ZnIn2S4/C3N4 decorated Ag2S/Ag (Ag2S/Ag/ZnIn2S4/C3N4) as a microelectrode active material to detect telomerase (Figure 4C). Deoxyribonucleoside triphosphates (dNTP) were used to extend the telomerase primer sequence (TS) immobilized in Ag2S/Ag/ZnIn2S4/C3N4. Moreover, the sulfhydryl-modified complementary (cDNA) bounded to the dNTP was linked to the Au/Cu2+@BNNs through an Au-S bond, forming an aptamer-based telomerase electrochemical sensor. The linked nanozyme could catalyze the 4-chloro-1-naphthol (4-CN) to produce 4-CD (insoluble precipitates) in the presence of H2O2, resulting in the suppression of electrochemical signals.68 In addition to the above analytes, aptamer-based electrochemical assays are also available for other clinical biomarkers such as vascular endothelial growth factor 165 (VEGF165),71 and CTnI.72 Nanozyme-based electrochemical assays have the advantages of a wide linear range, high sensitivity, and the possibility of automation, which is widely used for quantitative and qualitative assays. However, it usually requires excellent analyte electroactivity, dedicated instrumentation, and specialised technicians, which greatly increases the cost of the assay. Moreover, the stability and reproducibility of electrochemical assays are poor due to the easy fouling of electrodes induced fluctuant signal, which requires further development and exploitation of novel electrochemical sensors.
3.4 POC assay
In recent years, clinical biomarker detection based on nanozymes has been widely studied in laboratories. Nowadays, there is an urgent need to develop rapid, convenient, and efficient technology for use in the field and emergency rooms.73 POC assays, such as lateral flow tests (FLA)74 and blood glucose meters,75 eliminate the need of pre-processing complex samples and allow the analysis of samples in the field.
For antibody-based FLA, Xiong et al. constructed Fe3O4@MIL-100(Fe)@Pt as a POD-like nanozyme for the detection of procalcitonin (PCT), which is one of the common inflammatory biomarkers.76 By utilizing magnetic separation in conjunction with nanozyme-catalyzed AEC to generate colorimetric signals, the T-line on double antibody sandwich immunoassay strips underwent a discernible color change. This approach significantly improved the detection sensitivity of PCT in serum samples, achieving an LOD approximately 2280-fold lower than that of conventional gold nanozyme-based FLA (Figure 5A). Similarly, the detection of prostate specific antigen (PSA) has also been reported by the same method. For aptamer-based FLA, Nara et al. successfully designed gold nanozymes to detect carbohydrate antigen 125 (CA125),79 which is a common ovarian cancer biomarker. This nanoplatform has been verified as a low cost and short detection time assay in serum samples. Using the photometer, Li et al. constructed a convenient ELISA-based nanozyme sensor for the rapid detection of SARS-CoV-2 and the results were transmitted to a smartphone in real time via Bluetooth11 (Figure 5B). Meanwhile, Pang and co-workers reported a novel ELISA immunosensor for the detection of urothelial carcinoma biomarkers78 (Figure 5C). Due to the whole reaction integrated in a vial, this nanozyme-based immunosensor was considered to be a convenient and simple POC assay. Similarly, in the framework of fluorescent signal-based POC assays, Omer et al. designed Cu-MOFs as a POD-like nanozyme combined with the thrombin-binding aptamer (ssDNA), to detect thrombin in the diagnosis of the COVID-19 pandemic.80 The ssDNA on the surface of Cu-MOF inhibited the catalytic and fluorescent activity of the nanozyme, while thrombin bound to ssDNA, resulting in the recovery of fluorescent signals. The coupling of aptamers and fluorescence detection platforms improved the sensitivity of detection methods. The low cost and ease of operation provided significant potential in POC assay applications. As for the electrochemical signal-based POC assays, Zhang et al. developed MOF-818 as a POD-like nanozyme for the on-site detection of H2S and H2O2,81 utilizing a smartphone and a miniature electrochemical analyzer. Specifically, the electrochemical analyzer detects H2O2 present in MCF-7 cells, visibly displaying the data on the smartphone. It is worth noting that the catalytic activity of MOF-818 would be impeded by the binding of H2S with Cu2+ on the MOF-818, thereby introducing a novel concept in the field of POC assays. Colorimetric assay, in comparison with fluorescence and electrochemical assays, stands out for its low cost, speed, and ease of use. While fluorescence and electrochemical assay exhibit greater sensitivity, fluorescence is limited in versatility due to its high selectivity and electrochemical assay poses complexities in operation and requires substantial instrument maintenance costs. Therefore, more efforts are required in the future for the POC assay. Taken together, although the POC assay has great advantages in real-time testing such as convenience, rapidity, etc.,82 great efforts, such as accuracy and quality control systems, are still needed for the practical applications.
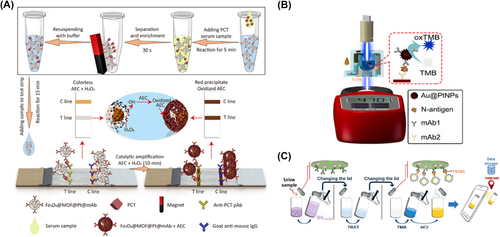
Nanozyme based POC testing of clinical biomarkers. (A) Colorimetric lateral flow immunoassay with Fe3O4@MOF@Pt as the nanozyme used in the POC assay of procalcitonin. Reproduced with permission.76 Copyright 2022, American Chemical Society. (B) Combining photometer with Au@PtNPs77 for the immunoassay of SARS-CoV-2 nucleocapsid protein. Reproduced with permission.11 Copyright 2021, Elsevier B.V. (C) Using a vial and a smartphone for the nanozyme-based detection of UC biomarkers. Reproduced with permission.78 Copyright 2020, American Chemical Society.
4 CONCLUSION AND PERSPECTIVE
The study of nanozymes has been a hot topic since the discovery of nanomaterials with POD activity in 2007. Nanozymes are now being successfully applied in the environmental, medical, and biological fields due to their high stability, low cost, and good catalytic properties in comparison with natural enzymes. At present, nanomaterials with catalytic activity have been designed and constructed as nanozymes to detect clinical biomarkers combined with advanced techniques, such as colorimetric, fluorescent, and electrochemical methods. Thus, this review summaried various clinical biomarker biosensors based on nanozymes and analytical methods, including colorimetric, fluorescent, electrochemical, and POC assays. Considering that suitable recognition molecules and intermolecular forces are crucial for high sensitivity and specificity, signal amplification strategies based on antibodies, nucleic acid aptamers, and various enzymes have been used to enhance the response signal of the biosensors. These nanozyme-based biosensors enable the rapid and simple clinical biomarker analysis with high sensitivity and wide linear range. However, it is challenging for nanozyme-based biosensors to further apply in real-time detection. Therefore, in future research and development, we need to focus on (1) biosensor miniaturization and integration of portable devices for POC detection, such as smartphones; (2) design of nanozymes with multiple properties, such as POD and OXD, and application in clinical biomarker detection; and (3) construction of recognition molecule-modified nanozymes specific to clinical biomarkers. Taken together, nanozyme-based clinical biomarker assays present promising applications in clinical disease diagnosis (Table 1).
| Methods | Nanozyme | Biomarkers | LOD (unit) | Ref. |
|---|---|---|---|---|
| Colorimetric assay | h-Fe3O4@ppy | H2O2 | 0.18 (μM) | 33 |
| 2D Co3O4@Rh | Urea, p-Ap | 1.1, 0.68 (μM) | 34 | |
| Prussian blue | Glycated albumin | 7.32 (mg mL−1) | 83 | |
| PP2DMs | UA | 295 (nM) | 40 | |
| Fluorescence assay | Ln-MOFs | Cholesterol | 10.5 (μM) | 59 |
| Pd-Ir nanocubes | CTnI | 0.31 (pg mL−1) | 57 | |
| MnO2 | Sarcosine | 0.36 (μM) | 55 | |
| MFNPs | Fructose | 32 (μM) | 48 | |
| Electrochemical assay | PtPd@MoS2 | HBs Ag | 10.2 (fg mL−1) | 67 |
| PbS | AFP | 3.0 (fg mL−1) | 69 | |
| PdPt@MoO2 | VEGF165 | 8.2 (pg mL−1) | 84 | |
| Au/OMCS | Xanthine | 6 (nM) | 66 |
ACKNOWLEDGMENTS
The authors gratefully acknowledge financial support from the Project 2022YFC2502800 by National Key R&D Program of China, Medical-Engineering Joint Funds of Shanghai Jiao Tong University (YG2021ZD09, YG2022QN107, YG2023QNA12), Projects 81971771, 22204103 by NSFC, and Project 2021-01-07-00-02-E00083 by Shanghai Institutions of Higher Learning. This work was also sponsored by Innovation Group Project of Shanghai Municipal Health Commission (2019CXJQ03), Innovation Research Plan by the Shanghai Municipal Education Commission (ZXWF082101), and National Research Center for Translational Medicine Shanghai (TMSK-2021-124, NRCTM(SH)-2021-06).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Biographies

Dingyitai Liang received his B.S. degree from the School of Biotechnology, Jiangnan University. He is currently studying for a master degree in the School of Biomedical Engineering, Shanghai Jiao Tong University, under the supervision of Prof. Kun Qian. His current research is focused on the development of novel nanodevices for in vitro diagnostics.

Yuning Wang received her Ph.D. in analytical chemistry from Fudan University. Currently, she is an assistant professor at the School of Biomedical Engineering, Shanghai Jiao Tong University. Her research interests focus on the development of novel signal amplification methods based on mass spectrometry for biomolecule detection.

Kun Qian received his B.S./M.S. from Fudan University, his Ph.D. from University of Queensland, and held the position of research fellow at Stanford University. He is now a principal investigator (PI) and distinguished professor at the School of Biomedical Engineering and School of Medicine, Shanghai Jiao Tong University. His research interests and efforts are in the design, synthesis, engineering, and advanced biomedical applications of materials and devices. His group aims at the development and commercialization of bioanalytical methods and mass spectrometry techniques for diverse biomolecules in biomedical fields.




