An integrated structural perspective on morphogenesis of crustacean epidermis and gut by complementary microscopy
Abstract
To understand developmental processes it is essential to consider both, molecular / genetic data and structural aspects of morphogenesis and differentiation. Integration of both approaches is beneficial to interpret the data obtained and unravel tissue and organism formation. Sequential morphological changes during animal development can be followed by different microscopic methods that enable us to combine imaging of intact embryos, elucidation of the tissue and cell architecture at different size scales, localization of tissue constituents in their original location and correlative imaging, supplemented by analytical microscopy at the specific stages of development.
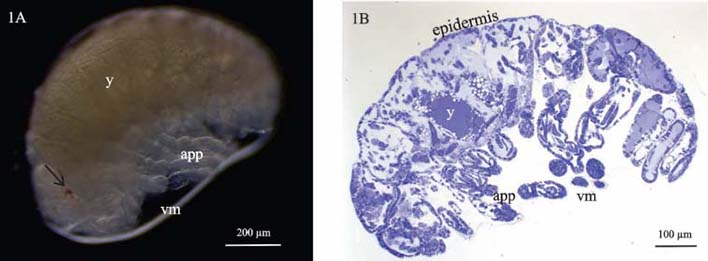
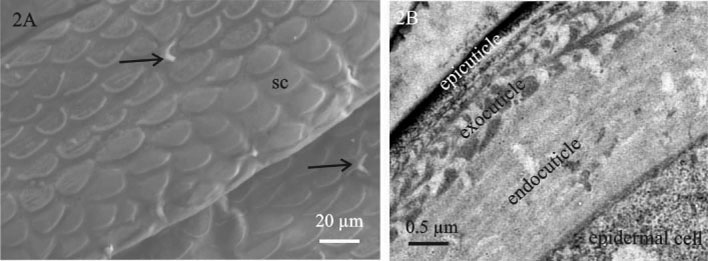
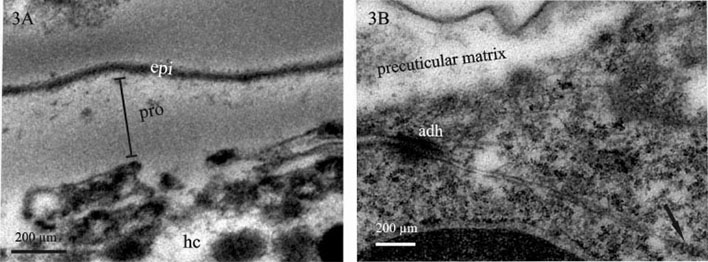
Here we present a combined use of light microscopy, histology, transmission (TEM) and scanning electron microscopy (SEM), labelling with specific ligands and analytical microscopy to unravel the differentiation of epidermis and gut epithelium in a crustacean model. Our study addresses the interrelation of tissue morphogenesis, epithelial cell differentiation at the ultrastructural level and differentiation of the corresponding apical extracellular matrix, the cuticle, examined in the embryos and marsupial larvae of Porcellio scaber. Cuticle is a chitin-based matrix, secreted apically by ectodermal epithelia during development and renewal in arthropods. We integrate structural information at different scale levels with data on matrix elemental and molecular composition in the selected developmental stages. Developmental stages and gross morphological changes in epidermal and gut tissues were identified by imaging the intact embryos and larvae (Fig. 1A), fluorescence labelling of nuclei and imaging of histological sections (Fig. 1B). Architecture of tissues, cells and matrix was characterized at the levels of light and electron microscopy. To determine the organic scaffold composition of the matrix, we performed localization of macromolecules containing N-acetyl-glucosamine (chitin) by labelling with lectin wheat germ agglutinin. The epidermal cuticle in crustaceans is in addition mineralized, forming a stiff exoskeleton. Analytical microscopy was applied to examine calcification of the forming cuticular matrix in marsupial larvae, performed by energy dispersive X-ray spectroscopy (EDS) to analyse elemental composition and Raman spectroscopy to determine mineral forms in the cuticle. The results show that several apical matrices are produced sequentially during epidermis and gut morphogenesis and that matrix renewal is coupled with major morphological modifications such as growth of appendages, elongation or bending of embryo body and hatching from egg envelopes. The early stages of apical matrix formation are similar in the epidermis and gut. Later in marsupial larval stages, specialization of both cuticles is evident, implying the establishment of their specific functions. Exoskeletal cuticle with elaborate surface structures (Fig. 2A) differentiates in several layers displaying chitin-protein fibres patterns (Fig. 2B) and is already prominently calcified. Hindgut cuticle displays differentiation in a homogenous electron lucent procuticle and prominent electron dense epicuticle (Fig. 3A).In concert with the stages of apical matrix formation, a gradual formation of the subapical cell junctions, one of the key factors that determines cell polarity, was evidenced (Fig. 3B). We consider our model system as valuable system to study tissue and cell differentiation in their native environment, with applying the integrated structure-function perspective.
Figures