First Synthesis of Optically Pure Propargylic N-Hydroxylamines by Direct, Highly Diastereoselective Addition of Terminal Alkynes to Nitrones†
We thank the ETH, Roche Research Foundation, the Swiss National Science Foundation, Merck, and Aventis for their generous support.
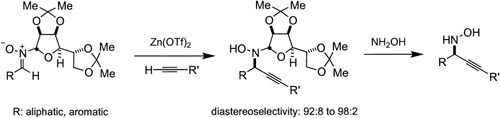
Graphical Abstract
In situ generation of nucleophilic alkynilides by using substoichiometric amounts of Zn(OTf)2 (OTf = trifluoromethanesulfonate) followed by removal of the chiral auxiliary enables the direct use of a wide range of terminal acetylenes in the synthesis of optically pure propargylic N-hydroxylamines (see scheme).
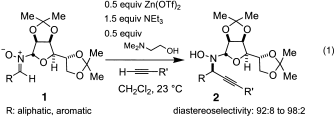 (1)
(1)There are scant, scattered reports that document diastereoselective additions to chiral nitrones. These typically involve additions of Grignard or organolithium compounds to chiral starting materials, and are limited in scope.4b, 5–8 Moreover, because they are generally single-substrate studies, the generality of these additions as a means to provide broad access to chiral N-hydroxylamines is unclear.
We recently reported a method for the catalytic in situ generation of ZnII–alkynilides and their addition to achiral nitrones.9 Accordingly, we were interested in extending the methodology to include an asymmetric version. Preliminary screening of auxiliaries for use in diastereoselective nitrone additions led us to single out carbohydrate-based structures, such as 3, derived from mannose, acetone, and N-hydroxylamine.
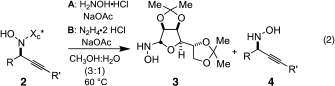 (2)
(2)The requisite nitrones for study (1) were conveniently prepared by treating a solution of glycosyl N-hydroxylamine 3 and aldehyde in CH2Cl2 with a desiccant such as Na2SO4 or MgSO4. As shown in Table 1
|
Entry |
R |
R′ |
Yield [%] |
dr[b] |
|---|---|---|---|---|
|
1 |
Me |
Ph |
88 |
95:5 |
|
2 |
Ph(CH2)2 |
45 |
96:4[c] |
|
|
3 |
99 |
95:5[d] |
||
|
4 |
Me3Si |
75 |
92:8 |
|
|
5 |
n-C4H9 |
80 |
97:3[e] |
|
|
6 |
iPr |
Ph |
89 |
92:8 |
|
7 |
Ph(CH2)2 |
87 |
97:3 |
|
|
8 |
98 |
96:4[d] |
||
|
9 |
94 |
96:4 |
||
|
10 |
c-C6H11 |
Ph |
91 |
94:6 |
|
11 |
Ph(CH2)2 |
91 |
95:5 |
|
|
12 |
94 |
93:7[d] |
||
|
13 |
n-C4H9 |
82 |
98:2 |
|
|
14 |
c-C3H5 |
Ph |
92 |
94:6 |
|
15 |
Ph(CH2)2 |
73 |
96:4 |
|
|
16 |
54 |
96:4 |
||
|
17 |
tBu |
Ph |
91 |
97:3 |
|
18 |
Ph |
Ph |
82 |
95:5[f,g] |
|
19 |
Me3Si |
88 |
95:5[g] |
|
|
20 |
83 |
95:5[d,g] |
||
|
21 |
Ph |
79 |
95:5 |
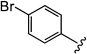
- [a] Addition reactions were conducted as described in the text. For additional details, see the Supporting Information. [b] The diastereomeric ratio of the adducts was assayed by 1H NMR spectroscopy. [c] The corresponding isoxazoline, generated by cyclization of the adduct, was isolated as the main product in 48 % yield. [d] 2-Methyl-3-butyne-2-ol was utilized as the solvent, and gave much higher yields than the standard reaction protocols (entry 16). [e] Besides the product (80 %), 12 % of the corresponding isoxazoline was isolated. [f] In the course of the reaction, the adduct entirely cyclized to the corresponding isoxazoline which was then isolated. [g] The reaction was conducted using Zn(OTf)2 (1.1 equiv), NEt3 (1.5 equiv), and 2-dimethylaminoethanol (1.1 equiv).
, the additions can be carried out on a wide variety of branched, unbranched, aromatic, and C-substituted nitrones as well as with an equally broad range of terminal alkynes. Thus, treatment of a solution of a terminal alkyne and a nitrone 1 with 0.5 equivalents of Zn(OTf)2, 0.5 equivalents of 2-dimethylaminoethanol, and 1.5 equivalents of NEt3 in CH2Cl2 (0.33 M) at 23 °C affords adducts 2 in up to 99 % yield and excellent diastereomeric ratios ranging from 92:8 to 98:2.10, 11 In the course of optimization studies, we observed that the use of 2-dimethylaminoethanol was beneficial, because not only did its use lead to enhanced reaction rates (fivefold), it also furnished homogenous solutions throughout the course of the reaction.
A convenient aspect of the process is the fact that removal of the auxiliary is effected upon heating a MeOH:H2O (3:1) solution of adducts 2 with 1.6 equivalents of H2NOH⋅HCl and 1.5 equivalents of NaOAc at 50–60 °C (Table 2
|
Entry |
R |
R′ |
t [h] |
Method |
Yield [%] |
|---|---|---|---|---|---|
|
1 |
Me |
Ph |
4.5 |
B |
99 |
|
2 |
Me3Si |
3 |
A |
86 |
|
|
3 |
iPr |
Ph |
1.5 |
A |
96 |
|
4 |
4.5 |
A |
95 |
||
|
5 |
Ph |
Me3Si |
2 |
A |
85 |
|
6 |
Ph |
3.5 |
B |
73[b] |
|
|
7 |
Me |
1 |
A |
54[b] |
- [a] For reaction conditions, see text. [b] The moderate yield in entries 6 and 7 is caused by the high polarity and low stability of the product. The work-up and isolation procedures are currently being optimized.
, method A).5c In certain cases (entries 1 and 6) more forcing conditions were necessary to effect removal of the auxiliary, wherein N2H4⋅2 HCl was substituted for H2NOH⋅HCl (Table 2, method B). Optically active propargylic N-hydroxylamines 4 can be isolated in useful yields by using either method.
In summary, we report a novel, general, practical method for the highly stereoselective synthesis of optically active secondary propargylic N-hydroxylamines. A key salient feature of this process is the fact that a wide range of terminal acetylenes and nitrones participate in additions under conditions where the nucleophilic alkynilides are generated in situ by utilizing substoichiometric amounts of Zn(OTf)2; as such, the terminal alkynes were used directly without requiring a separate deprotonation or activation step. Given the fact that acetylenes are readily and conveniently converted into numerous other functional groups, the method provides access to a large range of N-hydroxylamines in optically active form for the first time. Such compounds are of increasing importance in medicinal chemistry, where the corresponding hydroxamic acids, for example, have been shown to possess potent broad-spectrum activities against matrix metalloproteases and tumor necrosis factor α (TNF-α) converting enzymes.12
Dedicated to Professor Andrea Vasella



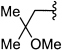

41:16<>1.0.co;2-7.cover.gif)
