Total Synthesis of the Natural Enantiomer of (−)-Lepadiformine and Determination of Its Absolute Stereochemistry†
This work was supported in part by a Grant for Private Universities provided by the Ministry of Education, Sports, and Culture of Japan and the Promotion and Mutual Aid Corporation for Private Schools of Japan. We are grateful to Professor J. F. Biard for a sample of natural lepadiformine.
Graphical Abstract
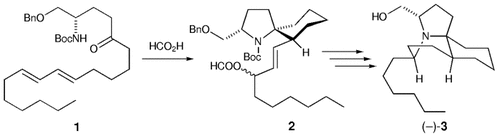
A short synthesis: The naturally occurring (−)-lepadiformine ((−)-3) was prepared in nine steps in 31.4 % overall yield. The key step involved the formation of 2 by the spirocyclization of the N-acyliminium ion generated from 1. Furthermore, HPLC analysis of the synthetic material and the natural product established the absolute configuration of 3 as 3S,5R,7aS,11aS. Bn=benzyl, Boc=tert-butoxycarbonyl.



41:16<>1.0.co;2-7.cover.gif)
