Rhenium(VII) Oxo Complexes as Extremely Active Catalysts in the Dehydration of Primary Amides and Aldoximes to Nitriles
Kazuaki Ishihara Prof. Dr.
Graduate School of Engineering, Nagoya University SORST, Japan and Science Technology Corporation (JST) Furo-cho, Chikusa, Nagoya 464-8603 (Japan) Fax: (+81) 52-789-3222
Search for more papers by this authorYoshiro Furuya
Graduate School of Engineering, Nagoya University SORST, Japan and Science Technology Corporation (JST) Furo-cho, Chikusa, Nagoya 464-8603 (Japan) Fax: (+81) 52-789-3222
Search for more papers by this authorHisashi Yamamoto Prof. Dr.
Graduate School of Engineering, Nagoya University SORST, Japan and Science Technology Corporation (JST) Furo-cho, Chikusa, Nagoya 464-8603 (Japan) Fax: (+81) 52-789-3222
Search for more papers by this authorKazuaki Ishihara Prof. Dr.
Graduate School of Engineering, Nagoya University SORST, Japan and Science Technology Corporation (JST) Furo-cho, Chikusa, Nagoya 464-8603 (Japan) Fax: (+81) 52-789-3222
Search for more papers by this authorYoshiro Furuya
Graduate School of Engineering, Nagoya University SORST, Japan and Science Technology Corporation (JST) Furo-cho, Chikusa, Nagoya 464-8603 (Japan) Fax: (+81) 52-789-3222
Search for more papers by this authorHisashi Yamamoto Prof. Dr.
Graduate School of Engineering, Nagoya University SORST, Japan and Science Technology Corporation (JST) Furo-cho, Chikusa, Nagoya 464-8603 (Japan) Fax: (+81) 52-789-3222
Search for more papers by this authorGraphical Abstract
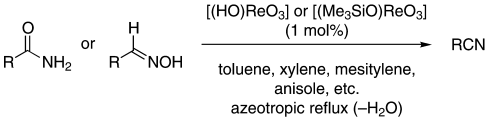
An economical and environmentally benign process for the preparation of nitriles by the dehydration of primary amides and aldoximes is catalyzed by rhenium(VII) oxo complexes such as perrhenic acid and trimethylsilylperrhenate (see scheme). The reaction proceeds at azeotropic reflux (with the removal of water) under essentially neutral conditions.
References
- 1
- 1aR. E. Kent, S. M. McElvain, Org. Synth. 1945, 25, 61;
- 1bD. T. Mowry, Chem. Rev. 1948, 42, 189.
- 2
- 2aS. Patai in The Chemistry of Functional Groups: Amides (Ed.: ), Wiley, New York, 1970;
- 2bR. C. Larock, Comprehensive Orgaanic Transformations, VCH Publishers, New York, 1989.
- 3
- 3aY. Watanabe, F. Okuda, Y. Tsuji, J. Mol. Catal. 1990, 58, 87;
- 3bM.-P. Heck, A. Wagner, C. Mioskowski, J. Org. Chem. 1996, 61, 6486;
- 3cD. S. Bose, B. Jayalakshmi, J. Org. Chem. 1999, 64, 1713;
- 3dD. S. Bose, B. Jayalakshmi, Synthesis 1999, 1724; for other dehydration methods of primary amides, see references in refs. [3b] and [3c].
- 4
- 4aK. Ishihara, S. Ohara, H. Yamamoto, J. Org. Chem. 1996, 61, 4196;
- 4bK. Ishiahra, S. Ohara, H. Yamamoto, Macromolecules 2000, 33, 3511.
- 5
- 5aK. Ishihara, S. Ohara, H. Yamamoto, Science 2000, 290, 1140;
- 5bK. Ishihara, M. Nakayama, S. Ohara, H. Yamamoto, Synlett 2001, 1117;
- 5cK. Ishihara, M. Nakayama, S. Ohara, H. Yamamoto, Tetrahedron 2002, in press..
- 6
- 6aK. Ishihara, H. Kurihara, H. Yamamoto, Synlett 1997, 597;
- 6bS. Saito, T. Nagahara, H. Yamamoto, Synlett 2001, 1690.
- 7For isomerization of allylic alcohols or allylic ethers catalyzed by [(R3SiO)ReO3], see:
- 7aS. Bellemin-Laponnaz, H. Gisie, J. P. Le Ny, J. A. Osborn, Angew. Chem. 1997, 109, 1011;
10.1002/ange.19971090915 Google ScholarAngew. Chem. Int. Ed. Engl. 1997, 36, 976;
- 7bS. Bellemin-Laponnaz, J. P. Le Ny, J. A. Osborn, Tetrahedron Lett. 2000, 41, 1549.
- 8For a Beckmann rearrangement catalyzed by [Bu4NReO4] and trifluoromethanesulfonic acid, see:
- 8aK. Narasaka, H. Kusama, Y. Yamasita, H. Sato, Chem. Lett. 1993, 489;
- 8bH. Kusama, Y. Yamashita, K. Narasaka, Bull. Chem. Soc. Jpn. 1995, 68, 373.
- 9Trimethylsilylperrhenate was purchased from Gelest-Azmax; perrhenic acid and rhenium(VII) oxide were purchased from Aldrich.
- 10The catalysts, aqueous [(HO)ReO3] (colorless), and [(Me3SiO)ReO3] (white), were often recovered as a dark solid by partial reduction to lower valent rhenium oxides such as [ReO3⋅x H2O](black), [ReO2] (brown), [ReO3] (red), or [Re2O5] (blue). However, the recovered catalyst was still active for the dehydration. Lower valent rhenium oxides could be oxidized to perrhenic acid (colorless) or its anhydride (yellow) by treatment with hydrogen peroxide (35 %) at 50 °C for 12 h and removal of excess hydrogen peroxide and water under vacuum at ambient temperature; for another oxidation method, see: A. D. Melaven, J. N. Fowle, W. Brickell, C. F. Hiskey, Inorg. Synth. 1950, 3, 188.
- 11
- 11aS. H. Yang, S. Chang, Org. Lett. 2001, 3, 4209;
- 11bD. C. Barman, A. J. Thakur, D. Prajapati, J. S. Sandhu, Chem. Lett. 2000, 1196;
- 11cX.-S. Fei, J. G. Verkade, Heteroat. Chem. 1999, 10, 541;
- 11dN. Iranpoor, B. Zeynizadeh, Synth. Commun. 1999, 29, 2747;
- 11eB. P. Bandgar, V. S. Sadavarte, K. R. Sabu, Synth. Commun. 1999, 29, 3409;
- 11fG. Sabitha, M. Syamala, Synth. Commun. 1998, 28, 4577;
- 11gJ. Hart-Davis, P. Battioni, J.-L. Boucher, D. Mansuy, J. Am. Chem. Soc. 1998, 120, 12 524;
- 11hO. Attanasi, P. Palma, F. Serra-Zanetti, Synthesis 1983, 741;
- 11iH. M. Meshram, Synthesis 1992, 943.
- 12E. W. Bousquet, Org. Synth. Coll. Vol. 1947, 2, 313.
- 13For the oxophilicity of rhenium(VII) oxo complexes, see:
- 13aS. Rezgui, R. Jentoft, B. C. Gates, J. Catal. 1996, 163, 496;
- 13bA. S. Fung, M. J. Kelley, D. C. Koningsberger, B. C. Gates, J. Am. Chem. Soc. 1997, 119, 5877.
- 14For the coordination of N,N-dimethylformamide to rhenium complexes through its oxygen atom, see:
- 14aV. H.-A. Lehamann, C. Ringel, Z. Anorg. Allg. Chem. 1969, 366, 73;
10.1002/zaac.19693660108 Google Scholar
- 14bT. Nichikson, Zubieta, J. Polyhedron 1988, 7, 171.
- 15
- 15aC. R. Hauser, D. S. Hoffenberg, J. Org. Chem. 1955, 20, 1491;
- 15bD. S. Hoffenberg, C. R. Hauser, J. Org. Chem. 1955, 20, 1496;
- 15cG. Zvilichovsky, L. Heller, Synthesis 1972, 563;
- 15dH. Sharghi, M. H. Sarvari, Synlett 2001, 99.
- 16S. L. Scott, J.-M. Basset, J. Am. Chem. Soc. 1994, 116, 12 069.



41:16<>1.0.co;2-7.cover.gif)
