An Efficient and Highly Enantio- and Diastereoselective Cyclopropanation of Olefins Catalyzed by Schiff-Base Ruthenium(II) Complexes†
Jason A. Miller
Department of Chemistry Northwestern University 2145 Sheridan Road, Evanston, IL 60208-3113 (USA) Fax: (+1) 847-467-5123
Search for more papers by this authorWiechang Jin Dr.
Department of Chemistry Northwestern University 2145 Sheridan Road, Evanston, IL 60208-3113 (USA) Fax: (+1) 847-467-5123
Search for more papers by this authorSonBinh T. Nguyen Prof. Dr.
Department of Chemistry Northwestern University 2145 Sheridan Road, Evanston, IL 60208-3113 (USA) Fax: (+1) 847-467-5123
Search for more papers by this authorJason A. Miller
Department of Chemistry Northwestern University 2145 Sheridan Road, Evanston, IL 60208-3113 (USA) Fax: (+1) 847-467-5123
Search for more papers by this authorWiechang Jin Dr.
Department of Chemistry Northwestern University 2145 Sheridan Road, Evanston, IL 60208-3113 (USA) Fax: (+1) 847-467-5123
Search for more papers by this authorSonBinh T. Nguyen Prof. Dr.
Department of Chemistry Northwestern University 2145 Sheridan Road, Evanston, IL 60208-3113 (USA) Fax: (+1) 847-467-5123
Search for more papers by this authorWe thank the reviewers for their helpful comments. Support from the DuPont Company and the Beckman, Dreyfus, and Packard Foundations are gratefully acknowledged. S.T.N. is an Alfred P. Sloan Fellow.
Graphical Abstract
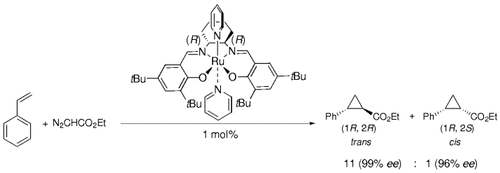
Both electron-rich and electron-deficient olefins—such as styrene and methyl methacrylate—undergo efficient (yields >90 %) cyclopropanation with ethyl diazoacetate as the carbene source to give predominantly trans products with exceptionally high enantioselectivity when the (salen)Ru catalyst shown is used (see scheme).
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2002/2002/z18536_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1M. P. Doyle, M. A. McKervey, T. Ye, Modern Catalytic Methods for Organic Synthesis with Diazo Compounds, Wiley, New York, 1998.
- 2M. P. Doyle, D. C. Forbes, Chem. Rev. 1998, 98, 911–935.
- 3J. Salaün, Chem. Rev. 1989, 89, 1247–1270.
- 4H. Nozaki, S. Moriuti, H. Takaya, R. Noyori, Tetrahedron Lett. 1966, 7, 5239–5244.
- 5T. Aratani, Y. Yoneyoshi, T. Nagase, Tetrahedron Lett. 1975, 1707–1710.
- 6A. Nakamura, A. Konishi, R. Tsujitani, M.-A. Kudo, S. Otsuka, J. Am. Chem. Soc. 1978, 100, 3449–3461.
- 7R. Noyori, Asymmetric Catalysis, Wiley, New York, 1994.
- 8H.-U. Reissig, Angew. Chem. 1996, 108, 1049–1051;
10.1002/ange.19961080907 Google ScholarAngew. Chem. Int. Ed. Engl. 1996, 35, 971–973.
- 9R. Schumacher, F. Dammast, H.-U. Reissig, Chem. Eur. J. 1997, 3, 614–619.
- 10T. Aratani, Pure Appl. Chem. 1985, 57, 1839–1844.
- 11A. Pfaltz, Acc. Chem. Res. 1993, 26, 339–345.
- 12R. E. Lowenthal, S. Masamune, Tetrahedron Lett. 1991, 32, 7373–7376.
- 13M. M.-C. Lo, G. C. Fu, J. Am. Chem. Soc. 1998, 120, 10 270–10 271.
- 14D. A. Evans, K. A. Woerpel, M. M. Hinman, M. M. Faul, J. Am. Chem. Soc. 1991, 113, 726–728.
- 15D. Müller, G. Umbricht, B. Weber, A. Pfaltz, Helv. Chim. Acta. 1991, 74, 232–240.
- 16M. P. Doyle, V. Bagheri, T. J. Wandless, N. K. Harn, D. B. Brinker, C. T. Eagle, K.-L. Loh, J. Am. Chem. Soc. 1990, 112, 1906–1912.
- 17M. P. Doyle, B. D. Brandes, A. P. Kazala, R. J. Pieters, Tetrahedron Lett. 1990, 31, 6613–6616.
- 18J. L. Maxwell, S. O'Malley, K. C. Brown, T. Kodadek, Organometallics 1992, 11, 645–652.
- 19C.-M. Che, J.-S. Huang, F.-W. Lee, Y. Li, T.-S. Lai, H.-L. Kwong, P.-F. Teng, W.-S. Lee, W.-C. Lo, S.-M. Peng, Z.-Y. Zhou, J. Am. Chem. Soc. 2001, 123, 4119–4129.
- 20E. Galardon, S. Roue, P. Le Maux, G. Simonneaux, Tetrahedron Lett. 1998, 39, 2333–2334.
- 21M. Frauenkron, A. Berkessel, Tetrahedron Lett. 1997, 38, 7175–7176.
- 22H. Nishiyama, Y. Itoh, H. Matsumoto, S.-B. Park, K. Itoh, J. Am. Chem. Soc. 1994, 116, 2223–2224.
- 23H. Nishiyama, Y. Itoh, Y. Sugawara, H. Matsumoto, K. Aoki, K. Itoh, Bull. Chem. Soc. Jpn. 1995, 68, 1247–1262.
- 24H. Nishiyama, S.-B. Park, K. Itoh, Chem. Lett. 1995, 599–600.
- 25S.-B. Park, K. Murata, H. Matsumoto, H. Nishiyama, Tetrahedron: Asymmetry 1995, 6, 2487–2494.
- 26T. Uchida, R. Irie, T. Katsuki, Tetrahedron 2000, 56, 3501–3509.
- 27I. J. Munslow, K. M. Gillespie, R. J. Deeth, P. Scott, Chem. Commun. 2001, 1638–1639.
- 28W. Tang, X. Hu, X. Zhang, Tetrahedron Lett. 2002, 43, 3075–3078.
- 29S. T. Nguyen, W. Jin, Abst. Pap. 218th ACS National Meeting (New Orleans, LA), 1999, INOR-104.
- 30S. T. Nguyen, W. Jin, USA Patent Appl. 2000, 22 pp.
- 31E. J. Hennessy, W. J. Marshall, M. A. Scialdone, S. T. Nguyen, unpublished data.
- 32T. Fukuda, T. Katsuki, Synlett 1995, 825–826.
- 33E. N. Jacobsen, W. Zhang, M. L. Guler, J. Am. Chen. Soc. 1991, 113, 6703–6704.
- 34Z. Zheng, X. Yao, M. Qiu, W. Lu, H. Chen, Tetrahedron: Asymmetry 2001, 12, 197–204.
- 35M. P. Doyle, R. L. Dorow, W. H. Tamblyn, J. Org. Chem. 1982, 47, 4059–4068.
- 36M. P. Doyle, M. R. Colsman, R. L. Dorow, J. Heterocycl. Chem. 1983, 20, 943–946.
- 37A. Nakamura, A. Konishi, Y. Tatsuno, S. Otsuka, J. Am. Chem. Soc. 1978, 100, 3443–3448.
- 38J. A. Miller, S. T. Nguyen, unpublished data.
- 39T. Uchida, R. Irie, T. Katsuki, Synlett 1999, 1793–1795.
- 40B. H. Hoff, T. Anthonsen, Chirality 1999, 11, 760–767.
10.1002/(SICI)1520-636X(1999)11:10<760::AID-CHIR4>3.0.CO;2-P CAS PubMed Web of Science® Google Scholar



41:16<>1.0.co;2-7.cover.gif)
