Chirality and Macroscopic Polar Order in a Ferroelectric Smectic Liquid-Crystalline Phase Formed by Achiral Polyphilic Bent-Core Molecules†
Gert Dantlgraber
Institute of Organic Chemistry Martin-Luther-University Halle-Wittenberg Kurt-Mothes-Strasse 2, 06120 Halle (Germany) Fax: (+49) 345-55-27223
Search for more papers by this authorAlexei Eremin
Institute of Physical Chemistry Martin-Luther-University Halle-Wittenberg
Search for more papers by this authorSiegmar Diele Dr.
Institute of Physical Chemistry Martin-Luther-University Halle-Wittenberg
Search for more papers by this authorAnton Hauser Dr.
Institute of Physical Chemistry Martin-Luther-University Halle-Wittenberg
Search for more papers by this authorHorst Kresse Prof. Dr.
Institute of Physical Chemistry Martin-Luther-University Halle-Wittenberg
Search for more papers by this authorGerhard Pelzl Prof. Dr.
Institute of Physical Chemistry Martin-Luther-University Halle-Wittenberg
Search for more papers by this authorCarsten Tschierske Prof. Dr.
Institute of Organic Chemistry Martin-Luther-University Halle-Wittenberg Kurt-Mothes-Strasse 2, 06120 Halle (Germany) Fax: (+49) 345-55-27223
Search for more papers by this authorGert Dantlgraber
Institute of Organic Chemistry Martin-Luther-University Halle-Wittenberg Kurt-Mothes-Strasse 2, 06120 Halle (Germany) Fax: (+49) 345-55-27223
Search for more papers by this authorAlexei Eremin
Institute of Physical Chemistry Martin-Luther-University Halle-Wittenberg
Search for more papers by this authorSiegmar Diele Dr.
Institute of Physical Chemistry Martin-Luther-University Halle-Wittenberg
Search for more papers by this authorAnton Hauser Dr.
Institute of Physical Chemistry Martin-Luther-University Halle-Wittenberg
Search for more papers by this authorHorst Kresse Prof. Dr.
Institute of Physical Chemistry Martin-Luther-University Halle-Wittenberg
Search for more papers by this authorGerhard Pelzl Prof. Dr.
Institute of Physical Chemistry Martin-Luther-University Halle-Wittenberg
Search for more papers by this authorCarsten Tschierske Prof. Dr.
Institute of Organic Chemistry Martin-Luther-University Halle-Wittenberg Kurt-Mothes-Strasse 2, 06120 Halle (Germany) Fax: (+49) 345-55-27223
Search for more papers by this authorThis work was supported by the Deutsche Forschungsgemeinschaft and the Fonds der Chemischen Industrie.
Graphical Abstract
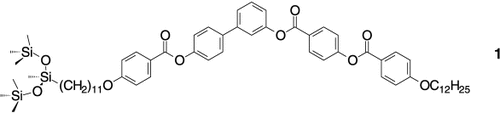
Nicht chiral und doch ferroelektrisch: Bei konventionellen fluiden Systemen heben sich die Dipolmomente der einzelnen Moleküle gegenseitig auf, und eine makroskopisch apolare Struktur entsteht. Hier wird gezeigt, wie sich durch gezieltes Moleküldesign und Nutzung von Mikrosegregationseffekten die Selbstorganisation von Molekülen wie 1 so steuern lässt, dass eine fluide Schichtstruktur mit makroskopisch polarer Ordnung entstehen kann.
References
- 1 M. C. Petty, M. R. Bryce, D. Bloor, Introduction to Molecular Electronics, Edward Arnold, London, 1995.
- 2
- 2a
B. I. Ostrovskii Structure and Bonding, 1999, 94, 200;
10.1007/3-540-68305-4_5 Google Scholar
- 2b T. Goldacker, V. Abetz, R. Stadler, I. Erukhimovich, L. Leibler, Nature 1999, 398, 137.
- 3
H.-S. Kitzerow, C. Bahr, Chirality in Liquid Crystals, Springer, Berlin, 2001.
10.1007/b97374 Google Scholar
- 4 S. T. Lagerwall, Ferroelectric and Antiferroelectric Liquid Crystals, Wiley-VCH, Weinheim, 1999.
- 5 T. Niori, F. Sekine, J. Watanabe, T. Furuwara, H. Takezoe, J. Mater. Chem. 1996, 6, 1231.
- 6 A related effect was observed in mixtures of side-chain polymers and low molecular-weight mesogens: Soto Bustamante, S. V. Yablonskii, B. I. Ostrovskii, L. A. Beresnev, L. M. Blinov, W. Haase, Liq. Cryst. 1996, 21, 829.
- 7 D. R. Link, G. Natale, R. Shao, J. E. Maclennan, N. A. Körblova, D. M. Walba, Science 1997, 278, 1924.
- 8 An orthogonal phase is reported in ref. [6] and was also reported for a rigid bent-core molecule: A. Eremin, S. Diele, G. Pelzl, H. Nadasi, W. Weissflog, J. Salfetnikova, H. Kresse, Phys. Rev. E 2001, 64, 51 707.
- 9
- 9a E. Gorecka, D. Pociecha, F. Araoka, D. R. Link, M. Nakata, J. Thisayukta, Y. Takanishi, J. Watanabe, H. Takezoe, Phys. Rev. E 2000, 62, R4524;
- 9b M. Nakata, D. R. Link, J. Thisayukta, Y. Takanishi, K. Ishikawa, J. Watanabe, H. Takezoe, J. Mater. Chem. 2001, 11, 2694;
- 9c D. R. Link, F. Araoka, J. Thisayukta, Y. Takanishi, K. Ishikawa, J. Watanabe, H. Takezoe, Liq. Cryst. 2001, 28, 1301.
- 10 D. M. Walba, E. Körblova, R. Shao, J. E. Maclennan, D. R. Link, M. A. Glaser, N. A. Clark, Science 2000, 288, 2181.
- 11 H. Nadasi, W. Weissflog, A. Eremin, G. Pelzl, S. Diele, B. Das, S. Grande, J. Mater. Chem. 2002, 12, 1316.
- 12 C. Tschierske J. Mater. Chem. 2001, 11, 2646.
- 13 D. Shen, A. Pegenau, S. Diele, I. Wirth, C. Tschierske, J. Am. Chem. Soc. 2000, 122, 1593.
- 14 Siloxane units were used for several calamitic polyphilic mesogens, for example,
- 14a M. Ibn-Elhaj, H. Möhwald, M. Z. Cherkaoui, R. Zniber, Langmuir 1998, 14, 504;
- 14b P. Sebastiao, S. Mery, M. Sieffert, J. F. Nicoud, Y. Galerne, D. Guillon, Ferroelectrics 1998, 212, 133;
- 14c H. J. Coles, S. Meyer, P. Lehmann, R. Deschenaux, I. Jauslin, J. Mater. Chem. 1999, 9, 1085;
- 14d G. H. Mehl, J. W. Goodby, Chem. Ber. 1996, 129, 521.
- 15
- 15a N. Miyaura, T. Yanagi, A. Suzuki, Synth. Commun. 1981, 11, 513;
- 15b N. Miyaura, A. Suzuki, Chem. Rev. 1995, 95, 2457;
- 15c M. Hird, G. W. Gray, K. J. Toyne, Mol. Cryst. Liq. Cryst. 1991, 206, 187.
- 16 C. Tschierske, H. Zaschke, J. Prakt. Chem. 1989, 331, 365.
- 17 N. W. Adams, J. S. Bradshaw, J. M. Bayona, K. E. Markides, M. L. Lee, Mol. Cryst. Liq. Cryst. 1987, 147, 43.
- 18 All analytical data are in accordance with the proposed structure, for example, compound 4: 1H NMR (400 MHz, CDCl3) δ=8.30 (d, J=8.8 Hz, 2 H, Ar-H), 8.15 (d, J=8.8 Hz, 4 H, Ar-H), 7.64 (d, J=8.6 Hz, 2 H, Ar-H), 7.49 (d, J=5.3 Hz, 2 H, Ar-H), 7.46–7.45 (m, 1 H, Ar-H), 7.38 (d, J=8.6 Hz, 2 H, Ar-H), 7.29 (d, J=8.6 Hz, 2 H, Ar-H), 7.23–7.20 (m, 1 H, Ar-H), 7.00 (d, J=9.0 Hz, 2 H, Ar-H), 6.97 (d, J=9.0 Hz, 2 H, Ar-H), 4.04 (t, J=6.6 Hz, 4 H, OCH2), 1.82 (m, 4 H, CH2), 1.45 (m, 4 H, CH2), 1.36–1.28 (m, 30 H, CH2), 0.89 (t, J=6.6 Hz, 3 H, CH3), 0.46 (t, J=7.4 Hz, 2 H, Si-CH2), 0.10 (s, 18 H, Si-CH3), 0.06 ppm (s, 3 H, Si-CH3); 13C NMR (100 MHz, CDCl3) δ=164.78, 164.34, 164.18, 163.75, 163.51, 155.46, 151.28, 150.81, 142.08, 137.66, 132.34, 132.23, 131.74, 129.75, 128.95, 128.15, 126.82, 124.58, 122.11, 122.04, 121.44, 120.94, 120.48, 120.35, 114.27, 68.39, 68.32, 33.21, 31.92, 29.65, 29.63, 29.62, 29.57, 29.37, 29.36, 29.12, 29.09, 26.00, 25.98, 23.08, 22.69, 17,65, 14.12, 1.88, −0.23 ppm; elemental analysis calcd (%) for C63H88O10Si3: C 69.44 %, H 8.14; found C 69.33 %, H 8.12.
- 19
- 19a G. Heppke, D. D. Parghi, H. Sawade, Liq. Cryst. 2000, 27, 313;
- 19b J. Thisayukta, H. Takezoe, J. Watanabe, Jpn. J. Appl. Phys. 2001, 40, 3277.
- 20
- 20a J. Thisayukta, Y. Nakayama, S. Kawauchi, H. Takezoe, J. Watanabe, J. Am. Chem. Soc., 2000, 122, 7441;
- 20b J. Thisayukta, H. Niwano, H. Takezoe, J. Watanabe, J. Mater. Chem. 2001, 11, 2717.
- 21 Determined by the three angular wave method as described in ref. [4].
- 22 Two distinct enantiomeric configurations, which differ in the tilt-direction and the direction of the polar axes of the molecules in the smectic layers (+/+ and −/−), are possible for the SmCSPF state.[7] They occur in different regions within the sample, in which the extinction crosses rotate either clockwise or anticlockwise.
- 23 Quite similarly, decreasing the temperature reduces the out-of-layer fluctuations and could lead to a transition from an AF to FE order as recently shown for a B5-phase.[11]
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.



114:13<>1.0.co;2-j.cover.gif)
