The Chemistry of the Oxychlorination Catalyst: an In Situ, Time-Resolved XANES Study†
Carlo Lamberti Dr.
Dipartimento di Chimica IFM Via P. Giuria 7, 10125 Torino (Italy) and INSTM Research unit of Turin University Fax: (+39) 011-670-7855
Search for more papers by this authorCarmelo Prestipino
Dipartimento di Chimica IFM Via P. Giuria 7, 10125 Torino (Italy) and INSTM Research unit of Turin University Fax: (+39) 011-670-7855
Search for more papers by this authorFrancesca Bonino
Dipartimento di Chimica IFM Via P. Giuria 7, 10125 Torino (Italy) and INSTM Research unit of Turin University Fax: (+39) 011-670-7855
Search for more papers by this authorLuciana Capello
Dipartimento di Chimica IFM Via P. Giuria 7, 10125 Torino (Italy) and INSTM Research unit of Turin University Fax: (+39) 011-670-7855
Search for more papers by this authorSilvia Bordiga Prof. Dr.
Dipartimento di Chimica IFM Via P. Giuria 7, 10125 Torino (Italy) and INSTM Research unit of Turin University Fax: (+39) 011-670-7855
Search for more papers by this authorGiuseppe Spoto Prof. Dr.
Dipartimento di Chimica IFM Via P. Giuria 7, 10125 Torino (Italy) and INSTM Research unit of Turin University Fax: (+39) 011-670-7855
Search for more papers by this authorAdriano Zecchina Prof. Dr.
Dipartimento di Chimica IFM Via P. Giuria 7, 10125 Torino (Italy) and INSTM Research unit of Turin University Fax: (+39) 011-670-7855
Search for more papers by this authorBarbara Cremaschi
EVC Technological Centre Via della Chimica 5, 30175 Porto Marghera/Venezia (Italy)
Search for more papers by this authorMarco Garilli
EVC Technological Centre Via della Chimica 5, 30175 Porto Marghera/Venezia (Italy)
Search for more papers by this authorAndrea Marsella
EVC Technological Centre Via della Chimica 5, 30175 Porto Marghera/Venezia (Italy)
Search for more papers by this authorDiego Carmello
EVC Technological Centre Via della Chimica 5, 30175 Porto Marghera/Venezia (Italy)
Search for more papers by this authorSandro Vidotto
EVC Technological Centre Via della Chimica 5, 30175 Porto Marghera/Venezia (Italy)
Search for more papers by this authorGiuseppe Leofanti
EVC Technological Centre Via della Chimica 5, 30175 Porto Marghera/Venezia (Italy)
Search for more papers by this authorCarlo Lamberti Dr.
Dipartimento di Chimica IFM Via P. Giuria 7, 10125 Torino (Italy) and INSTM Research unit of Turin University Fax: (+39) 011-670-7855
Search for more papers by this authorCarmelo Prestipino
Dipartimento di Chimica IFM Via P. Giuria 7, 10125 Torino (Italy) and INSTM Research unit of Turin University Fax: (+39) 011-670-7855
Search for more papers by this authorFrancesca Bonino
Dipartimento di Chimica IFM Via P. Giuria 7, 10125 Torino (Italy) and INSTM Research unit of Turin University Fax: (+39) 011-670-7855
Search for more papers by this authorLuciana Capello
Dipartimento di Chimica IFM Via P. Giuria 7, 10125 Torino (Italy) and INSTM Research unit of Turin University Fax: (+39) 011-670-7855
Search for more papers by this authorSilvia Bordiga Prof. Dr.
Dipartimento di Chimica IFM Via P. Giuria 7, 10125 Torino (Italy) and INSTM Research unit of Turin University Fax: (+39) 011-670-7855
Search for more papers by this authorGiuseppe Spoto Prof. Dr.
Dipartimento di Chimica IFM Via P. Giuria 7, 10125 Torino (Italy) and INSTM Research unit of Turin University Fax: (+39) 011-670-7855
Search for more papers by this authorAdriano Zecchina Prof. Dr.
Dipartimento di Chimica IFM Via P. Giuria 7, 10125 Torino (Italy) and INSTM Research unit of Turin University Fax: (+39) 011-670-7855
Search for more papers by this authorBarbara Cremaschi
EVC Technological Centre Via della Chimica 5, 30175 Porto Marghera/Venezia (Italy)
Search for more papers by this authorMarco Garilli
EVC Technological Centre Via della Chimica 5, 30175 Porto Marghera/Venezia (Italy)
Search for more papers by this authorAndrea Marsella
EVC Technological Centre Via della Chimica 5, 30175 Porto Marghera/Venezia (Italy)
Search for more papers by this authorDiego Carmello
EVC Technological Centre Via della Chimica 5, 30175 Porto Marghera/Venezia (Italy)
Search for more papers by this authorSandro Vidotto
EVC Technological Centre Via della Chimica 5, 30175 Porto Marghera/Venezia (Italy)
Search for more papers by this authorGiuseppe Leofanti
EVC Technological Centre Via della Chimica 5, 30175 Porto Marghera/Venezia (Italy)
Search for more papers by this authorL.C. was supported by an INFM grant for her stay at the ESRF. We are indebted to R. Weigel for his fundamental support during data acquisition and to M. Sanchez Del Rio for his important support on the complex data handling.
Graphical Abstract
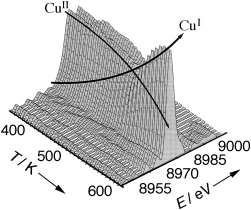
Der geschwindigkeitsbestimmende Schritt der Ethylen-Oxychlorierung am CuCl2/γ-Al2O3-Katalysator ließ sich in einer zeitaufgelösten In-situ-XANES-Studie identifizieren. Nach diesen Daten (siehe Bild; E=Photonenenergie) und der ebenfalls ermittelten Katalysatoraktivität ist es die Oxidation von CuCl. Das den industriellen Katalysatoren als Dotierung zugesetzte Kaliumchlorid verringert die Geschwindigkeit der Reduktion, die beim KCl/CuCl2/γ-Al2O3-Katalysator zum geschwindigkeitsbestimmenden Schritt wird.
Supporting Information
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author. Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2001/2002/z18372_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1a
J. S. Naworski, E. S. Evil, Applied Industrial Catalysis, Vol. 1 ( ), Academic Press, New York, 1983, p. 239;
10.1016/B978-0-12-440201-0.50014-X Google Scholar
- 1b M. N. Newmann in Encyclopedia of Polymer Science and Engineering, Vol. 17, Wiley, New York, 1985, p. 245, and references therein.
- 2 W. D. Mross, Catal. Rev. Sci. Eng. 1983, 25, 591–637.
- 3 A. Arcoya, A. Cortes, X. L. Seoane, Can. J. Chem. Eng. 1982, 60, 55–60.
- 4
- 4a A. Baiker, W. L. Holstein, J. Catal. 1983, 84, 178–188;
- 4b K. Rollins, P. A. Sermon, J. Chem. Soc. Chem. Commun. 1986, 1171–1172;
- 4c E. M. Fortini, C. L. Garcia, D. E. Resasco, J. Catal. 1986, 99, 12–18;
- 4d P. A. Sermon, K. Rollins, P. N. Reyes, S. A. Lawrence, M. A. Martin Luengo, M. J. Davies, J. Chem. Soc. Faraday Trans. 1 1987, 83, 1347–1353;
- 4e P. S. Sai Prasad, P. Kanta Rao, J. Chem. Soc. Chem. Commun. 1987, 951–952;
- 4f C. L. Garcia, D. E. Resasco, J. Catal. 1990, 122, 151–165.
- 5 G. Leofanti, M. Padovan, M. Garilli, D. Carmello, A. Zecchina, G. Spoto, S. Bordiga, G. Turnes Palomino, C. Lamberti, J. Catal. 2000, 189, 91–104, and references therein.
- 6 G. Leofanti, M. Padovan, M. Garilli, D. Carmello, G. L. Marra, A. Zecchina, G. Spoto, S. Bordiga, C. Lamberti, J. Catal. 2000, 189, 105–116.
- 7 G. Leofanti, A. Marsella, B. Cremaschi, M. Garilli, A. Zecchina, G. Spoto, S. Bordiga, P. Fisicaro, G. Berlier, C. Prestipino, G. Casali, C. Lamberti, J. Catal. 2001, 202, 279–295.
- 8 G. Leofanti, A. Marsella, B. Cremaschi, M. Garilli, A. Zecchina, G. Spoto, S. Bordiga, P. Fisicaro, C. Prestipino, F. Villain, C. Lamberti, J. Catal. 2002, 205, 375–381.
- 9 Although the exact position of the X-ray photoelectron edge of an element in a material is also defined by the coordinating ligands and the local symmetry around the absorbing atom the main factor is the oxidation state of this atom.
- 10
- 10a L. S. Kau, D. J. Spira-Solomon, J. E. Penner-Hahn, K. O. Hodgson, E. I. Solomon, J. Am. Chem. Soc. 1987, 109, 6433–6442;
- 10b N. J. Blackburn, R. W. Strange, J. Reedijk, A. Volbeda, A. Farooq, A. Karlin, J. Zubieta, Inorg. Chem. 1989, 28, 1349–1357.
- 11a C. Lamberti, G. Spoto, D. Scarano, C. Pazé, M. Salvalaggio, S. Bordiga, A. Zecchina, G. Turnes Palomino, F. D'Acapito, Chem. Phys. Lett. 1997, 269, 500–508;
- 11b G. Turnes Palomino, P. Fisicaro, S. Bordiga, A. Zecchina, E. Giamello, C. Lamberti, J. Phys. Chem. B 2000, 104, 4064–4073;
- 11c V. Bolis, S. Maggiorini, L. Meda, F. D'Acapito, G. Turnes Palomino, S. Bordiga, C. Lamberti, J. Chem. Phys. 2000, 113, 9248–9261.
- 12
- 12a M. Fernández-García, I. Rodríguez-Ramos, P. Ferreira-Aparicio, A. Guerrero-Ruiz, J. Catal. 1998, 178, 253–263;
- 12b P. Kappen, J.-D. Grunwaldt, B. S. Hammershøi, L. Tröger, B. S. Clausen, J. Catal. 2001, 198, 56–65.
- 13
C. Lamberti, G. Turnes Palomino, S. Bordiga, G. Berlier, F. D'Acapito, A. Zecchina, Angew. Chem. 2000, 112, 2222–2225;
10.1002/1521-3757(20000616)112:12<2222::AID-ANGE2222>3.0.CO;2-2 Google ScholarAngew. Chem. Int. Ed. 2000, 39, 2138–2141.10.1002/1521-3773(20000616)39:12<2138::AID-ANIE2138>3.0.CO;2-9 CAS PubMed Web of Science® Google Scholar
- 14 XANES spectroscopy is one of the most informative techniques for determining oxidation states and local symmetries of transition metal ions [see for example A. Bianconi in X-Ray Absorption ( ), Wiley, New York, 1988, p. 573]. This is particularly true when model compounds with well-defined oxidation and coordination states are available for comparison. In this regard, the reader should refer to refs. [7, 8]. It has been shown that the spectra of the as-activated catalyst and of the catalyst after interaction with O2 [that is, at the end of the cycle described in Equations (2)–(4)] are very close to that of the CuCl2 model compound. In addition, the XANES spectra of the catalyst after interaction with C2H4 under static conditions are in fair agreement with that of CuCl, except for a less pronounced 1s→4p transition. This difference has been explained in terms of the high dispersion of the CuCl particles on the reduced catalyst, showing 50 % of surface CuI as determined in a combined IR/CO chemisorption study.[7]
- 15 In a parallel experiment, in which only HCl was dosed to the oxychloride phase, it was demonstrated that the chlorination of the oxychloride occurs already at 373 K. This implies that reaction (4) is immediate at the temperature of interest, thus it is not responsible for the presence of CuII.
- 16 The ability of potassium and copper to form the phase KCuCl3 is well known, see for example: N. N. Greenwood, A. Earnshaw, Chemistry of the Elements, Pergamon, Oxford, 1986, p. 1385.
- 17 A. Zecchina, D. Scarano, S. Bordiga, G. Spoto, C. Lamberti, Adv. Catal. 2001, 46, 265–397, and references therein.
- 18 D. Scarano, P. Galletto, C. Lamberti, R. De Franceschi, A. Zecchina, Surf. Sci. 1997, 387, 236–242.
- 19
- 19a http://www.esrf.fr/exp_facilities/ID24/ID24.html;
- 19b M. Hagelstein, A. San Miguel, T. Ressler, A. Fontaine, J. Goulon, J. Phys. IV 1997, 7, C2–303–308.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.



114:13<>1.0.co;2-j.cover.gif)
