Proton-Induced, Reversible Evolution of O2 from the OsIV–Sulfoximido Complex [OsIV(tpy)(Cl)2{NS(O)-3,5-Me2C6H3}]†
My Hang V. Huynh Dr.
Director-Funded Postdoctoral Fellow Los Alamos National Laboratory Chemistry Division MS J514 Los Alamos, NM 87545 (USA) Fax: (+1) 505-667-3314
Search for more papers by this authorDavid E. Morris Dr.
Actinide, Catalysis, and Separations Group Chemistry Division MS J514 and the Glenn T. Seaborg Institute for Transactinium Science Los Alamos National Laboratory Los Alamos, NM 87545 (USA)
Search for more papers by this authorPeter S. White Dr.
Department of Chemistry University of North Carolina at Chapel Hill Chapel Hill, NC 27599-3290 (USA)
Search for more papers by this authorThomas J. Meyer Dr.
Associate Director for Strategic Research Los Alamos National Laboratory, MS A127 Los Alamos, NM 87545 (USA) Fax: (+1) 505-667-5450
Search for more papers by this authorMy Hang V. Huynh Dr.
Director-Funded Postdoctoral Fellow Los Alamos National Laboratory Chemistry Division MS J514 Los Alamos, NM 87545 (USA) Fax: (+1) 505-667-3314
Search for more papers by this authorDavid E. Morris Dr.
Actinide, Catalysis, and Separations Group Chemistry Division MS J514 and the Glenn T. Seaborg Institute for Transactinium Science Los Alamos National Laboratory Los Alamos, NM 87545 (USA)
Search for more papers by this authorPeter S. White Dr.
Department of Chemistry University of North Carolina at Chapel Hill Chapel Hill, NC 27599-3290 (USA)
Search for more papers by this authorThomas J. Meyer Dr.
Associate Director for Strategic Research Los Alamos National Laboratory, MS A127 Los Alamos, NM 87545 (USA) Fax: (+1) 505-667-5450
Search for more papers by this authorWe are grateful to the Laboratory Directed Research and Development Program for support of this research. M.H.V.H. gratefully acknowledges postdoctoral fellowship support from the Director's Office of Los Alamos National Laboratory. Los Alamos National Laboratory is operated by the University of California for the U.S. Department of Energy under Contract W-7405-ENG-36. tpy=2,2′:6′,2″-terpyridine.
Graphical Abstract
Freiheit dem Sauerstoff! In einem neuen Beispiel für die O2-Freisetzung/-Aktivierung an einem Liganden, der in diesem Fall durch die Os-N-Mehrfachbindung elektronisch aktiviert ist, wird 2 durch Protonierung reversibel in 1+ überführt. Beide Reaktionen sind nicht nur bemerkenswert, sondern auch bemerkenswert schnell.
References
- 1
- 1a H. Nohl, A. V. Kozlov, K. Staniek, L. Gille, Bioorg. Chem. 2001, 29, 1–13;
- 1b J. P. Klinman, J. Biol. Inorg. Chem. 2001, 6, 1–13;
- 1c S. Shiva, P. S. Brookes, R. P. Patel, P. G. Anderson, V. M. Darley-Usmar, Proc. Natl. Acad. Sci. USA 2001, 98, 7212–7217;
- 1d A. E. Martell, R. J. Motekaitis, R. Menif, D. A. Rockcliffe, A. Llobet, J. Mol. Catal. A-Chem. 1997, 117, 205–213;
- 1e D. E. Edmondson, B. H. Huynh, Inorg. Chim. Acta 1996, 252, 399–404;
- 1f D. H. Lee, N. Wei, N. N. Murthy, Z. Tyeklar, K. D. Karlin, S. Kaderli, B. Jung, A. D. Zuberbuhler, J. Am. Chem. Soc. 1995, 117, 12 498–12 513;
- 1g H. Niknahad, S. Khan, P. Obrien, Chem.-Biol. Interact. 1995, 98, 27–44.
- 2
- 2a I. Yamanaka, K. Otsuka, J. Chem. Soc. Faraday Trans. 1993, 89, 1791–1797;
- 2b I. Yamanaka, K. Otsuka, J. Alloys Compd. 1993, 193, 56–58;
- 2c K. Asano, T. Hibino, H. Iwahara, J. Electrochem. Soc. 1995, 142, 3241–3245;
- 2d H. M. Saffarian, R. Srinivasan, D. Chu, S. Gilman, J. Electrochem. Soc. 2001, 148, A559–A564;
- 2e S. Z. Wang, Y. Jiang, W. Z. Li, J. W. Yan, Studies in Surf. Sci. and Catal. 1997, 112, 401–410.
- 3
- 3a C. Tommos, G. T. Babcock, Acc. Chem. Res. 1998, 31(1), 18–25;
- 3b V. K. Yachandra, K. Sauer, M. P. Klein, Chem. Rev. 1996, 96, 2927–2950;
- 3c K. Lindberg, L. E. Andreasson, Biochem. 1996, 35(45), 14 259–14 267;
- 3d P. E. M. Siegbahn, R. H. Crabtree, J. Am. Chem. Soc. 1999, 121, 117–127.
- 4
- 4a R. A. Binstead, C. W. Chronister, J. F. Ni, C. M. Hartshorn, T. J. Meyer, J. Am. Chem. Soc. 2000, 122, 8464–8473;
- 4b A. S. Arico, A. K. Shukla, H. Kim, S. Park, M. Min, V. Antonucci, Appl. Surf. Sci. 2001, 172, 33–40;
- 4c R. Z. Jiang, D. J. Chu, J. Electrochem. Soc. 2000, 147, 4605–4609;
- 4d M. Odgaard, E. Skou, Solid State Ionics 1996, 86, 1217–1222;
- 4e S. Y. Ye, A. K. Vijh, L. H. Dao, J. Electroanal. Chem. 1996, 415, 115–121.
- 5 M. H. V. Huynh, P. S. White, T. J. Meyer, J. Am. Chem. Soc. 2001, 123, 9170–9171.
- 6 1+-PF6:
- 6a elemental analysis calcd (%) for OsC23H21N4SCl2PF6: C 34.90, H 2.67, N 7.08; found: C 35.03, H 2.61, N 7.18;
- 6b cyclic voltammetry in 0.1 M Bu4NPF6/CH3CN (V vs sodium saturated calomel electrode (SSCE)): E1/2 (OsV/IV)=+1.21 V and E1/2 (OsIV/III)=−0.09 V;
- 6c UV/Vis spectra in CH3CN λmax [nm] (ε, M−1 cm−1): 460 (9.47×103), 314 (1.99×104), 272 (2.37×104), 228 (3.16×104);
- 6d
IR (Nujol mull):
 =ν(SH) 1994, ν(3,5-Me2C6H3HS) 1601 (vs), and 1558 (s) ν(tpy) 1469 (vs), 1449 (vs), and 1390 cm−1 (vs); ν(14NS) 1023 and ν(15NS) 991 cm−1;
=ν(SH) 1994, ν(3,5-Me2C6H3HS) 1601 (vs), and 1558 (s) ν(tpy) 1469 (vs), 1449 (vs), and 1390 cm−1 (vs); ν(14NS) 1023 and ν(15NS) 991 cm−1;
- 6e 1H NMR data (δ=DMSO): 9.0–6.9 (m, 14 aromatic protons (11 H of tpy and 3 H of the aryl group)) 2.3 (s, 6H, methyl protons), 3.4 ppm (s, 1H, proton on the S atom);
- 6f CCDC-177717 (1+) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Centre, 12, Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223-336-033; or [email protected]).
- 7 2:
- 7a Elemental analysis calcd (%) for OsC23H20N4SOCl2: C 41.76, H 3.05, N 8.47; found: C 42.07, H 3.08, N 8.19;
- 7b cyclic voltammetry in 0.1 M Bu4NPF6/CH3CN (V vs SSCE): E1/2 (OsVI/V)=1.23 V, E1/2 (OsV/IV)=0.30 V, E1/2 (OsIV/III)=−0.89 V, and E1/2 (OsIII/II)=−1.19 V;
- 7c UV/Vis spectra in CH3CN λmax [nm] (ε, M−1 cm−1): 696 (3.17×103), 592 (2.79×103), 444 (9.16×103), 320 (1.32×104), 272 (2.07×104), 212 (2.73×104);
- 7d
IR (Nujol mull):
 =ν(3,5-Me2C6H3HS) 1603 (vs) and 1578 (s), ν(tpy) 1477 (vs), 1449 (vs), and 1435 (vs), and ν(SO) 1277 cm−1;
=ν(3,5-Me2C6H3HS) 1603 (vs) and 1578 (s), ν(tpy) 1477 (vs), 1449 (vs), and 1435 (vs), and ν(SO) 1277 cm−1;
- 7e 1H NMR (DMSO) δ=8.95–8.91 (d, 6 and 6″-positions of tpy), 8.64–8.61 (d, 3 and 3″-positions of tpy), 7.83–7.81 (d, 3′ and 5′-positions of tpy), 7.48–7.42 (t, 5 and 5″-positions of tpy), 7.44–7.41 (q, 2 and 6-positions of benzene ring), 7.14–7.10 (t, 4 and 4″-positions of tpy), 6.97–6.94 (t, 4′-position of tpy), 6.90 (s, 4-position of benzene ring), and 1.71 ppm (d, 6 methyl protons on benzene ring).
- 8
- 8a
M. H. V. Huynh, P. S. White, T. J. Meyer, Angew. Chem. 2000, 112, 4267–4270;
10.1002/1521-3757(20001117)112:22<4267::AID-ANGE4267>3.0.CO;2-5 Google ScholarAngew. Chem. Int. Ed. 2000, 39, 4101–4104;10.1002/1521-3773(20001117)39:22<4101::AID-ANIE4101>3.0.CO;2-E CAS PubMed Web of Science® Google Scholar
- 8b Selected bond lengths and angles of the S-protonated trans isomer of the OsIV–sulfilimido (trans-1+) complex are listed for comparison: OsN(tpy) 2.015(10), 2.108(9), and 2.129(9) Å (with the shortest OsN(tpy) bond trans to the sulfilimido ligand); OsN(sulfilimido) 1.906(10) Å; N(1)S(1) 1.706(9) Å (single bond); ∢Os(1)-N(1)-S(1) 130.4(6)°; and ∢N(1)-S(1)-C(1) 101.6(5)°.
- 9a M. H. V. Huynh, E.-S. El-Samanody, K. D. Demadis, P. S. White, T. J. Meyer, Inorg. Chem. 2000, 39, 3075–3085;
- 9b M. H. V. Huynh, P. S. White, T. J. Meyer, Inorg. Chem. 2001, 40, 5231–5235.
- 10 The oxygen produced was measured by a Thermal Orion Model 1230 waterproof dissolved oxygen meter both with and without salinity correction modes. Each measurement of dissolved oxygen was corrected against a blank air-saturated acidic CH3CN solution.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.



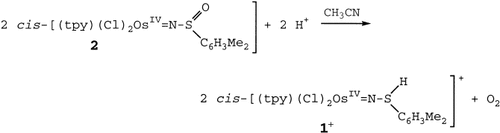
114:13<>1.0.co;2-j.cover.gif)
