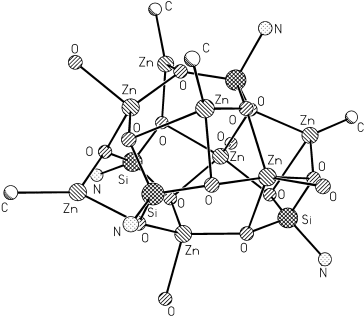
[Zn4(thf)4(MeZn)4(O3SiR)4] (R=2,6-iPr2C6H3N(SiMe3)), A Compound Containing Trigonal-Planar, Tetrahedral, and Trigonal-Bipyramidal Metal Atoms: A New Route to Larger Aggregates
Ganapathi Anantharaman
Institut für Anorganische Chemie Universität Göttingen Tammannstrasse 4, 37077 Göttingen (Germany) Fax: (+49) 551-393373
Search for more papers by this authorHerbert W. Roesky
Institut für Anorganische Chemie Universität Göttingen Tammannstrasse 4, 37077 Göttingen (Germany) Fax: (+49) 551-393373
Search for more papers by this authorJörg Magull
Institut für Anorganische Chemie Universität Göttingen Tammannstrasse 4, 37077 Göttingen (Germany) Fax: (+49) 551-393373
Search for more papers by this authorGanapathi Anantharaman
Institut für Anorganische Chemie Universität Göttingen Tammannstrasse 4, 37077 Göttingen (Germany) Fax: (+49) 551-393373
Search for more papers by this authorHerbert W. Roesky
Institut für Anorganische Chemie Universität Göttingen Tammannstrasse 4, 37077 Göttingen (Germany) Fax: (+49) 551-393373
Search for more papers by this authorJörg Magull
Institut für Anorganische Chemie Universität Göttingen Tammannstrasse 4, 37077 Göttingen (Germany) Fax: (+49) 551-393373
Search for more papers by this authorThis work was supported by the Deutsche Forschungsgemeinschaft and the Göttinger Akademie der Wissenschaften.
Graphical Abstract
Zinkatome in drei unterschiedlichen Koordinationsmodi und chemisch verschiedenen Koordinationssphären sind bei der Titelverbindung zu erkennen (siehe Struktur). Diese achtkernige Zink-Silicium-Verbindung wurde durch Zugabe von Me2Zn zu RSi(OH)3 (R=2,6-iPr2C6H3N(SiMe3)) im Molverhältnis 2:1 in THF/Hexan bei Raumtemperatur erhalten. Die Methode ermöglicht auch die Synthese größerer Aggregate.
Supporting Information
Supporting information for this article is available on the WWW under http://www.angewandte.com or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1a R. Murugavel, A. Voigt, M. G. Walawalkar, H. W. Roesky, Chem. Rev. 1996, 96, 2205;
- 1b L. King, A. C. Sullivan, Coord. Chem. Rev. 1999, 189, 19;
- 1c
F. Schindler, H. Schmidbaur, Angew. Chem. 1967, 79, 697;
10.1002/ange.19670791602 Google ScholarAngew. Chem. Int. Ed. Engl. 1967, 6, 683.
- 2
- 2a A. R. Barron, L. K. Cheatham, A. W. Apblett, J. Mater. Chem. 1991, 10, 143;
- 2b M. Lazell, M. Motevalli, S. A. A. Shah, A. C. Sullivan, J. Chem. Soc. Dalton Trans. 1997, 3363.
- 3
- 3a Y. I. Yermakov, B. N. Kuznetsov, V. A. Zakharkov, Catalysis by Supported Complexes, Elsevier, New York, 1981;
- 3b
F. R. Hartley, Supported Metal Complexes, Reidel, Boston, 1985;
10.1007/978-94-009-5247-8 Google Scholar
- 3c
Y. Iwasawa, Tailored metal catalysts, Reidel, Boston, 1986;
10.1007/978-94-009-5261-4_1 Google Scholar
- 3d F. J. Feher, T. A. Budzichowski, Polyhedron 1995, 14, 3239;
- 3e
F. T. Edelmann, Angew. Chem. 1992, 102, 600;
10.1002/ange.19921040510 Google ScholarAngew. Chem. Int. Ed. Engl. 1992, 31, 586;
- 3f
R. Fandos, A. Otero, A. Rodriguez, M. J. Ruiz, P. Terreros, Angew. Chem. 2001, 113, 2968;
10.1002/1521-3757(20010803)113:15<2968::AID-ANGE2968>3.0.CO;2-M Google ScholarAngew. Chem. Int. Ed. 2001, 40, 2884.10.1002/1521-3773(20010803)40:15<2884::AID-ANIE2884>3.0.CO;2-7 CAS PubMed Web of Science® Google Scholar
- 4
- 4a J. A. Biscardi, G. D. Meitzner, E. Iglesia, J. Catal. 1998, 179, 192;
- 4b M. F. Circolo, P. Norby, J. C. Hanson, D. R. Corbin, C. P. Grey, J. Phys. Chem. B 1999, 103, 346;
- 4c D. K. Murray, T. Howard, P. W. Goguen, T. R. Krawietz, J. F. Haw, J. Am. Chem. Soc. 1994, 116, 6354;
- 4d D. K. Murray, J. W. Chang, J. F. Haw, J. Am. Chem. Soc. 1993, 115, 4732;
- 4e M. Isao, Y. Z. Czarny, J. Oszczudlowski, J. Appl. Chem. 1991, 3, 187.
- 5
- 5a M. G. Voronkov, E. A. Maletina, V. K. Roman, Heterosiloxanes (Soviet Scientific Review Supplement, Series Chemistry, Vol. 1), Academic Press, London, 1988;
- 5b F. J. Feher, T. A. Budzichowski, Polyhedron 1995, 14, 329.
- 6
- 6a A. I. Gouzyr, H. Wessel, C. E. Barnes, H. W. Roesky, M. Teichert, I. Usón, Inorg. Chem. 1997, 36, 3392;
- 6b
A. Voigt, R. Murugavel, M. L. Montero, H. Wessel, F. Q. Liu, H. W. Roesky, I. Usón, T. Albers, E. Parisini, Angew. Chem. 1997, 109, 1020;
10.1002/ange.19971090919 Google ScholarAngew. Chem. Int. Ed. Engl. 1997, 36, 1001;
- 6c A. Klemp, H. W. Roesky, H.-G. Schmidt, H. S. Park, M. Noltemeyer, Organometallics 1998, 17, 5225;
- 6d R. Siefken, M. Teichert, D. Chakraborty, H. W. Roesky, Organometallics 1999, 18, 2321.
- 7
- 7a M. L. Montero, I. Usón, H. W. Roesky, Angew. Chem. 1994, 106, 2198; Angew. Chem. Int. Ed. Engl. 1994, 33, 2103;
- 7b
M. L. Montero, A. Voigt, M. Teichert, I. Usón, H. W. Roesky, Angew. Chem. 1995, 107, 2761;
10.1002/ange.19951072232 Google ScholarAngew. Chem. Int. Ed. Engl. 1995, 34, 2504.
- 8 R. Murugavel, M. G. Walawalkar, G. Prabushankar, P. Davis, Organometallics 2001, 20, 2639.
- 9 F. Schindler, H. Schmidbaur, U. Krüger, Angew. Chem. 1965, 77, 865; Angew. Chem. Int. Ed. Engl. 1965, 4, 876.
- 10
- 10a M. Driess, K. Merz, S. Rell, Eur. J. Inorg. Chem. 2000, 2517;
- 10b K. Su, T. D. Tilley, M. J. Sailor, J. Am. Chem. Soc. 1996, 118, 3459.
- 11 G. Anantharaman, N. D. Reddy, H. W. Roesky, J. Magull, Organometallics 2001, 20, 5777.
- 12
- 12a V. Chandrasekhar, R. Murugavel, A. Voigt, M. Noltemeyer, H.-G. Schmidt, H. W. Roesky, Organometallics 1996, 15, 918;
- 12b
A. Voigt, R. Murugavel, E. Parisini, H. W. Roesky, Angew. Chem. 1996, 108, 823;
10.1002/ange.19961080718 Google ScholarAngew. Chem. Int. Ed. Engl. 1996, 35, 748;
- 12c
A. Voigt, M. G. Walawalkar, R. Murugavel, H. W. Roesky, E. Parisini, P. Lubini, Angew. Chem. 1997, 109, 2313;
10.1002/ange.19971092019 Google ScholarAngew. Chem. Int. Ed. Engl. 1997, 36, 2203.
- 13
Crystal data for compound 2⋅3 C7H8: C101H172N4O16Si8Zn8, Mr=2446.11, triclinic, space group P
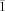 , a=14.5274(7), b=16.0523(10), c=27.4893(14) Å, α=99.197(4), β=91.338(4), γ=112.005(4)°, V=5842.7(5) Å3, Z=2, ρcalcd=1.390 mg m−3, F(000)=2572, λ=0.71073 Å, T=133(2) K, μ(MoKα)=0.904 mm−1. Crystal size 0.2×0.3×0.3 mm. The data were collected by using the ω scan mode in the range of 1.52≤θ≤24.82, −17≤h≤17, −18≤k≤18, −32≤l≤32. Of 39 805 reflections collected, 17 747 were unique. Final R1 (I>2σ(I))=0.0393; wR2 (all data)=0.0961. Maximum and minimum heights in the final Fourier difference map were 0.877 and −0.653 e Å−3. The single crystals suitable for X-ray diffraction studies of compound 2⋅3 C7H8 were obtained from toluene at 0 °C. Diffraction data were collected on a IPDS II Stoe image-plate diffractometer with graphite-monochromated MoKα radiation (λ=0.71073 Å). The structure was solved by direct methods (SHELX-97)[17] and refined against F 2 on all data by full-matrix least-squares with SHELX-97.[18] The heavy atoms were refined anisotropically. Hydrogen atoms were included by using the riding model with Uiso tied to Uiso of the parent atoms. CCDC-173318 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Centre, 12, Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223-336-033; or [email protected]).
, a=14.5274(7), b=16.0523(10), c=27.4893(14) Å, α=99.197(4), β=91.338(4), γ=112.005(4)°, V=5842.7(5) Å3, Z=2, ρcalcd=1.390 mg m−3, F(000)=2572, λ=0.71073 Å, T=133(2) K, μ(MoKα)=0.904 mm−1. Crystal size 0.2×0.3×0.3 mm. The data were collected by using the ω scan mode in the range of 1.52≤θ≤24.82, −17≤h≤17, −18≤k≤18, −32≤l≤32. Of 39 805 reflections collected, 17 747 were unique. Final R1 (I>2σ(I))=0.0393; wR2 (all data)=0.0961. Maximum and minimum heights in the final Fourier difference map were 0.877 and −0.653 e Å−3. The single crystals suitable for X-ray diffraction studies of compound 2⋅3 C7H8 were obtained from toluene at 0 °C. Diffraction data were collected on a IPDS II Stoe image-plate diffractometer with graphite-monochromated MoKα radiation (λ=0.71073 Å). The structure was solved by direct methods (SHELX-97)[17] and refined against F 2 on all data by full-matrix least-squares with SHELX-97.[18] The heavy atoms were refined anisotropically. Hydrogen atoms were included by using the riding model with Uiso tied to Uiso of the parent atoms. CCDC-173318 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Centre, 12, Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223-336-033; or [email protected]).
- 14 Other examples, where zinc possesses different geometries in a structure:
- 14a Y. Yang, J. Pinkas, M. Noltemeyer, H.-G. Schmidt, H. W. Roesky, Angew. Chem. 1999, 111, 706; Angew. Chem. Int. Ed. Engl. 1997, 38, 664;
- 14b M. G. Gardiner, S. M. Lawrence, C. L. Ratson, B. A. Skelton, A. H. White, Chem. Commun. 1996, 2491.
- 15 For compounds having Zn⋅⋅⋅Zn interaction:
- 15a A. J. Elias, H.-G. Schmidt, M. Noltemeyer, H. W. Roesky, Organometallics 1992, 11, 462;
- 15b H. Li, M. Eddaoudi, T. L. Groy, O. M. Yaghi, J. Am. Chem. Soc. 1998, 120, 8571.
- 16 J. Arnold, T. D. Tilley, A. L. Rheingold, S. J. Geib, Inorg. Chem. 1987, 26, 2106.
- 17 G. M. Sheldrick, Acta Crystallogr. Sect. A 1990, 46, 467.
- 18 G. M. Sheldrick, SHELX-97, Program for Crystal Structure Refinement, University of Göttingen, Göttingen (Germany), 1997.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.



114:7<>1.0.co;2-b.cover.gif)
