Self-Complementary Metal Complexes Containing a DNA Base Pair
Clayton Price
Department of Chemistry, University of Newcastle upon Tyne Newcastle upon Tyne NE1 7RU (UK) Fax: (+44) 191-222-6829
Search for more papers by this authorBenjamin R. Horrocks
Department of Chemistry, University of Newcastle upon Tyne Newcastle upon Tyne NE1 7RU (UK) Fax: (+44) 191-222-6829
Search for more papers by this authorAnnabelle Mayeux
Department of Chemistry, University of Newcastle upon Tyne Newcastle upon Tyne NE1 7RU (UK) Fax: (+44) 191-222-6829
Search for more papers by this authorMark R. J. Elsegood
Department of Chemistry, University of Newcastle upon Tyne Newcastle upon Tyne NE1 7RU (UK) Fax: (+44) 191-222-6829
Search for more papers by this authorWilliam Clegg
Department of Chemistry, University of Newcastle upon Tyne Newcastle upon Tyne NE1 7RU (UK) Fax: (+44) 191-222-6829
Search for more papers by this authorAndrew Houlton
Department of Chemistry, University of Newcastle upon Tyne Newcastle upon Tyne NE1 7RU (UK) Fax: (+44) 191-222-6829
Search for more papers by this authorClayton Price
Department of Chemistry, University of Newcastle upon Tyne Newcastle upon Tyne NE1 7RU (UK) Fax: (+44) 191-222-6829
Search for more papers by this authorBenjamin R. Horrocks
Department of Chemistry, University of Newcastle upon Tyne Newcastle upon Tyne NE1 7RU (UK) Fax: (+44) 191-222-6829
Search for more papers by this authorAnnabelle Mayeux
Department of Chemistry, University of Newcastle upon Tyne Newcastle upon Tyne NE1 7RU (UK) Fax: (+44) 191-222-6829
Search for more papers by this authorMark R. J. Elsegood
Department of Chemistry, University of Newcastle upon Tyne Newcastle upon Tyne NE1 7RU (UK) Fax: (+44) 191-222-6829
Search for more papers by this authorWilliam Clegg
Department of Chemistry, University of Newcastle upon Tyne Newcastle upon Tyne NE1 7RU (UK) Fax: (+44) 191-222-6829
Search for more papers by this authorAndrew Houlton
Department of Chemistry, University of Newcastle upon Tyne Newcastle upon Tyne NE1 7RU (UK) Fax: (+44) 191-222-6829
Search for more papers by this authorThis work was financially supported by BBSRC and EPSRC.
Graphical Abstract
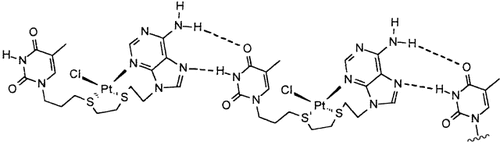
Hoogsteen-Paarbildung zwischen den Thymin(T)- und koordinierten Adenin(A)-Resten des Pt-Komplexes eines Dithioether-Liganden, an den diese Reste gebunden sind, führt im festen Zustand zur Bildung unendlich ausgedehnter Ketten (siehe Bild). Bei den Ketten wurden auch π-π-Stapel-Wechselwirkungen festgestellt.
References
- 1
- 1a G. M. Whitesides, E. E. Simanek, J. P. Mathias, C. T. Seto, D. N. Chin, M. Mammen, D. M. Gordon, Acc. Chem. Res. 1995, 28, 37–44;
- 1b J. L. Sessler, B. Wang, A. Harriman, J. Am. Chem. Soc. 1993, 115, 10 418–10 419;
- 1c A. D. Burrows, C.-W. Chan, M. M. Chowdhry, J. E. McGrady, D. M. P. Mingos, Chem. Soc. Rev. 1995, 24, 351–358.
- 2
- 2a I. Dieter-Wurm, M. Sabat, B. Lippert, J. Am. Chem. Soc. 1992, 114, 357–359;
- 2b A. Schreiber, M. S. Luth, A. Erxleben, E. C. Fusch, B. Lippert, J. Am. Chem. Soc. 1996, 118, 4124–4132;
- 2c J. A. R. Navarro, B. Lippert, Coord. Chem. Rev. 1999, 185, 653–667, and references therein.
- 3 Nucleic Acids in Chemistry and Biology ( ), IRL Press, Oxford, 1990.
- 4
- 4a C. Price, M. R. J. Elsegood, W. Clegg, A. Houlton, Chem. Commun. 1995, 2285–2286;
- 4b
C. Price, M. R. J. Elsegood, W. Clegg, N. H. Rees, A. Houlton, Angew. Chem. 1997, 109, 1823–1825;
10.1002/ange.19971091624 Google ScholarAngew. Chem. Int. Ed. Engl. 1997, 36, 1762–1764;
- 4c
M. A. Shipman, C. Price, A. E. Gibson, M. R. J. Elsegood, W. Clegg, A. Houlton, Chem. Eur. J. 2000, 6, 4371–4378;
10.1002/1521-3765(20001201)6:23<4371::AID-CHEM4371>3.0.CO;2-X CAS PubMed Web of Science® Google Scholar
- 4d
M. A. Shipman, C. Price, M. R. J. Elsegood, W. Clegg, A. Houlton, Angew. Chem. 2000, 112, 2450–2452;
10.1002/1521-3757(20000703)112:13<2450::AID-ANGE2450>3.0.CO;2-2 Google ScholarAngew. Chem. Int. Ed. 2000, 39, 2360–2362;10.1002/1521-3773(20000703)39:13<2360::AID-ANIE2360>3.0.CO;2-6 CAS PubMed Web of Science® Google Scholar
- 4e C. Price, M. A. Shipman, S. L. Gummerson, A. Houlton, W. Clegg, M. R. J. Elsegood, J. Chem. Soc. Dalton Trans. 2001, 353–354;
- 4f
C. Price, M. A. Shipman, N. H. Rees, M. R. J. Elsegood, A. J. Edwards, W. Clegg, A. Houlton, Chem. Eur. J. 2001, 7, 1194–1200.
10.1002/1521-3765(20010316)7:6<1194::AID-CHEM1194>3.0.CO;2-3 CAS PubMed Web of Science® Google Scholar
- 5 Synthetic nucleobase strands linked to flexible and rigid organic framework have been reported previously:
- 5a D. T. Browne, J. Eisinger, N. J. Leonard, J. Am. Chem. Soc. 1968, 90, 7302–7312;
- 5b J. L. Sessler, R. Wang, J. Am. Chem. Soc. 1996, 118, 9808–9809;
- 5c J. L. Sessler, R. Wang, J. Org. Chem. 1998, 63, 4079–4091.
- 6 AT bases have been attached to diazacrowns that can bind metal ions:
- 6a O. F. Schall, G. W. Gokel, J. Am. Chem. Soc. 1994, 116, 6089–6100;
- 6b O. F. Schall, G. W. Gokel, J. Org. Chem. 1996, 61, 1449–1458.
- 7
- 7a C. Meiser, B. Song, E. Freisinger, M. Peilert, H. Sigel, B. Lippert, Chem. Eur. J. 1997, 3, 388–398;
- 7b
Y. Lui, C. Pacifico, G. Natile, E. Sletten, Angew. Chem. 2001, 113, 1266–1268;
10.1002/1521-3757(20010401)113:7<1266::AID-ANGE1266>3.0.CO;2-Z Google ScholarAngew. Chem. Int. Ed. 2001, 40, 1226–1228.10.1002/1521-3773(20010401)40:7<1226::AID-ANIE1226>3.0.CO;2-U CAS PubMed Web of Science® Google Scholar
- 8
- 8a Crystal data for 2: C17H32ClF3N7O6.5PdS2Si0.5, Mr=715.51, monoclinic, space group I2/a, a=24.3737(16), b=7.9006(5), c=29.2031(19) Å, β=97.894(2)°, V=5570.3(6) Å3, Z=8, ρcalcd=1.706 g cm−3, MoKα radiation, λ=0.71073 Å, μ=1.00 mm−1, T=160 K. 18 467 measured reflections were corrected for absorption; 4903 were unique (Rint=0.0451, θ≤25.0°); R=0.0829 (F values, F 2>2 σ), Rw=0.1771 (F 2 values, all data), GOF 1.312 for 350 parameters, max./min. residual electron density 2.58/−1.47 e Å−3.
- 8b Crystal data for 3: C17H32ClF3N7O6.5PtS2Si0.5, Mr=804.20, monoclinic, space group I2/a, a=24.4619(10), b=7.9190(3), c=29.1288(10) Å, β=98.073(2)°, V=5586.7(4) Å3, Z=8, ρcalcd=1.912 g cm−3, synchrotron radiation at Darebury Laboratory station 9.8, λ=0.6942 Å, μ=5.36 mm−1, T=160 K. 18 024 measured reflections were corrected for absorption; 6866 were unique (Rint=0.0360, θ≤27.5°); R=0.0388 (F values, F 2>2 σ), Rw=0.1053 (F 2 values, all data), GOF 1.185 for 350 parameters, max./min. residual electron density 2.49/−1.19 e Å3.
- 8c The anion in both crystal structures wass identified as SiF62−, generated by etching of the glass by the aqueous BF4− ions. Detailed evidence is available from the authors.
- 8d Programs: standard Bruker AXS control and integration software and SHELXTL. CCDC-170471 (2) and CCDC-170472 (3) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223-336-033; or [email protected]).
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.



114:6<>1.0.co;2-7.cover.gif)
