Aluminum-Catalyzed Asymmetric Addition of TMSCN to Aromatic and Aliphatic Ketones Promoted by an Easily Accessible and Recyclable Peptide Ligand
Hongbo Deng Dr.
Department of Chemistry, Merkert Chemistry Center Boston College Chestnut Hill, MA 02467 (USA) Fax: (+1) 617-552-1442
Search for more papers by this authorMarkus P. Isler Dr.
Department of Chemistry, Merkert Chemistry Center Boston College Chestnut Hill, MA 02467 (USA) Fax: (+1) 617-552-1442
Search for more papers by this authorMarc L. Snapper Prof.
Department of Chemistry, Merkert Chemistry Center Boston College Chestnut Hill, MA 02467 (USA) Fax: (+1) 617-552-1442
Search for more papers by this authorAmir H. Hoveyda Prof.
Department of Chemistry, Merkert Chemistry Center Boston College Chestnut Hill, MA 02467 (USA) Fax: (+1) 617-552-1442
Search for more papers by this authorHongbo Deng Dr.
Department of Chemistry, Merkert Chemistry Center Boston College Chestnut Hill, MA 02467 (USA) Fax: (+1) 617-552-1442
Search for more papers by this authorMarkus P. Isler Dr.
Department of Chemistry, Merkert Chemistry Center Boston College Chestnut Hill, MA 02467 (USA) Fax: (+1) 617-552-1442
Search for more papers by this authorMarc L. Snapper Prof.
Department of Chemistry, Merkert Chemistry Center Boston College Chestnut Hill, MA 02467 (USA) Fax: (+1) 617-552-1442
Search for more papers by this authorAmir H. Hoveyda Prof.
Department of Chemistry, Merkert Chemistry Center Boston College Chestnut Hill, MA 02467 (USA) Fax: (+1) 617-552-1442
Search for more papers by this authorThis research was supported by the United States National Institutes of Health (GM-57212), Novartis, and the Swiss National Science Foundation (postdoctoral fellowships to M.P.I.). TMSCN=trimethylsilyl cyanide.
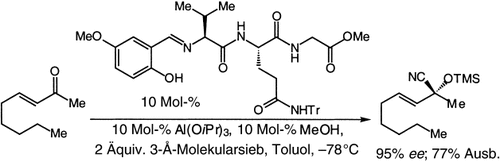
Graphical Abstract
Von Peptiden abgeleitete chirale Liganden fördern die Al-katalysierte enantioselektive Addition von Trimethylsilylcyanid an aromatische und aliphatische, cyclische und acyclische Ketone (Beispiel siehe Schema). Sie sind einfach herzustellen, werden leicht wiedergewonnen und lassen sich ohne Aktivitäts- oder Selektivitätsverlust erneut einsetzen.
Supporting Information
Supporting information for this article is available on the WWW under http://www.angewandte.com or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 For representative examples, see:
- 1a J. R. Porter, J. F. Traverse, A. H. Hoveyda, M. L. Snapper, J. Am. Chem. Soc. 2001, 123, 984–985;
- 1b J. R. Porter, J. F. Traverse, A. H. Hoveyda, M. L. Snapper, J. Am. Chem. Soc. 2001, 123, 10 409–10 410;
- 1c S. J. Degrado, H. Mizutani, A. H. Hoveyda, J. Am. Chem. Soc. 2001, 123, 755–756;
- 1d
C. A. Luchaco-Cullis, H. Mizutani, K. E. Murphy, A. H. Hoveyda, Angew. Chem. 2001, 113, 1504–1508;
10.1002/1521-3757(20010417)113:8<1504::AID-ANGE1504>3.0.CO;2-V Google ScholarAngew. Chem. Int. Ed. 2001, 40, 1456–1460; see also refs. [2, 3, 17d].10.1002/1521-3773(20010417)40:8<1456::AID-ANIE1456>3.0.CO;2-T CAS PubMed Web of Science® Google Scholar
- 2
- 2a
B. M. Cole, K. D. Shimizu, C. A. Krueger, J. P. A. Harrity, M. L. Snapper, A. H. Hoveyda, Angew. Chem. 1996, 108, 1776–1779;
10.1002/ange.19961081509 Google ScholarAngew. Chem. Int. Ed. Engl. 1996, 35, 1668–1671;
- 2b K. D. Shimizu, B. M. Cole, C. A. Krueger, K. W. Kuntz, M. L. Snapper, A. H. Hoveyda, Angew. Chem. 1997, 109, 1781–1785; Angew. Chem. Int. Ed. Engl. 1997, 36, 1704–1707.
- 3
- 3a C. A. Krueger, K. W. Kuntz, C. D. Dzierba, W. G. Wirschun, J. D. Gleason, M. L. Snapper, A. H. Hoveyda, J. Am. Chem. Soc. 1999, 121, 4284–4285;
- 3b J. R. Porter, W. G. Wirschun, K. W. Kuntz, M. L. Snapper, A. H. Hoveyda, J. Am. Chem. Soc. 2000, 122, 2657–2658.
- 4
- 4a D. J. Ramon, M. Yus, Tetrahedron Lett. 1998, 39, 1239–1242;
- 4b D. J. Ramon, M. Yus, Tetrahedron 1998, 54, 5651–5666;
- 4c P. I. Dosa, G. C. Fu, J. Am. Chem. Soc. 1998, 120, 445–446;
- 4d S. Casolari, D. D'Addario, E. Tagliavini, Org. Lett. 1999, 1, 1061–1063.
- 5 For a review on the utility of cyanohydrins, see: R. J. H. Gregory, Chem. Rev. 1999, 99, 3649–3682.
- 6
- 6a Y. N. Belokon, B. Green, N. S. Ikonnikov, M. North, V. I. Tararov, Tetrahedron Lett. 1999, 40, 8147–8150;
- 6b Y. N. Belokon, B. Green, N. S. Ikonnikov, M. North, T. Parsons, V. I. Tararov, Tetrahedron 2001, 57, 771–779.
- 7
- 7a Y. Hamashima, M. Kanai, M. Shibasaki, J. Am. Chem. Soc. 2000, 122, 7412–7413;
- 7b Y. Hamashima, M. Kanai, M. Shibasaki, Tetrahedron Lett. 2001, 42, 691–694;
- 7c K. Yabu, S. Masumoto, S. Yamasaki, Y. Hamashima, M. Kanai, W. Du, D. P. Curran, M. Shibasaki, J. Am. Chem. Soc. 2001, 123, 9908–9909.
- 8 S-K. Tian, L. Deng, J. Am. Chem. Soc. 2001, 123, 6195–6196.
- 9 For an Al-catalyzed asymmetric addition of TMSCN to aldehydes, see:
- 9a H. Ohno, H. Nitta, K. Tanaka, A. Mori, S. Inoue, J. Am. Chem. Soc. 1992, 114, 6778–6783; for representative reports on catalytic asymmetric addition of cyanide to aldehydes, see:
- 9b H. Nitta, D. Yu, M. Kudo, A. Mori, S. Inoue, J. Am. Chem. Soc. 1992, 114, 7969–7975;
- 9c E. J. Corey, Z. Wang, Tetrahedron Lett. 1993, 34, 4001–4004;
- 9d Y. Hamashima, D. Sawada, M. Kanai, M. Shibasaki, J. Am. Chem. Soc. 1999, 121, 2641–2642;
- 9e Y. N. Belokon, S. Caveda-Cepas, B. Green, N. S. Ikonnikov, V. N. Khrustalev, V. S. Larichev, M. A. Moskalenko, M. North, C. Orizu, V. I. Tararov, M. Tassinazzo, G. I. Timofeeva, L. V. Yashkina, J. Am. Chem. Soc. 1999, 121, 3968–3973;
- 9f X.-G. Zhou, J.-S. Huang, P.-H. Ko, K.-K. Cheung, C.-M. Che, J. Chem. Soc. Perkin Trans. 1 1999, 3303–3309; for review articles covering this transformation, see:
- 9g K. Soai, S. Niwa, Chem. Rev. 1992, 92, 833–856;
- 9h M. North, Synlett 1993, 807–820;
- 9i
F. Effenberger, Angew. Chem. 1994, 104, 1609–1619;
10.1002/ange.19941061504 Google ScholarAngew. Chem. Int. Ed. Engl. 1994, 33, 1555–1564.
- 10 For catalytic asymmetric cyanide additions to ketimines, see:
- 10a P. Vachal, E. N. Jacobsen, Org. Lett. 2000, 2, 867–870;
- 10b J. J. Byrne, M. Chavarot, P.-Y. Chavant, Y. Vallee, Tetrahedron Lett. 2000, 41, 873–876.
- 11
- 11a
K. D. Shimizu, M. L. Snapper, A. H. Hoveyda, Chem. Eur. J. 1998, 4, 1885–1889; for reviews regarding screening strategies for optimal catalyst identification, see:
10.1002/(SICI)1521-3765(19981002)4:10<1885::AID-CHEM1885>3.0.CO;2-D CAS Web of Science® Google Scholar
- 11b M. B. Francis, T. F. Jamison, E. N. Jacobsen, Curr. Opin. Chem. Biol. 1998, 2, 422–428;
- 11c H. B. Kagan, J. Organomet. Chem. 1998, 567, 3–6;
- 11d K. W. Kuntz, M. L. Snapper, A. H. Hoveyda, Curr. Opin. Chem. Biol. 1999, 3, 313–319;
- 11e
B. Jandeleit, D. J. Schaefer, T. S. Powers, H. W. Turner, W. H. Weinberg, Angew. Chem. 1999, 111, 2648–2689;
10.1002/(SICI)1521-3757(19990903)111:17<2648::AID-ANGE2648>3.0.CO;2-N Google ScholarAngew. Chem. Int. Ed. 1999, 38, 2494–2532;10.1002/(SICI)1521-3773(19990903)38:17<2494::AID-ANIE2494>3.0.CO;2-# CAS PubMed Web of Science® Google Scholar
- 11f M. L. Snapper, A. H. Hoveyda, Combinatorial Chemistry ( ), Oxford University Press, Oxford, 2000, pp. 433–455.
- 12 See the Supporting Information for experimental details, including the results of screening studies.
- 13 For a review on the effect of additives on asymmetric catalytic processes, see:
- 13a
E. M. Vogel, H. Groger, M. Shibasaki, Angew. Chem. 1999, 111, 1672–1680;
10.1002/(SICI)1521-3757(19990601)111:11<1672::AID-ANGE1672>3.0.CO;2-2 Google ScholarAngew. Chem. Int. Ed. 1999, 38, 1570–1577; for additional recent examples, see:10.1002/(SICI)1521-3773(19990601)38:11<1570::AID-ANIE1570>3.0.CO;2-Y CAS PubMed Web of Science® Google Scholar
- 13b H. Ishitani, M. Ueno, S. Kobayashi, J. Am. Chem. Soc. 2000, 122, 8180–8186;
- 13c S. Yamasaki, M. Kanai, M. Shibasaki, J. Am. Chem. Soc. 2001, 123, 1256–1257;
- 13d S. Kobayashi, H. Ishitani, Y. Yamashita, M. Ueno, H. Shimizu, Tetrahedron 2001, 57, 861–866;
- 13e S. Ribe, P. Wipf, Chem. Commun. 2001, 299–307, and references therein.
- 14
D. J. Berrisford, C. Bolm, K. B. Sharpless, Angew. Chem. 1995, 107, 1159–1170;
10.1002/ange.19951071004 Google ScholarAngew. Chem. Int. Ed. Engl. 1995, 34, 1059–1070.
- 15 N. S. Josephsohn, K. W. Kuntz, M. L. Snapper, A. H. Hoveyda, J. Am. Chem. Soc. 2001, 123, 11 594–11 599.
- 16 This hypothesis is based on the scenario that HCN adds to the ketone and the resulting cyanohydrin reacts with TMSCN to generate the product and reproduce HCN.
- 17 α,β-Unsaturated enones can be prepared easily by recyclable Ru-catalyzed cross-metathesis reactions; see:
- 17a S. B. Garber, J. S. Kingsbury, B. L. Gray, A. H. Hoveyda, J. Am. Chem. Soc. 2000, 122, 8168–8179;
- 17b J. Cossy, S. BouzBouz, A. H. Hoveyda, J. Organomet. Chem. 2001, 624, 327–332;
- 17c
J. S. Kingsbury, S. B. Garber, J. M. Giftos, B. L. Gray, M. M. Okamoto, R. A. Farrer, J. T. Fourkas, A. H. Hoveyda, Angew. Chem. 2001, 113, 4381–4386;
10.1002/1521-3757(20011119)113:22<4381::AID-ANGE4381>3.0.CO;2-J Google ScholarAngew. Chem. Int. Ed. 2001, 40, 4251–4256;10.1002/1521-3773(20011119)40:22<4251::AID-ANIE4251>3.0.CO;2-L CAS PubMed Web of Science® Google Scholar
- 17d H. Mizutani, S. J. Degrado, A. H. Hoveyda, J. Am. Chem. Soc. 2002, 124, 779–781.
- 18
For a review of catalytic enantioselective methods for the synthesis of quaternary carbon stereogenic centers, see: E. J. Corey, A. Guzman-Perez, Angew. Chem. 1998, 110, 402–405;
10.1002/(SICI)1521-3757(19980216)110:4<402::AID-ANGE402>3.0.CO;2-6 Google ScholarAngew. Chem. Int. Ed. 1998, 37, 388–401.10.1002/(SICI)1521-3773(19980302)37:4<388::AID-ANIE388>3.0.CO;2-V PubMed Web of Science® Google Scholar
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.



114:6<>1.0.co;2-7.cover.gif)
