Remote Stereocontrol in Carbonyl Additions Promoted by Vinylstannanes
Asunción Barbero Dr.
Departamento de Química Orgánica, Universidad de Valladolid 47011 Valladolid (Spain) Fax: (+34) 983-423013
Search for more papers by this authorFrancisco J. Pulido Dr.
Departamento de Química Orgánica, Universidad de Valladolid 47011 Valladolid (Spain) Fax: (+34) 983-423013
Search for more papers by this authorJuan A. Rincón Dr.
Departamento de Química Orgánica, Universidad de Valladolid 47011 Valladolid (Spain) Fax: (+34) 983-423013
Search for more papers by this authorPurificación Cuadrado Dr.
Departamento de Química Orgánica, Universidad de Valladolid 47011 Valladolid (Spain) Fax: (+34) 983-423013
Search for more papers by this authorDiego Galisteo Dr.
Departamento de Química Orgánica, Universidad de Valladolid 47011 Valladolid (Spain) Fax: (+34) 983-423013
Search for more papers by this authorHenar Martínez-García Dr.
Departamento de Química Orgánica, Universidad de Valladolid 47011 Valladolid (Spain) Fax: (+34) 983-423013
Search for more papers by this authorAsunción Barbero Dr.
Departamento de Química Orgánica, Universidad de Valladolid 47011 Valladolid (Spain) Fax: (+34) 983-423013
Search for more papers by this authorFrancisco J. Pulido Dr.
Departamento de Química Orgánica, Universidad de Valladolid 47011 Valladolid (Spain) Fax: (+34) 983-423013
Search for more papers by this authorJuan A. Rincón Dr.
Departamento de Química Orgánica, Universidad de Valladolid 47011 Valladolid (Spain) Fax: (+34) 983-423013
Search for more papers by this authorPurificación Cuadrado Dr.
Departamento de Química Orgánica, Universidad de Valladolid 47011 Valladolid (Spain) Fax: (+34) 983-423013
Search for more papers by this authorDiego Galisteo Dr.
Departamento de Química Orgánica, Universidad de Valladolid 47011 Valladolid (Spain) Fax: (+34) 983-423013
Search for more papers by this authorHenar Martínez-García Dr.
Departamento de Química Orgánica, Universidad de Valladolid 47011 Valladolid (Spain) Fax: (+34) 983-423013
Search for more papers by this authorWe Thank the M.E.C. of Spain (PB96/0357) and the Junta de Castilla y León (VA43/98) for financial support. We are very much indebted to Dr. Santiago García-Granda, Oviedo (Spain) for X-ray crystallographic assistance.
Graphical Abstract
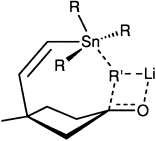
Syn zum Zinn – dies ist die bevorzugte Orientierung bei der Addition von Organolithiumreagentien an die Carbonylgruppe cyclischer Ketone mit einer β-Stannylvinylgruppe. Diese stereochemische Fernkontrolle resultiert daraus, dass das Organolithiumreagens wie im Bild gezeigt zwischen Zinnatom und Carbonylgruppe verankert wird.
References
- 1 J. D. Morrison, Asymmetric Synthesis, Vol. 1–5, Academic Press, New York, 1985.
- 2 M. Nógrádi, Stereoselective Synthesis, VCH, Weinheim, 1987.
- 3 G. Procter, Asymmetric Synthesis, Oxford University Press, Oxford, 1996.
- 4
- 4a E. J. Thomas, A. H. McNeill, Tetrahedron Lett. 1990, 31, 6239;
- 4b E. J. Thomas, J. S. Carey, J. Chem. Soc. Chem. Commun. 1994, 283;
- 4c E. J. Thomas, J. S. Carey, Synlett 1992, 585;
- 4d E. J. Thomas, J. S. Carey, Tetrahedron Lett. 1993, 34, 3935;
- 4e E. J. Thomas, S. J. Stanway, J. Chem. Soc. Chem. Commun. 1994, 285;
- 4f E. J. Thomas, A. H. McNeill, Synthesis 1994, 322;
- 4g E. J. Thomas, D. J. Hallet, Tetrahedron: Asymmetry 1995, 6, 2575;
- 4h E. J. Thomas, Chem. Commun. 1997, 411;
- 4i E. J. Thomas, L. A. Hobson, M. A. Vincent, I. H. Hillier, Chem. Commun. 1998, 899; see also Y. Nishigaichi, M. Kuramoto, A. Takuwa, Tetrahedron Lett. 1995, 36, 3353 and Y. Nishigaichi, M. Kuramoto, A. Takuwa, Chem. Lett. 1996, 961.
- 5 J. A. Marshall, Chem. Rev. 1996, 96, 31.
- 6
- 6a P. Cuadrado, A. M. González, F. J. Pulido, I. Fleming, M. Rowley, Tetrahedron 1989, 45, 413;
- 6b A. Barbero, P. Cuadrado, A. M. González, F. J. Pulido, I. Fleming, J. Chem. Soc. Chem. Commun. 1990, 1030;
- 6c A. Barbero, P. Cuadrado, A. M. González, F. J. Pulido, I. Fleming, J. Chem. Soc. Perkin Trans. 1 1992, 327;
- 6d A. Barbero, P. Cuadrado, A. M. González, F. J. Pulido, I. Fleming, J. Chem. Res. 1990, 297, 291.
- 7
- 7a A. Barbero, P. Cuadrado, A. M. González, F. J. Pulido, I. Fleming, J. Chem. Soc. Chem. Commun. 1992, 351;
- 7b A. Barbero, P. Cuadrado, A. M. González, F. J. Pulido, R. Rubio, I. Fleming, J. Chem. Soc. Perkin Trans. 1 1993, 1657;
- 7c A. Barbero, P. Cuadrado, C. García, F. J. Pulido, J. A. Rincón, J. Org. Chem. 1998, 63, 7531; see also, F. J. Pulido, I. Fleming, A. Barbero, P. Cuadrado, A. M. González, R. Rubio, Tetrahedron Lett. 1992, 33, 5841.
- 8
- 8a S. Sharma, A. C. Oehlschlager, J. Org. Chem. 1991, 56, 770; S. Sharma, A. C. Oehlschlager, J. Org. Chem. 1991, 56, 4993;
- 8b E. Piers, A. V. Gavai, J. Org. Chem. 1990, 55, 2374; E. Piers, A. V. Gavai, J. Org. Chem. 1990, 55, 2380;
- 8c B. H. Lipshutz, S. Sharma, D. C. Reuter, Tetrahedron Lett. 1990, 31, 7253;
- 8d J. P. Marino, M. V. Edmonds, P. J. Stengel, A. R. Oliveira, F. Simonelli, J. T. Ferreira, Tetrahedron Lett. 1992, 33, 49;
- 8e O. Z. Pereira, T. H. Chan, J. Org. Chem. 1996, 61, 5406.
- 9 A. G. Davies, Organotin Chemistry, VCH, Weinheim, 1997.
- 10 R. J. Taylor, Organocopper Reagents: A Practical Approach, Oxford University Press, New York, 1994.
- 11a (−)-(5R)-Carvone: [α]D=−61 (neat), Aldrich; (−)-(1S,5S)-verbenone: [α]D=−142 (neat), Aldrich; 2R,3R,5R-9: [α]D=−9.9 (c=1.01, CHCl3)—a 4 % yield of the C-2 epimeric ketone was also isolated; 1S,4S,5R-11: [α]D=−23 (c=1.03, CHCl3);
- 11b (1R,2R,3R,5R)-14 a: [α]D=−29.5 (c=0.4, CHCl3).
- 12 Chemical confirmation of the stereochemistry assigned to 12 a was obtained as follows: a diastereomeric mixture of 1,3-dimethyl-3-vinylcyclopentan-1-ol was obtained by adding lithium divinyl cuprate to 2, and reacting the resulting ketone with MeLi. The two diastereomeric cyclopentanol compounds were separated: the 1-methyl/3-vinyl syn cyclopentanol is identical to that obtained from protodestannylation (HI/THF) of 12 a, whereas the other cyclopentanol (1-methyl/3-vinyl anti) was converted into the bicyclic lactone 1,4-dimethyl-3-oxa-bicyclo[2,2,1]heptan-2-one by ozonolysis followed by oxidation (PCC/CH2Cl2) of the resulting hydroxyaldehyde.
- 13 Crystallographic data (excluding structure factors) for the structures reported in this paper have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication no. CCDC-139911. Copies of the data can be obtained free of charge on application to CCDC, 12 Union Road, Cambridge CB2 1EZ, UK (fax: (+44) 1223-336-033; e-mail: [email protected]).
- 14 The pseudo-axial position of the methyl group in ketone 7 should favor the attack of the organometallic reagent from the less hindered side, anti to the methyl group and hence syn to vinyltin group. Similarly, for the ketone 8, equatorial attack would be favored which could explain the diastereoselectivity observed.
- 15 Ab initio analysis of cyclohexanone 9 predicts equatorial positions for methyl and isopropenyl groups and an axial position for the stannylvinyl group. Coupling constants and NOE data from NMR spectroscopic analysis corroborate this.
- 16 Gaussian 98 (Revision A.7), M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, V. G. Zakrzewski, J. A. Montgomery, R. E. Stratmann, J. C. Burant, S. Dapprich, J. M. Millam, A. D. Daniels, K. N. Kudin, M. C. Strain, O. Farkas, J. Tomasi, V. Barone, M. Cossi, R. Cammi, B. Mennucci, C. Pomelli, C. Adamo, S. Clifford, J. Ochterski, G. A. Petersson, P. Y. Ayala, Q. Cui, K. Morokuma, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. Cioslowski, J. V. Ortiz, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. Gomperts, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, C. Gonzalez, M. Challacombe, P. M. W. Gill, B. G. Johnson, W. Chen, M. W. Wong, J. L. Andres, M. Head-Gordon, E. S. Replogle, J. A. Pople, Gaussian, Inc., Pittsburgh, PA, 1998. Theoretical level: MP2(fc)/3-21G*//HF/3-21G*. Naked methyl anions were used for simplicity.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.



113:11<>1.0.co;2-#.cover.gif)
