Abstract
[108-24-7] C4H6O3 (MW 102.09)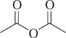
InChI = 1S/C4H6O3/c1-3(5)7-4(2)6/h1-2H3
InChIKey = WFDIJRYMOXRFFG-UHFFFAOYSA-N
(useful for the acetylation of alcohols,2 amines,3 and thiols,4 oxidation of alcohols,5 dehydration,6 Pummerer7 reaction, Perkin8 reaction, Polonovski9 reaction, N-oxide reaction,10 Thiele11 reaction, ether cleavage,12 enol acetate formation,13 gem-diacetate formation14)
Physical Data: bp 138–140 °C; mp −73 °C; d 1.082 g cm−3.
Solubility: sol most organic solvents. Reacts with water rapidly and alcohol solvents slowly.
Form Supplied in: commercially available in 98% and 99+% purities. Acetic anhydride-d6 is also commercially available.
Analysis of Reagent Purity: IR, NMR.15
Preparative Methods: acetic anhydride is prepared industrially by the acylation of Acetic Acid with Ketene.1b A laboratory preparation of acetic anhydride involves the reaction of sodium acetate and Acetyl Chloride followed by fractional distillation.16
Purification: adequate purification is readily achieved by fractional distillation. Acetic acid, if present, can be removed by refluxing with CaC2 or with coarse magnesium filings at 80–90 °C for 5 days. Drying and acid removal can be achieved by azeotropic distillation with toluene.17
Handling, Storage, and Precautions: acetic anhydride is corrosive and a lachrymator and should be handled in a fume hood.
Bibliography
- 1 (a) Kim, D. H. J. Heterocycl. Chem. 1976, 13, 179. (b) Cook, S. L. Chemical Industries 1993, 49, 145. (c) Joy, E. F.; Barnard, A. J., Jr. Encyclopedia of Industrial Chemical Analysis; Interscience: New York, 1967; Vol. 4, p 102.
- 2 (a) Weber, H.; Khorana, H. G. J. Mol. Biol. 1972, 72, 219 (b) Zhdanov, R. I.; Zhenodarova, S. M. Synthesis 1975, 222.
- 3 Mariella, R. P.; Brown, K. H. J. Org. Chem. 1971, 36, 735 and references cited therein.
- 4 Zervas, L.; Photaki, I.; Ghelis, N. J. Am. Chem. Soc. 1963, 85, 1337.
- 5 Albright, J. D.; Goldman, L. J. Am. Chem. Soc. 1967, 89, 2416.
- 6 (a) Buck, J. S.; Ide, W. S. Org. Synth., Coll. Vol 1943, 2, 622. (b) White, D. M. J. Org. Chem. 1974, 39, 1951. (c) Nicolet, B. H.; Pelc, J. J. J. Am. Chem. Soc. 1922, 44, 1145. (d) Bell, M. R.; Johnson, J. R.; Wildi, B. S.; Woodward, R. B. J. Am. Chem. Soc. 1958, 80, 1001.
- 7 (a) Pummerer, R. Biochemistry 1910, 43, 1401. (b) Parham, W. E.; Edwards, L. D. J. Org. Chem. 1968, 33, 4150. (c) Tanikaga, R.; Yabuki, Y.; Ono, N.; Kaji, A. Tetrahedron 1976, 2257.
- 8
(a)
Rosen, T.
Comprehensive Organic Synthesis
1991,
2,
395.
10.1016/B978-0-08-052349-1.00034-2 Google Scholar(b) Merchant, J. R.; Gupta, A. S. Chem. Ind. (London) 1978, 628.
- 9 Polonovski, M.; Polonovski, M. Bull. Soc. Claim. Fr. 1927, 41, 1190.
- 10 Katada, M. J. Pharm. Soc. Jpn. 1947, 67, 51.
- 11
(a)
Thiele, J.
Biochemistry
1898,
31,
1247.
(b)
Thiele, J.;
Winter, E.
Justus Liebigs Ann. Chem./Liebigs Ann. Chem.
1899,
311,
341.
10.1002/jlac.19003110305 Google Scholar
- 12 (a) Peet, N. P.; Cargill, R. L. J. Org. Chem. 1973, 38, 1215. (b) Goldsmith, D. J.; Kennedy, E.; Campbell, R. G. J. Org. Chem. 1975, 40, 3571.
- 13 (a) Bedoukin, P. Z. Org. Synth., Coll. Vol 1955, 3, 127. (b) Cousineau, T. J.; Cook, S. L.; Secrist, J. A., III Synth. Commun. 1979, 9, 157.
- 14 Kochhar, K. S.; Bal, B. S.; Deshpande, R. P.; Rajadhyaksha, S. N.; Pinnick, H. W. J. Org. Chem. 1983, 48, 1765.
- 15 For analysis by titration, see Ref. 1c. Spectra available from the Aldrich Library.
- 16 Vogel's Textbook of Practical Organic Chemistry, 4th ed.; Longman: Harlow, 1978; p 499.
- 17 Perrin, D. D.; Armarego, W. L. F.; Perrin, D. R. Purification of Laboratory Chemicals, 2nd ed.; Pergamon: Oxford, 1985; p 77.
- 18 Stork, G.; Takahashi, T.; Kawamoto, I.; Suzuki, T. J. Am. Chem. Soc. 1978, 100, 8272.
- 19 Hölfe, G.; Steglich, W.; Vorbrüggen, H. Angew. Chem. Int. Ed. Engl. 1978, 17, 569.
- 20 Vedejs, E.; Diver, S. T. J. Am. Chem. Soc. 1993, 115, 3358.
- 21 Oppenauer, R. V. Monatsh. Chem. 1966, 97, 62.
- 22 Dauben, W. G.; Bunce, R. A.; Gerdes, J. M.; Henegar, K. E.; Cunningham, A. F., Jr.; Ottoboni, T. B. Tetrahedron 1983, 24, 5709.
- 23 Nagao, Y.; Fujita, E.; Kohno, T.; Yagi, M. Chem. Pharm. Bull. 1981, 29, 3202.
- 24 Vogel's Textbook of Practical Organic Chemistry, 4th ed.; Longman: Harlow, 1978; pp 454–455.
- 25 See Ref. 1(b) and Candoros, F., Rom. Patent 85 726, 1984; Chem. Abstr. 1985, 103, 104 715.
- 26 Barrett, A. G. M.; Lana, J. C. A. Chem. Commun./J. Chem. Soc., Chem. Commun. 1978, 471.
- 27 Tiffeneau, M.; Fuhrer, K. Bull. Soc. Claim. Fr. 1914, 15, 162.
- 28 Fujita, T.; Suga, K.; Watanabe, S. Aust. J. Chem. 1974, 27, 531.
- 29 Freter, K.; Zeile, K. Chem. Commun./J. Chem. Soc., Chem. Commun. 1967, 416.
- 30 (a) Dakin, H. D.; West, R. J. Biol. Chem. 1928, 78, 745, 757 (b) Allinger, N. L.; Wang, G. L.; Dewhurst, B. B. J. Org. Chem. 1974, 39, 1730. (c) Buchanan, G. L. Chem. Scr. 1988, 17, 91.
- 31 (a) Ferles, M. Collect. Czech. Chem. Commun. 1964, 29, 2323. (b) Rueppel, M. L.; Rapoport, H. J. Am. Chem. Soc. 1972, 94, 3877.
- 32 Glebova, Z. I.; Uzlova, L. A.; Zhdanov, Y. A. Zh. Obshch. Khim. 1985, 55, 1435; Chem. Abstr. 1986, 104, 69 072.
- 33 Hanessian, S.; Rancourt, G. Can. J. Chem. 1977, 55, 1111.
- 34 Newman, M. S.; Davis, C. C. J. Org. Chem. 1967, 32, 66.
- 35 (a) Albright, J. D.; Goldman, L. J. Am. Chem. Soc. 1965, 87, 4214. (b) J. Org. Chem. 1965, 30, 1107.
- 36 (a) Blanc, H. G. C.R. Hebd. Seances Acad. Sci. 1907, 144, 1356. (b) Ruzicka, L.; Prelog, V.; Meister, P. Helv. Chim. Acta 1945, 28, 1651.
- 37 Beringer, F. M.; Ugelow, I. J. Am. Chem. Soc. 1953, 75, 2635, and references cited therein.
- 38
Newnan, M. S.;
Hung, W. M.
J. Org. Chem.
1973,
38,
4073.
10.1021/jo00987a029 Google Scholar
- 39 Boar, R. B.; McGhie, J. F.; Robinson, M.; Barton, D. H. R.; Horwell, D. C.; Stick, R. V. J. Chem. Soc., Perkin Trans. 1 1975, 1237.
- 40 Zentmyer, D. T.; Wagner, E. C. J. Org. Chem. 1949, 14, 967.
- 41 Iruichijima, S.; Maniwa, K.; Tsuchihashi, G. J. Am. Chem. Soc. 1974, 96, 4280.
- 42 Iriuchijima, S.; Maniwa, K.; Tsuchihashi, G. J. Am. Chem. Soc. 1975, 97, 596.
- 43 (a) Monteiro, H. J.; de Souza, J. P. Tetrahedron 1975, 921. (b) Monteiro, H. J.; Gemal, A. L. Synthesis 1975, 437.
- 44 Watanabe, M.; Nakamori, S.; Hasegawa, H.; Shirai, K.; Kumamoto, T. Bull. Chem. Soc. Jpn. 1981, 54, 817.
- 45 Morin, R. B.; Jackson, B. G.; Mueller, R. A.; Lavagnino, E. R.; Scanlon, W. S.; Andrews, S. L. J. Am. Chem. Soc. 1963, 85, 1896.
- 46 Chauvette, R. R.; Pennington, P. A.; Ryan, C. W.; Cooper, R. D. G.; Jose, F. L.; Wright, I. G.; Van Heyningen, E. M.; Huffman, G. W. J. Org. Chem. 1971, 36, 1259.
- 47 Smith, A. B., III; Dorsey, B. D.; Visnick, M.; Maeda, T.; Malamas, M. S. J. Am. Chem. Soc. 1986, 108, 3110.
- 48 (a) See Ref. 9. (b) Polonovski, M.; Polonovski, M. Bull. Soc. Claim. Fr. 1926, 39, 147. (c) Wenkert, E. Experientia 1954, 10, 346. (d) Cave, A.; Kan-Fan, C.; Potier, P.; LeMen, J. Tetrahedron 1967, 23, 4681.
- 49 Stütz, P.; Stadler, P. A. Tetrahedron 1973, 5095.
- 50 Tamagaki, S.; Kozuka, S.; Oae, S. Tetrahedron 1970, 26, 1795.
- 51 (a) Kobayashi, G.; Furukawa, S. Chem. Pharm. Bull. 1953, 1, 347. (b) Boekelheide, V.; Lim, W. J. J. Am. Chem. Soc. 1954, 76, 1286. (c) Bullit, O. H., Jr.; Maynard, J. T. J. Am. Chem. Soc. 1954, 76, 1370. (d) Berson, J. A.; Cohen, T. J. Am. Chem. Soc. 1955, 77, 1281.
- 52 Knoevenagel, E. Justus Liebigs Ann. Chem./Liebigs Ann. Chem. 1914, 402, 111.
- 53 Ganem, B.; Small, V. R., Jr. J. Org. Chem. 1974, 39, 3728.
- 54 (a) Youssefyeh, R. D.; Mazur, Y. Tetrahedron 1962, 1287. (b) Narayanan, C. R.; Iyer, K. N. Tetrahedron 1964, 759.
- 55 Goldsmith, D. J.; Kennedy, E.; Campbell, R. G. J. Org. Chem. 1975, 40, 3571.
- 56 (a) Voaden, D. J.; Waters, R. M. Org. Prep. Proced. Int. 1976, 8, 227. (b) For conversion of ROTBS to ROAc using Ac2O and FeCl3, see Ganem, B.; Small, V. R. J. Org. Chem. 1974, 39, 3728.
- 57 Babler, J. H.; Olsen, D. O. Tetrahedron 1974, 351.
- 58
Eggette, T. A.;
deBoer, J. J. J.;
deKoning, H.;
Huisman, H. O.
Synth. Commun.
1978,
8,
353.
10.1080/00397917808063557 Google Scholar
- 59 Rosenmund, K. W.; Herzberg, H.; Schutt CB 1954, 87, 1258.
- 60 (a) Rigby, J. H.; Senanayake, C. J. Am. Chem. Soc. 1987, 109, 3147. (b) Rigby, J. H.; Senanayake, C. J. Org. Chem. 1988, 53, 440.
- 61 Ahmad, S.; Iqbal, J. Chem. Commun./J. Chem. Soc., Chem. Commun. 1987, 692.
- 62 Fry, A. J.; Rho, A. K.; Sherman, L. R.; Sherwin, C. S. J. Org. Chem. 1991, 56, 3283.
- 63 Friary, R. J., Jr.; Gilligan, J. M.; Szajewski, R. P.; Falci, K. J.; Franck, R. W. J. Org. Chem. 1973, 38, 3487.
- 64 Meth-Cohn, O.; Suschitzky, H. In Advances in Heterocyclic Chemistry; A. R. Katritzky; J. Boulton, Eds.; Academic Press: New York, 1972; Vol. 14, p 213.
- 65 Bélanger, A.; Lambert, Y.; Deslongchamps, P. Can. J. Chem. 1969, 47, 795.
- 66 Rao, Y. S.; Filler, R. Tetrahedron 1975, 1457.
- 67 (a) Rhoads, S. J.; Raulins, N. R. Org. React. 1975, 22, 1. (b) Karanewsky, D. S.; Kishi, Y.; J. Org. Chem. 1976, 41, 3026.
- 68 Gryer, R. I.; Brust, B.; Earley, J. V.; Sternbach, L. H. J. Chem. Soc. (C) 1967, 366.
- 69 Kablaoui, M. S. J. Org. Chem. 1974, 39, 2126.
- 70 Kablaoui, M. S. J. Org. Chem. 1974, 39, 3696.
- 71 Büchi, G.; Wüest, H. J. Org. Chem. 1979, 44, 4116.
- 72 Edwards, J. D.; McGuire, S. E.; Hignite, C. J. Org. Chem. 1964, 29, 3028.



