Ticks From Mink and Their Associated Microorganisms in Spain
Abstract
Wildlife is an important source of emerging zoonotic agents, including tick-borne ones. Wild carnivores such as mink are commonly parasitized by ticks, which are vectors and reservoirs of zoonotic diseases. Besides the importance of these arthropods as potential sources of diseases in mink, and the role of these mammals as reservoirs of infectious diseases, scarce studies of microorganisms of ticks from mink have been performed. In the present work, ticks collected from mink (European mink [Mustela lutreola] and American mink [Neogale vison]) from 2007 to 2021 in the North of Spain, and their associated microorganisms (bacteria, protozoan, and viruses), were studied. A total of 916 specimens (154 Ixodes acuminatus, 761 Ixodes hexagonus, and one Rhipicephalus sanguineus sensu lato) were processed in 165 pools (31 I. acuminatus, 133 I. hexagonus, and one R. sanguineus s.l.). The microorganisms detected in I. acuminatus pools were Ehrlichia sp. (8), Neoehrlichia mikurensis (4), Coxiella spp. (23), Rickettsiella spp. (7), and Ixovirus spp. (3). In I. hexagonus pools, Coxiella spp. (131), Rickettsiella spp. (5), Hepatozoon martis (5), and Ixovirus spp. (6) were amplified. Infection with Coxiella spp. was found in the R. sanguineus s.l. specimen. Mink are involved in the epidemiology of tick-borne microorganisms, including important pathogens. The role of these tick species as vectors and mink as reservoirs of these microorganisms should be further investigated.
1. Introduction
At least 60% of the human emerging infectious disease events (the first detection of case/s of emerging infectious diseases in a human population) are zoonotic and are increasing significantly over time [1]. Around 72% of these events have originated in wildlife, a source of diseases that not only threatens human health (zoonotic spillover) but also domestic and other free-ranging animals (cross-species transmission), especially endangered species [1–3]. Thus, the range of host species susceptible to some of the zoonosis that are current threats to global health, COVID-19 or influenza among others, is increasing and includes several animal species, such as mink, that are not involved in the origin of the pathogens but are involved in their evolution [4–6]. Vector-borne diseases, including those transmitted by ticks, are also responsible for emerging disease events [1]. Wildlife vertebrate species are involved in the enzootic cycles of tick-borne diseases as hosts of tick-vectors (amplifiers and dispersers of ticks) and as hosts, amplifiers and/or reservoirs of tick-borne pathogens. As an example, recent studies have demonstrated that farmed mink also suffer from severe fever with thrombocytopenia syndrome (SFTS), an emerging zoonotic viral tick-borne disease endemic in Asia [7]. Tick-borne diseases represent a worldwide health concern, being ticks the most important vectors of infectious diseases for animals and the second most important for humans, only after mosquitoes [8]. Thus, Crimean-Congo haemorrhagic fever (CCHF), a widespread disease recognized by the World Health Organization as a priority disease for research and development in emergency contexts [9] is worth mentioning.
The European mink (Mustela lutreola [Linnaeus 1761]; hereafter EM) is one of the most threatened small carnivores in the world. This mustelid (Carnivora; Mustelidae) is classified as critically endangered worldwide, and only isolated fragmented populations occur in Russia, Estonia, Ukraine, Germany, Romania, France, and Spain [10]. The latest estimation is of just a couple 1000 individuals [11]. The reduction of populations and the range of distribution of the EM in Europe involves several causes, mainly dependent on human intervention, such as the habitat loss/degradation, road-kills, overhunting, and introduction of alien species [12–14]. Regarding the latter matter, the invasive American mink (Neogale vison [von Schreber, 1776]; hereafter AM), introduced in Europe for fur farming in the 1920s and by the early 2000s, is one of the main factors in the reduction of EM [14]. The intraguild aggression, but also competition for habitats and food resources, and possibly the introduction of diseases, particularly that caused by the Aleutian Disease Virus, make the AM eradication one of the strengths of the EM conservation programmes [11, 14, 15].
Despite the importance of mustelids as hosts of arthropod vectors, studies of ticks from mink and their associated microorganisms, including those that could adversely affect them, are scarce [7, 16–23]. In the present work, ticks collected in Spain from two mustelid species, the critically endangered EM and the alien AM, and their associated microorganisms were studied.
2. Materials and Methods
The current study was carried out from 2007 to 2021 in the north of Spain, in the distribution area of the EM. The Spanish EM population was estimated in 500 specimens in 2014 in territories occupied routinely or sporadically by the AM [11, 13]. In this period, the Spanish Government and Regional Governments implemented different EM conservation programmes, as well as EU-financed programmes were carried out (LIFE GERVE [2007], LIFE Territorio Vison [2010–2015], LIFE LUTREOLA SPAIN/LIFE13NAT/ES/001171 [2014–2019]; in the frame of “Estrategia Nacional de Conservación del Visón Europeo” [MMARM, 2009]). During the different interventions carried out in these projects, live-trapping was used as a principal method for EM monitoring and AM culling. Captured EM were anesthetized and clinically examined in accordance to the Spanish Policy for Animal Protection RD 53/2013, Law 32/2007 and Law 6/2013 (which meets the European Union legislation), and when possible, they were inspected for the presence of ticks. After recovering from anaesthesia, the specimens were released at the capture site. AM were also anesthetized, and after data collection when the specimens were still under anesthesia, they were sacrificed following the welfare legal standards [15].
Ticks were removed from anesthetized EM individuals or from the AM carcasses using forceps. The inspection of captured mink specimens, as well as the removal and storage of ticks, was carried out in five wildlife rescue centres (Martioda [(Alava], Cabárceno [Cantabria], La Fombera [La Rioja], Ilundain [Navarra] and La Alfranca [Zaragoza]) by a responsible veterinarian, assisted by a field technician. Tick specimens from each host were collected separately. Data about the identification of the mink specimens, the area, and the date of collection were recorded. From 2007 to 2014, all the ticks were preserved in ethanol 70%. From 2015 to 2021, they were kept alive until tick identification, and immediately frozen at −80°C (or sporadically preserved in ethanol 70% if they were not received in the lab within seven days after collection).
Ticks were classified using taxonomic keys [24, 25]. Morphological identification was confirmed in selected specimens using molecular biology techniques (Table S1) [26, 27].
Ticks were pooled according to the species, stage, host, area, and preservation method (ethanol/frozen). Tick individuals were surface-sterilized by immersion in benzalkonium chloride 0.1% (5 min), ethanol 70% (1 min), rinsed twice in sterile deionized water and air-dried. Each pool containing immature specimens or halves of adult specimens was used for nucleic acid extraction. Those pools containing specimens preserved in ethanol were only processed for DNA extraction using the DNeasy Blood and Tissue kit (Qiagen, Hilden, Germany) following the manufacturer’s recommendations with an overnight incubation. Pools with frozen samples were homogenized in 300 μL of Dulbecco’s Modified Eagle Medium (DMEM) with 10,000 units penicillin and 10 mg streptomycin/mL (Sigma, USA). DNA was obtained from one-third of the homogenate as mentioned above. In addition, RNA was extracted from a 100 μL-aliquot using the RNeasy Mini kit (Qiagen, Hilden, Germany), and the remaining homogenate (100 μL) was preserved at −80°C for future analysis. RNA was retrotranscribed using Omniscript (Qiagen, Hilden, Germany).
Quality of nucleic acid extracts and control of PCR inhibitors were checked using a PCR assay that amplified partial mitochondrial 16S rRNA gene of ticks (Table S1) [26]. Positive samples were then analyzed for the presence of microorganisms. All pools were studied for the presence of bacteria (Anaplasma, Ehrlichia, Neoehrlichia, Borrelia, Rickettsia, Coxiella, Rickettsiella, Francisella, and Spiroplasma species) and protozoa (Babesia, Theileria, Hepatozoon, and Trypanosoma species) using specific PCR assays. Moreover, pools from frozen ticks were also analyzed for Orthonairovirus, Flaviviridae, and Phenuiviridae viruses (Table S1) [28–52].
Negative controls of extraction, negative controls of PCR (water instead of nucleic acid extracts) and positive controls containing DNA of Anaplasma phagocytophilum [53], Ehrlichia sp. [54], Neoehrlichia mikurensis [53], Coxiella sp. [55], Rickettsia amblyommatis [56], Babesia bigemina [54], or Spiroplasma sp. [54] from the Centre of Rickettsioses and Arthropod-Borne Diseases (CRETAV) collection (“Zoonosis collection” registered in the National Registry of Biobanks from Carlos III Health Institute [Reference: C.0006409], located at CRETAV-CIBIR [Biomedical Research Center of La Rioja], Spain) or synthetic DNA from Coxiella burnetii or Francisella tularensis (AMPLIRUN, Vircell, Spain) or DNA/RNA extracts of Borrelia spielmannii, Trypanosoma brucei, Orthoflavivirus japonicum or Uukuniemi uukuniemiense, or lyophilized sera specimens from CCHF-infected humans, kindly provided by colleagues (please see acknowledgements section), were used.
PCR products were visualized by 1% agarose gel electrophoresis. Amplicons of the expected size were subjected to Sanger sequencing at the Molecular Diagnostic Department (CIBIR, Logroño, Spain). Nucleotide sequences were analyzed using the BLAST search (https://blast.ncbi.nlm.nih.gov/Blast.cgi), and new sequences were submitted to GeneBank (https://submit.ncbi.nlm.nih.gov/). The Clustal Omega online software (https://www.ebi.ac.uk/Tools/msa/clustalo/) was used for multiple sequence alignment. Phylogenetic analyses were conducted with MEGA XI (http://www.megasoftware.net) using the maximum likelihood method, including all sites. The nucleotide substitution model was selected according to the Akaike information criterion implemented in MEGA XI. Confidence values for the individual branches of the resulting trees were determined by bootstrap analysis with 500 replicates.
3. Results
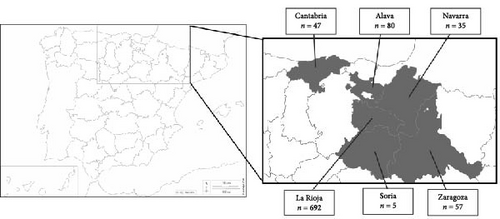
A total of 916 ticks were collected from mink in northern Spain from 2007 to 2021: 755 ticks were removed from 147 EM individuals (13 of them were recaptured, 12 twice and one three times), and 161 from 51 AM individuals (Tables S2 and S3). According to the area, 80 were collected in Alava, 47 in Cantabria, 692 in La Rioja, 35 in Navarra, five in Soria and 57 in Zaragoza (Figure 1, Table 1 and Tables S2 and S3). All but one Rhipicephalus sanguineus sensu lato specimen belonged to Ixodes genus, represented with two species, Ixodes acuminatus (n = 154) and Ixodes hexagonus (n = 761). The number of ticks collected in a single EM and AM ranged from 1 to 39 and from 1 to 13, respectively (Tables S2 and S3).
| Host | Tick species | Tick stage | Area | Preserved in ethanol | Preserved frozen | ||
|---|---|---|---|---|---|---|---|
| No. of ticks | No. of pools | No. of ticks | No. of pools | ||||
| EM | I. acuminatus | Females | La Rioja | 64 | 14 | 41 | 5 |
| Alava | 1 | 1 | 1 | 1 | |||
| Zaragoza | 1 | 1 | 12 | 2 | |||
| Navarra | — | — | 4 | 1 | |||
| Males | La Rioja | 1 | 1 | — | — | ||
| I. hexagonus | Females | Alava | 15 | 6 | — | — | |
| La Rioja | 139 | 47 | 61 | 13 | |||
| Navarra | — | — | 5 | 2 | |||
| Males | Alava | 1 | 1 | — | — | ||
| La Rioja | — | — | 1 | 1 | |||
| Nymphs | Alava | 17 | 2 | 6 | 1 | ||
| La Rioja | 185 | 19 | 91 | 5 | |||
| Navarra | — | — | 19 | 2 | |||
| Zaragoza | 5 | 1 | — | — | |||
| Larvae | Alava | 39 | 4 | — | — | ||
| La Rioja | 11 | 2 | 16 | 2 | |||
| Navarra | — | — | 7 | 1 | |||
| Zaragoza | 11 | 1 | — | — | |||
| R. sanguineus s.l. | Male | La Rioja | 1 | 1 | — | — | |
| AM | I. acuminatus | Females | La Rioja | 25 | 3 | 2 | 1 |
| Cantabria | — | — | 2 | 1 | |||
| I. hexagonus | Females | Cantabria | — | — | 21 | 4 | |
| La Rioja | 9 | 3 | 19 | 3 | |||
| Soria | — | — | 4 | 1 | |||
| Zaragoza | 7 | 2 | — | — | |||
| Male | La Rioja | — | — | 1 | 1 | ||
| Nymphs | Cantabria | — | — | 24 | 2 | ||
| La Rioja | 14 | 2 | 11 | 1 | |||
| Soria | — | — | 1 | 0a | |||
| Zaragoza | 16 | 3 | — | — | |||
| Larvae | Zaragoza | 5 | 1 | — | — | ||
- Abbreviations: AM, American mink; EM, European mink; I. acuminatus, Ixodes acuminatus; I. hexagonus, Ixodes hexagonus; R. sanguineus s.l., Rhipicephalus sanguineus sensu lato.
- aThe nymph specimen collected from an AM in Soria was processed with nymphs from La Rioja.
The morphological classification was conclusive for all the samples. Nevertheless, two I. acuminatus and three I. hexagonus female specimens were genetically characterized using the 16S rRNA and 12S rRNA fragment genes. On the one hand, the two obtained 16S rRNA sequences of I. acuminatus were 99.5% identical to each other. One of these sequences (90% query cover) was homologous, and the other one showed 99.75% identity with available sequences of I. acuminatus (GenBank accession number [GenBank acc. No.] OL352912 and MH708166, respectively). There were no available public sequences of the 12S rRNA gene of I. acuminatus, and the analysis of the two sequences, identical to each other, showed identities <92% with other Ixodes species. On the other hand, the 16S rRNA sequences obtained from I. hexagonus were identical with each other and identical to those from public I. hexagonus sequences (GenBank acc. No. MW114503 and AF549844). Moreover, one of the 12S rRNA sequences belonging to this species was homologous to a public I. hexagonus sequence (GenBank acc. No. AF081828), while the remaining two sequences showed a single change, reaching 99.7% identity with other public sequences.
Ticks were grouped in 165 pools, 115 corresponding to the specimens preserved in ethanol (R. sanguineus s.l.: 1 pool/1 specimen; I. acuminatus: 20 pools/92 specimens; I. hexagonus: 94 pools/474 specimens) and 50 corresponding to those preserved frozen (I. acuminatus: 11 pools/62 specimens; I. hexagonus: 39 pools/287 specimens) (Table 1). Individual male specimens and pools including 5–11 larvae, 2–21 nymphs, 1–12 I. acuminatus females, and 1–6 I. hexagonus females were processed.
All the pools gave positive results in the 16S rRNA-PCR assays and were analyzed for the microorganism screening. Table 2 summarizes the obtained results according to host, tick species and stage, and area. The detailed data obtained in each pool are shown in the Table S4.
| Tick species | Host | Stage | No. of ticks/No. of pools | Microorganisms, No. of positive pools/MIR (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| For bacterial and protozoan screening | For viruses screening | Ehrlichia sp. | N. mikurensis | Coxiella spp. | Rickettsiella spp. | H. martis | Phenuiviruses | |||
| I. acuminatus | EM | Females | 124/25 | 58/9 | 6/4.8 | 3/2.4 | 19/15.3 | 5/4.0 | 0 | 2/3.5 |
| AM | Females | 29/5 | 4/2 | 2/6.9 | 1/3.5 | 3/10.4 | 2/6.9 | 0 | 1/25 | |
| Male | 1/1 | 0 | 0 | 0 | 1/100 | 0 | 0 | 0 | ||
| Total I. acuminatus | — | — | 154/31 | 61/11 | 8/5.2 | 4/2.6 | 23/14.8 | 7/4.5 | 0 | 3/4.8 |
| I. hexagonus | EM | Larvae | 84/10 | 23/3 | 0 | 0 | 10/11.9 | 0 | 0 | 0 |
| Nymphs | 323/30 | 116/8 | 0 | 0 | 30/9.3 | 0 | 1/0.3 | 0 | ||
| Females | 220/68 | 66/15 | 0 | 0 | 68/30.9 | 2/0.9 | 3/1.4 | 4/6.1 | ||
| Males | 2/2 | 1/1 | 0 | 0 | 2/100 | 0 | 0 | 0 | ||
| AM | Larvae | 5/1 | 0 | 0 | 0 | 1/20.0 | 0 | 0 | 0 | |
| Nymphs | 66/8 | 36/3 | 0 | 0 | 7/10.6 | 2/3.0 | 0 | 0 | ||
| Females | 60/13 | 44/8 | 0 | 0 | 12/20.0 | 1/1.7 | 1/1.7 | 2/4.6 | ||
| Male | 1/1 | 1/1 | 0 | 0 | 1/100 | 0 | 0 | 0 | ||
| Total I. hexagonus | — | — | 761/133 | 287/39 | 0 | 0 | 131/17.2 | 5/0.7 | 5/0.7 | 6/2.1 |
| R. sanguineus s.l. | EM | Male | 1/1 | 0 | 0 | 0 | 1/100 | 0 | 0 | 0 |
| Total | — | — | 916/165 | 349/50 | 8/0.9 | 4/0.4 | 155/16.9 | 12/1.3 | 5/0.6 | 9/2.6 |
- Abbreviations: AM, American mink (Neogale vison); EM, European mink (Mustela lutreola); H., Hepatozoon; I., Ixodes; MIR, minimum infection rate (No. of positive pools/No. of ticks analyzed); N., Neoehrlichia; R., Rhipicephalus; s.l., sensu lato.
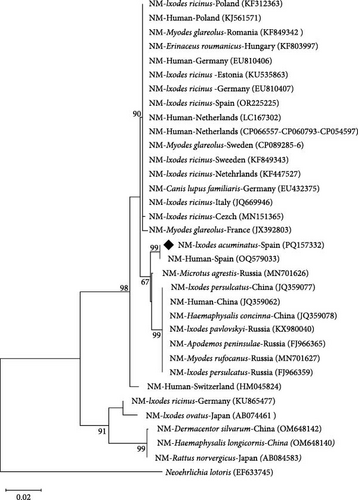
Ehrlichia sp. was amplified from 25.8% of the I. acuminatus pools (n = 8 from La Rioja; Table 2 and Table S4). Identical sequences were obtained for each of the four fragment genes amplified in all these pools, and the analysis of the nucleotide sequences showed the highest identity with Ehrlichia sp. HF [57]. Specifically, groESL, gltA, 16S rRNA, and rpoB gene fragments reached identities of 99.21%, 97.48%, 99.59%, and 97.57%, respectively, with the available complete genome of Ehrlichia sp. HF (GenBank acc. No. CP007474), proposed as Ehrlichia japonica sp. nov. [58]. The gltA and 16S rRNA genes were homologous (100% identity) with partial sequences of this strain (GenBank acc. No. DQ647319 and DQ647318, respectively). The phylogenetic analysis of groESL fragment gene supported the clustering with Ehrlichia sp. HF (Figure 2).

N. mikurensis was detected in four (from La Rioja) out of 31 I. acuminatus pools (12.9%) (Table 2 and Table S4). The same genotype was amplified in all the positive pools. The obtained groEL sequences were identical to that detected in a patient from Spain (GenBank acc. No. OQ579033 [59]), an isolate related to Asian strains (GenBank acc. No. MN701626 [60]). The 305 bp 16S rRNA fragment gene of this human isolate from Spain (GenBank acc. No. OQ581737) was also homologous to the one amplified herein. Taking into account a query cover >90%, the analysis of the 16S rRNA fragment gene showed 100% identity with sequences obtained from European ticks different from I. acuminatus (GenBank acc. No. KF155493, OP269536, and KF155487, among others), and with 100% query cover they showed a 99.9% identity with sequences from Europe and Asia, including sequences from humans (GenBank acc. No. MN736127, KU535862, CP089286, and CP066557, among others). The rpoB sequences showed a 98.8% as highest identity with any of the scarce available sequences from GenBank (only those with complete genome: CP089285-6, CP066557, CP054597, and CP060793).
A maximum likelihood tree based on groEL sequences was inferred. The sequences obtained in this study formed an independent cluster with sequences obtained from a patient that suffered for neoehrlichiosis from Spain (GenBank acc. No. OQ579033 [59]) (Figure 3).
Coxiella and/or Rickettsiella were detected in all but two Ixodes pools (30 I. acuminatus and 132 I. hexagonus pools), in all the studied areas, and in the R. sanguineus s.l. sample using the selected PCR assays targeting 16S rRNA, rpoB, and groEL genes (Table 2 and Table S4).
Coxiella was found in the R. sanguineus s.l. pool, in 23 pools of I. acuminatus and in 131 pools of I. hexagonus. The three PCR target genes (16S rRNA, rpoB, and groEL) showed amplicons of the expected size for R. sanguineus s.l. Unfortunately, only rpoB sequences were readable and showed 99.7% identity with an uncultured Coxiella sequence obtained from a questing R. sanguineus s.l. found in the same area (Unpublished data, GenBank acc. No. OM302467). According to the sequences of the 16S rRNA (n = 29), rpoB (n = 138), and groEL (n = 137) gene fragments, similar Coxiella genotypes were detected in both Ixodes species. The 29 sequences of the 16S rRNA gene fragment were identical to each other and identical to Coxiella endosymbiont sequences found in Ixodes ricinus (GenBank acc. No. KP994826 and KP994825) and showed two changes with those available from Coxiella endosymbiont of I. hexagonus (GenBank acc. No. KP994823 and KP994824). All but one of the rpoB sequences (n = 137) were identical to each other and indistinguishable (100% identity) from Coxiella endosymbionts of I. hexagonus (GenBank acc. No. KP985318, KP985319 and MK248731) and I. ricinus (GenBank acc. No. KP985320 and KP985321). The remaining rpoB sequence showed a single point mutation. All the 137 groEL sequences were homologous and showed 100% identity with Coxiella endosymbiont single sequences of I. ricinus and Ixodes uriae (GenBank acc. No. KP985502 and KJ459059, among others) and showed a single change with those available from I. hexagonus (GenBank acc. No. KP985500 and KP985501). Sequences of Coxiella amplified from I. acuminatus were not available for any of the primer pairs.
Rickettsiella sp. was detected in seven I. acuminatus and five I. hexagonus pools, based on the analysis of five 16S rRNA, one rpoB and 11 groEL nucleotide sequences obtained. The 16S rRNA sequences were homologous to that from Candidatus Rickettsiella isopodorum (GenBank acc. No. JX406180). The rpoB sequence showed the highest identity (90.4%) with Rickettsiella melolonthae (GenBank acc. No. EF694042). The analysis of the groEL sequences, all identical with each other, revealed that they were homologous to others previously found in Ixodes trianguliceps, Ixodes ventalloi, and Ixodes arboricola (GenBank acc. No. KY678003, KY677996, and KY677997, among others). Neither Rickettsiella sequences from I. hexagonus nor from I. acuminatus were available.
Anaplasma, Borrelia, Rickettsia, Francisella, or Spiroplasma species were not amplified in any samples.
Hepatozoon martis was the unique protozoan detected. It was found in five I. hexagonus pools from La Rioja, one formed with nymphs and four with females (Table 2 and Table S4). The 18S RNA sequences were identical with each other, reaching 100% of identity with the public sequences of H. martis (GenBank acc. No. EF222257 and KU198330, among others) amplified from mustelids from different European countries, including Spain, such as Martes foina (MG136688), Martes martes (GenBank acc. No. MG136687, OM256567, EF222257, and EU686690) and Meles meles (GenBank acc. No. KU198330), and from a Haemaphysalis concinna tick from Hungary (GenBank acc. No. OM256566).
Neither Trypanosoma, Babesia, or Theileria species were amplified in any samples.
Regarding viruses, the selected PCR assay for the Orthonairovirus detection yielded bands of the expected size in 32 out of 50 pools. Unfortunately, only a few sequences were readable, and the tick genome was detected (instead of the viruses). No Flaviviridae were detected using the selected PCR assays.
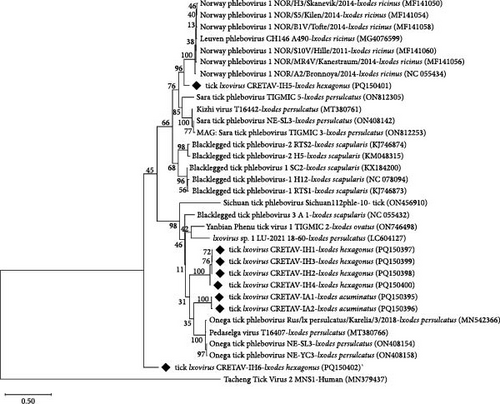
The two single PCR assays performed for the amplification of the L segment of the Phenuiviridae viruses yielded negative results. Using the same primer pairs in a semi-nested assay designed for this study (Table S1), positive results were obtained for 12 pools from La Rioja (Table 2 and Table S4). After sequence analysis, the presence of phenuiviruses was confirmed in nine out of 12 pools, three I. acuminatus female, and six I. hexagonus female pools. Sequences showed eight different genotypes, two of them from I. acuminatus pools (IA1 was amplified in two pools, and IA2 in one pool) and six from I. hexagonus pools (IH1-IH6, one per pool). The nucleotide and amino acid sequences reached the highest identities (76.1%−87.5% and 81.5%−98.9%, respectively) with Ixovirus spp. sequences previously detected in different Ixodes spp. (Tables 3 and 4 and Figure 4), not in I. acuminatus or in I. hexagonus. The nucleotide sequences of IA1 and IA2 (from I. acuminatus) showed a silent point mutation. Four of the six genotypes (IH1−IH4) found in I. hexagonus were closely related (≥98% and 99.4% identity for nucleotides and proteins). Genotypes IH5 and IH6 (from I. hexagonus) were not similar to each other or to any of the other detected genotypes (Tables 3 and 4). The inferred phylogeny showed two clusters close to Ixovirus ixodis (Blacklegged tick phlebovirus 3), one containing the IA genotypes and the other one with four of the IH genotypes (IH1, IH2, IH3, and IH4). Moreover, the genotype IH5 clustered with Ixovirus norvergiae (Norway phlebovirus 1), but separately. Lastly, the genotype IH6 belonged to Ixovirus spp. but was independent and far from all known species (Figure 4).
| Virus | IA1 | IA2 | IH1 | IH2 | IH3 | IH4 | IH5 | IH6 |
|---|---|---|---|---|---|---|---|---|
| Tick Ixovirus CRETAV-IA1 | 100.00 | 99.79 | 76.84 | 77.05 | 76.84 | 77.26 | 67.80 | 69.26 |
| Tick Ixovirus CRETAV-IA2 | 99.79 | 100.00 | 76.63 | 76.84 | 76.63 | 77.05 | 67.80 | 69.26 |
| Tick Ixovirus CRETAV-IH1 | 76.84 | 76.63 | 100.00 | 99.37 | 99.58 | 97.70 | 69.70 | 69.05 |
| Tick Ixovirus CRETAV-IH2 | 77.05 | 76.84 | 99.37 | 100.00 | 99.37 | 97.91 | 70.13 | 69.47 |
| Tick Ixovirus CRETAV-IH3 | 76.84 | 76.63 | 99.58 | 99.37 | 100.00 | 97.70 | 69.70 | 69.05 |
| Tick Ixovirus CRETAV-IH4 | 77.26 | 77.05 | 97.70 | 97.91 | 97.70 | 100.00 | 69.92 | 69.05 |
| Tick Ixovirus CRETAV-IH5 | 67.80 | 67.80 | 69.70 | 70.13 | 69.70 | 69.92 | 100.00 | 73.31 |
| Tick Ixovirus CRETAV-IH6 | 69.26 | 69.26 | 69.05 | 69.47 | 69.05 | 69.05 | 73.31 | 100.00 |
| Onega tick phlebovirus-MN542366 I. persulcatus | 80.81 | 80.81 | 79.74 | 79.96 | 79.74 | 79.96 | 70.17 | 68.66 |
|
80.63 | 80.63 | 79.29 | 79.71 | 79.29 | 80.75 | 72.25 | 72.63 |
|
70.13 | 70.34 | 69.92 | 70.34 | 69.92 | 69.28 | 87.50 | 72.88 |
|
68.86 | 68.86 | 69.47 | 69.47 | 69.47 | 69.26 | 81.36 | 76.06 |
| Blacklegged tick phlebovirus 2-KJ746874 I. scapularis | 69.70 | 69.70 | 69.26 | 69.26 | 69.26 | 69.26 | 81.36 | 74.36 |
- Note: Identity values (%) are shown in the table. Highest identities are highlighted in bold.
| Virus | IA1 | IA2 | IH1 | IH2 | IH3 | IH4 | IH5 | IH6 |
|---|---|---|---|---|---|---|---|---|
| Tick Ixovirus CRETAV-IA1 | 100.00 | 100.00 | 84.81 | 85.44 | 84.81 | 86.08 | 70.51 | 72.78 |
| Tick Ixovirus CRETAV-IA2 | 100.00 | 100.00 | 84.81 | 85.44 | 84.81 | 86.08 | 70.51 | 72.78 |
| Tick Ixovirus CRETAV-IH1 | 84.81 | 84.81 | 100.00 | 99.37 | 98.74 | 98.73 | 72.44 | 72.78 |
| Tick Ixovirus CRETAV-IH2 | 85.44 | 85.44 | 99.37 | 100.00 | 99.37 | 99.37 | 73.08 | 73.42 |
| Tick Ixovirus CRETAV-IH3 | 84.81 | 84.81 | 98.74 | 99.37 | 100.00 | 98.73 | 72.44 | 72.78 |
| Tick Ixovirus CRETAV-IH4 | 86.08 | 86.08 | 98.73 | 99.37 | 98.73 | 100.00 | 73.08 | 73.42 |
| Tick Ixovirus CRETAV-IH5 | 70.51 | 70.51 | 72.44 | 73.08 | 72.44 | 73.08 | 100.00 | 78.98 |
| Tick Ixovirus CRETAV-IH6 | 72.78 | 72.78 | 72.78 | 73.42 | 72.78 | 73.42 | 78.98 | 100.00 |
| Onega tick phlebovirus- QPD01619 I. persulcatus | 87.34 | 87.34 | 86.16 | 86.79 | 86.16 | 87.34 | 71.79 | 72.78 |
|
93.04 | 93.04 | 84.91 | 85.53 | 84.91 | 86.08 | 72.44 | 75.32 |
|
71.15 | 71.15 | 72.61 | 73.25 | 72.61 | 73.08 | 98.09 | 78.98 |
|
69.23 | 69.23 | 71.97 | 72.61 | 71.97 | 72.44 | 91.72 | 80.89 |
| Blacklegged tick phlebovirus 2-AII01807 I. scapularis | 69.87 | 69.87 | 72.61 | 73.25 | 72.61 | 73.08 | 92.99 | 81.53 |
- Note: Identity values (%) are shown in the table. Highest identities are highlighted in bold.
Following the International Committee on Taxonomy of Viruses (ICTV) species demarcation criteria (RdRP amino acid sequence >95% [61]), only genotype IH5 was identified at the species level, as I. norvergiae, and the remaining genotypes belonged to three potential new Ixovirus species (1. IA1 and IA2; 2. IH1, IH2, IH3, and IH4; 3. IH6). We propose the following names for these potential new species: Ixovirus vison for genotypes IA1-IA2, Ixovirus beronensis for genotypes IH1–IH2–IH3–IH4 and Ixovirus cretavensis for genotype IH6.
4. Discussion
To the best of our knowledge, the present work is the largest study of ticks from mink and their associated microorganisms. A total of 916 ticks collected from EM or AM during 15 years in the North of Spain have been analyzed for the presence of tick-borne bacteria, protozoan and viruses.
Apart from a single specimen of R. sanguineus s.l., the most prevalent tick species found infesting EM and AM in the North of Spain have been I. acuminatus and I. hexagonus. These two species, widely distributed in the Palearctic area, are common parasites of wild carnivores, including mustelids, and have been sporadically described biting humans. The later species also parasitize companion animals such as dogs and cats increasing the risk of exposure to pet owners [25, 62]. I. acuminatus and I. hexagonus have been previously reported in scarce specimens of AM and EM from Spain [16] and from other European countries [17, 19], and similarly with those studies, immature and adult specimens of the latter tick species have been found herein and only adult I. acuminatus specimens were collected. The host preferences for immature and adult stages of I. hexagonus are similar, but could be different for the stages of I. acuminatus [25]. The main hosts of I. acuminatus are insectivores and rodents, mink prey with which they share the habitat and favor the mink exposition to this tick species, but as our results suggest, preferably to adult specimens. At least five tick species had been found in mink in Europe (I. acuminatus, Ixodes canisuga, I. hexagonus, I. ricinus, and Ixodes rugicollis) and likewise in the present study, I. hexagonous had been the most prevalent [19]. I. ricinus, the most widespread tick species in Europe and in the study area, has not been detected in this work, but only a few specimens have been reported from mink [17, 19]. The semiaquatic habitat of mink and the use of dens as burrows are not favorable conditions for the development of exophilic tick species (such as I. ricinus) but they favor the presence of nest-dwelling tick species (such as I. acuminatus or I. hexagonus).
Herein, Ehrlichia HF has been amplified in I. acuminatus from mink. This Anaplasmataceae bacterium was discovered in Ixodes ovatus from Japan and has been recently proposed as new species, named E. japonica [57, 58, 63]. Its pathogenicity is still under study, but known virulence factors found in the highly pathogenic Ehrlichia chaffeensis affecting humans are also found in Ehrlichia HF [58]. Experimentally, this microorganism has been also proved to be highly virulent for immunocompetent mice [64]. Before this study, no Ehrlichia had been found in I. acuminatus. However, apart from I. ovatus in Japan, Ehrlichia HF had been detected in I. ricinus, Ixodes aphronophorus and R. sanguineus in Europe, but not in Spain [65–68]. Moreover, it had been detected in rodents and in a sick dog from Japan [63, 69]. A unique study about mink potentially infected with an Anaplasmataceae microorganism has been reported from Spain; unfortunately, it included only two mink specimens, and no sequence data for the microorganism identification was obtained [70]. The presence of this Ehrlichia in mink and the role of I. acuminatus as vector should be further investigated.
N. mikurensis is a tick-borne infectious agent responsible for the disease named neoehrlichiosis [71, 72]. The first human case in Spain, our study area, has been recently reported [59], and the obtained isolate from the clinical sample was identical to that detected in the present study. Unfortunately, the tick vector in this Spanish case was unknown, but the sequences differ from those previously detected in I. ricinus, including those available from Spain [53]. N. mikurensis had not been previously described in I. acuminatus, although it had been amplified in several tick species belonging to different genera (Ixodes, Dermacentor, Haemaphysalis, and Rhipicephalus), including I. hexagonus (negative in the present study). However, the recognized vectors are I. ricinus and Ixodes persulcatus, and the vector competence of other species should be demonstrated.
The finding of Ehrlichia HF and N. mikurensis in I. acuminatus ticks from mink infested with I. hexagonus or R. sanguineus s.l. specimens that were negative for these agents (Table S4) suggests the presence of E. japonica and N. mikurensis in the ticks instead of the blood meal. Nevertheless, further studies about the role of I. acuminatus as vector of these microorganisms are recommended.
Several maternally inherited tick endosymbionts are known. Among them, Coxiella-like (LE) and Rickettsiella-LE are nutritional providing intracellular bacteria required for tick survival and fitness. Ticks and their symbionts coevolve interdependently, but this association is not necessarily stable, and ancestral symbionts can be replaced by new ones [73]. The replacement of Coxiella-LE by Rickettsiella-LE in some tick species has been previously suggested [74], and our data support this fact since not previous detection of Rickettsiella has been reported in these tick species. Similar genotypes of each corresponding bacteria have been detected in I. acuminatus and I. hexagonus from mink in the studied area, with a higher prevalence of Coxiella-LE. Coxiella sp. detected in I. hexagonus and I. acuminatus belong to the same lineage as other Coxiella spp. from different Ixodes species, suggesting the ancient and stable coevolution of Coxiella endosymbionts with these tick species. Buysse et al. [75] showed tropism of this bacterium in the ovaries and Malpighian tubules in I. hexagonus, supporting its vertical (transovarial) transmission and the role of this bacterium as nutritional-providing. C. burnetii has been previously detected by qPCR assay in I. acuminatus from rodents in Italy [76]. According to our results and our own experience with qPCR assays designed for the C. burnetii detection prior to the knowledge of Coxiella-LE microorganisms, and the absence of sequencing confirmation in that work, a false C. burnetii positive result should be taken into account for that study. The presence of Rickettsiella has not been previously reported from these two Ixodes species but, identical groEL sequences have been detected in other species of the genus, such as I. arboricola, I. ventalloi, and I. trianguliceps [74]. Thus, Rickettsiella is prevalent in Ixodes genus, but it is not a microorganism exclusive to ticks and their role in tick functions is unclear [74, 77]. It has been suggested as a facultative nutritional symbiont but also as a reproductive symbiont, due to the possible cause of sex ratio distortions in parthenogenetic Ixodes woodi [78].
Until the first description of H. martis in European pine marten (M. martes) in Spain [79], this protozoon had been mainly found in mustelids through Europe, but also in other carnivores, rodents, and in several artiodactyl species [80, 81]. Moreover, its presence in AM specimens from Canada and in other mustelid species from Japan and Korea has been suggested due to the similarities in morphology, pathology and organ distributions, supported in specific cases by molecular biology [80]. The knowledge about the tick species involved in the transmission, via tick ingestion, of H. martis is scarce. A questing I. ricinus male and a pool of questing H. concinna nymphs have been found infected with H. martis in Europe, and a closely related genotype has also been detected in questing Haemaphysalis longicornis and Haemaphysalis hystricis in Japan [80–82]. The presence of H. martis in I. hexagonus has not been explicitly published, but it is worth mentioning that small sequence fragments obtained from specimens from foxes in Germany were identical to sequences obtained herein [83]. Our data support the implication of I. hexagonus in the epidemiology of this tick-borne microorganism, although their role as vectors remains unknown.
Viruses associated with ticks are widely distributed and are responsible for several emerging human diseases. Among them, the family Phenuiviridae includes many zoonotic species such as Dabie bandavirus (SFTS), Heartland virus (Heartland virus disease), Tacheng tick virus 2 (TcTV-2 infection), or Bhanja virus (BHAV infection) [84–88]. Thus, an outbreak of SFTS in farmed mink has been recently reported, and the virus transmission from infected mink to humans has been suggested [7, 89]. Moreover, the family Phenuiviridae also contains species with a high zoonotic potential prediction, such as I. norvergiae detected in this study [90]. Phenuiviruses have been previously detected in several tick species, although not from I. acuminatus neither I. hexagonus. Ixovirus species have been reported from I. ricinus and Ixodes scapularis in Europe, although not from the study area, possibly due to the lack of investigations [91, 92]. In the present research, two and six Ixovirus genotypes have been amplified in I. acuminatus and I. hexagonus, respectively. According to the ICTV species demarcation criteria, except for one representative of I. norvegiae, all the genotypes obtained in this study belong to novel Ixovirus species. Deeper studies should be performed, including the virus isolation, to better characterize these genotypes and to investigate their pathogenic potential.
5. Conclusions
This study supports the role of mink as important hosts for I. acuminatus and I. hexagonus and reports the sporadic infestation of mink by R. sanguineus s.l. The present research demonstrates the circulation of E. japonica and I. norvergiae in Spain, and the existence of potential new Ixovirus species (proposed as I. vison, I. beronensis and I. cretavensis) with unknown pathogenicity. More efforts should be made to investigate: (1) the role of I. acuminatus as a vector of E. japonica, N. mikurensis, and Ixovirus spp., (2) the role of I. hexagonus as a vector of H. martis and Ixovirus spp., (3) the role of mink as reservoirs of these tick-borne microorganisms, (4) the pathogenic potential of these microorganisms for mink and other animals and their zoonotic potential (already confirmed for N. mikurensis), (5) their presence in other areas, tick species or hosts, and (6) the potential of I. acuminatus and I. hexagonus in the enzootic cycle of tick-borne diseases and in the risk of pathogen spillover and disease emergence.
Disclosure
Partial results of this study were presented at: 4th Conference on Neglected Vectors and Vector-Borne Diseases (EURNEGVEC; COST Action TD1303) (Crete-Greece, 2017); International intracellular bacteria meeting 2022-ESCCAR International congress on Rickettsiae and 9th Meeting of the European Society for Chlamydia Research (ESCR) (Lausanne-Switzerland, 2022); (TTP11); 11th Tick and Tick-Borne Pathogen Conference (TTP11) (Havana/Varadero-Cuba, 2024).
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
José A. Oteo, Aránzazu Portillo, and Ana M. Palomar conceived, designed, and supervised the study. Ana M. Palomar, Asunción Gómez, Madis Põdra, Paula Santibáñez, Cristina Cervera-Acedo, Sonia Santibáñez, Elena López, and Manena Fayos conducted the research. José A. Oteo, Aránzazu Portillo, Asunción Gómez, and Madis Põdra provided resources. Ana M. Palomar, Aránzazu Portillo, and José A. Oteo contributed to data analysis. Ana M. Palomar, Aránzazu Portillo, Sonia Santibáñez, Paula Santibáñez, Cristina Cervera-Acedo, and José A. Oteo contributed to the visualization of the data. Ana M. Palomar wrote the first draft of the manuscript; All the authors provided critical review and revision of the manuscript, and approved the final version.
Funding
This work has been partially funded by European Regional Development Funds (Programa FEDER de La Rioja 2021-2027; No. 32, 00860-2024/116698) at 60% in the Specific Objective 1.1 and 1.2 of Health-Advanced (HEALTH-A), and the European Commission LIFE program (LIFE LUTREOLA SPAIN LIFE 13 NAT/ES/001171).
Acknowledgments
We thank Javier Pinedo, David Lacanal, César Aguilar (Tragsatec), Luis Lopo (Gobierno de La Rioja), Joseba Carreras (Diputación Foral de Álava), Manolo Alcántara (Gobierno de Aragón), Jesús Varas and Elena Álvarez (Gobierno de Cantabria), Enrique Castién (Gobierno de Navarra), José Manuel Meneses (Junta de Castilla y León), and Fernando Gómez (SERAFO) for providing tick samples, and Agustín Estrada-Peña for his kind help with the taxonomic classification of some tick specimens. We thank Spanish Autonomous Governments (Álava, Aragón Cantabria, Castilla y León, La Rioja, and Navarra) and the Ministry for the Ecological Transition and the Demigraphic Challenge for the support in the mink surveillance and tick collection. We are grateful to the Wildlife Rescue Centres in Alava (Martioda), in La Rioja (La Fombera), in Cantabria (Cabárceno), in Aragón (La Alfranca), and in Navarra (Ilundain) for their cooperation in collecting and storing samples. We thank Volker Fingerle (German National Reference Centre for Borrelia), Andrew P. Jackson (Institute of Infection, Veterinary and Ecological Sciences, University of Liverpool), Aitor Casas-Sanchez (Dep. Tropical Disease and Vector Biology, Liverpool School of Tropical Medicine), Matthias Niedrig (Centre for Biosafety, Robert Koch Institute, Germany), Luis M. Hernández-Triana (Wildlife Zoonoses and Vector-borne Diseases Research Group, Animal and Plant Health Agency, United Kingdom) and Houssam Attoui (UMR Virologie, National Institute for Agriculture, Food, and Environment [INRAE], France), for providing positive controls. This work has been partially funded by European Regional Development Funds (Programa FEDER de La Rioja 2021-2027; No. 32, 00860-2024/116698) at 60% in the Specific Objective 1.1 and 1.2 of Health- Advanced (HEALTH-A), and the European Commission LIFE program (LIFE LUTREOLA SPAIN LIFE 13 NAT/ES/001171).
Supporting Information
Additional supporting information can be found online in the Supporting Information section.
Open Research
Data Availability Statement
Sequences obtained in the present study were submitted to GenBank database under the following accession numbers: Tick: I. acuminatus: PQ145543 (12S rRNA gene), PQ144653-4 (16S rRNA gene), I. hexagonus: PQ145544-5 (12S rRNA gene), PQ144655 (16S rRNA gene); Ehrlichia sp.: PQ151704 (16S rRNA gene), PQ157327 (gltA), PQ157331 (rpoB), PQ157335 (groESL); N. mikurensis: PQ151705 (16S rRNA gene), PQ157332 (rpoB), PQ157336 (groESL); Coxiella spp.: PQ151703 (16S rRNA gene), PQ157328-30 (rpoB), PQ157334 (groEL); Rickettsiella spp.: PQ151706 (16S rRNA gene), PQ157333 (rpoB), PQ157337 (groEL); Hepatozoon martis: PQ144754 (18S rRNA gene); Ixovirus spp.: PQ150395-402 (L segment).




