Emergence of Canine Parvovirus Type 2a and Canine Enteric Coronavirus in Diarrheal Outbreaks Among Farmed Raccoon Dogs in China
Abstract
Canine parvovirus (CPV) and canine enteric coronavirus (CCoV) are primary viral pathogens responsible for causing diarrhea in carnivores. CCoV infection generally induces mild enteritis, whereas infections solely by CPV or coinfections involving both CPV and CCoV frequently result in severe diarrhea and can lead to fatal outcomes in affected animals. This study investigated CPV and CCoV infections in raccoon dogs farmed in China between 2018 and 2023. Among the 133 small intestine tissue samples from raccoon dogs with severe diarrhea, 107 of 133 (80.5%) were CPV nucleic acid positive, involving CPV-2 and CPV-2a subtypes. The CPV-2 amplicons maintained I418T, S927A, and T27S. Moreover, the CPV-2a subtype amplicons possessed G300S, N562V, and Y573F mutations compared to dog-ori CPV-2a. Moreover, 30 of the 133 (22.6%) samples were positive for CCoV, containing the CCoV-IIa and CCoV-IIb subtypes. Of these, 25 of 30 (83.3%) samples were coinfected with CPV and CCoV. In CCoV infection alone, mild inflammatory infiltration was observed in the lamina propria of the small intestine. Immunohistochemical staining revealed that CCoV antigens were mainly detected in the crypts of the small intestine. Furthermore, coronavirus-like particles were observed in small intestine samples. Next, we analyzed the detailed binding model for raccoon dog-origin (RD-ori) CCoV-IIa with host aminopeptidase N (APN). The receptor-binding domain (RBD) of RD-ori CCoV-IIa showed robust binding to raccoon dog APN but not to human APN. Our results demonstrated that CPV-2a and CCoV are emerging in farmed raccoon dogs in northern China. Further investigations should be performed to monitor the potential evolution and recombination events of RD-ori CPV and CCoVs.
Summary
- •
Canine parvoviruses (CPVs) are evolving among raccoon dogs and can contaminate the environment, resulting in cross-species transmission among canids.
- •
CPV and canine enteric coronavirus (CCoV) simultaneous infections occurred in raccoon dogs.
- •
Raccoon dog-CCoV infections cause mild pathological changes in the small intestine of young raccoon dogs.
- •
Raccoon dog-origin (RD-ori) CCoV-IIa showed a strong binding affinity for raccoon dog aminopeptidase N (APN), indicating that APN is probably the host receptor for RD-ori CCoV-IIa.
1. Introduction
Canine parvovirus (CPV) and canine enteric coronavirus (CCoV) are the main viral agents causing severe enteritis in dogs and other related carnivores, including raccoon dogs, foxes, and wolves [1]. CPV causes severe enteritis, often leading to the death of juvenile animals. CPV is a member of the species Protoparvovirus carnivoran1 (genus Protoparvovirus, family Parvoviridae), along with raccoon parvovirus (RaPV), feline panleukopenia virus (FPV), and mink enteritis virus (MEV) [2].
Since CPV was first detected in the 1970s, it has rapidly spread around the world. The original viral strain was designated as CPV-2 to distinguish it from the previously known minute virus of canines (CPV-1). Throughout its transmission process, CPV-2 has evolved variants, including CPV-2a, CPV-2b, and CPV-2c, in succession that show diverse host ranges and pathogenicity [3, 4]. The evolution of CPV is based on the substitution of key residues in the viral capsid protein 2 (VP2), which contains 90% of the viral capsid and determines the antigenic type [5].
Our and other groups reported that both farmed and wild raccoon dogs infected with CPV-2 often develop severe enteritis, which can be fatal [6–8]. Furthermore, raccoon dog-origin (RD-ori) CPV-2 evolved continuously among farmed raccoon dogs. As a result, the variants mostly retained the S297A mutation [6], similar to the evolutionary history of CPV-2a and CPV-2b [9].
In contrast, our understanding of CCoV infections in raccoon dogs remains limited. CCoV infection in dogs typically results in self-limiting enteritis characterized by mild diarrheal symptoms and low mortality [10]. CCoVs are enveloped, single-stranded positive-sense RNA viruses, possessing a genome ranging from 26 to 32 kb—the largest genome known among RNA viruses [11]. It is a member of the genus Alphacoronavirus, family Coronaviridae, order Nidovirales [12], together with the transmissible gastroenteritis virus of swine (TGEV) and feline infectious peritonitis virus (FIPV). These viruses display more than 96% sequence identity in the polyprotein (pp1ab) gene [13]. Furthermore, the receptor-binding domain (RBD) of these coronaviruses binds to the host aminopeptidase N (APN) to enter the cells; therefore, APN has been recognized as a functional receptor for coronaviruses [14, 15].
CCoV consists of two genotypes, CCoV-I and CCoV-II. Although CCoV-II was first identified in 1971, approximately three decades earlier than CCoV-I, it is hypothesized that CCoV-II originated from CCoV-I and feline coronavirus type I (FCoV-I) through genetic recombination and gene loss events (Buonavoglia et al., 2023; Dong et al., 2022; Lorusso et al., 2008; Vlasova et al., 2022) [16–19]. Despite sharing ~96% nucleotide sequence identity at the genomic level, CCoV-I and CCoV-II exhibit significant divergence in the spike (S) protein. Professor Canio Buonavoglia’s group developed a polymerase chain reaction (PCR) assay to differentiate between CCoV-I and CCoV-II based on primers targeting specific regions of the M gene [20]. The detection of recombinant TGEV/CCoV strains led to the classification of CCoV-II into two subtypes: classical (CCoV-IIa) and recombinant (CCoV-IIb) [21]. Pantropic hypervirulent CCoV-IIa was also detected [22].
CCoV infections in raccoon dogs were first reported in 2006 [23, 24]. During the COVID-19 pandemic, two reports based on next-generation sequencing methods revealed that CCoVs infected wild or farmed raccoon dogs [25, 26], which were distinct from SARS-CoV-2 at the genome level. However, the detailed molecular characteristics and antigen detection remain limited. Between 2018 and 2023, severe diarrheal diseases were observed in farmed raccoon dogs in northern China. To investigate potential infections of CPVs and CCoVs in these affected animals, an epidemiological study was conducted. The molecular characteristics of each virus were analyzed to assess the prevalence of different CPV and CCoV types. Additionally, gross and histopathological examinations were performed on CCoV-infected raccoon dogs, along with the detection of CCoV antigens. Further analysis included the sequencing of the RBD, and an all-atom molecular dynamic (MD) simulation was conducted to evaluate the binding affinity of RD-ori CCoV-IIa with raccoon dog or human APN receptors.
2. Material and Methods
2.1. Samples and Study Area
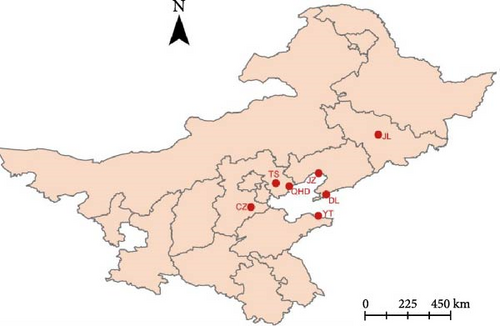
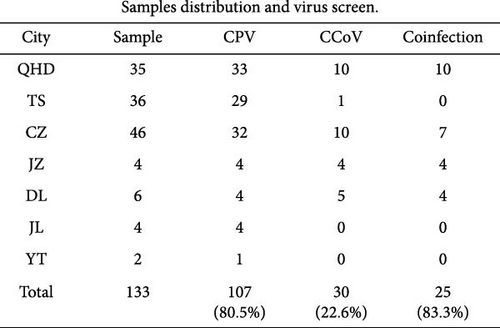
From 2018 to 2023, 133 small intestine tissue samples were collected from naturally dead farmed raccoon dogs with diarrhea. Of these, 35, 36, and 46 samples were from Qinhuangdao, Tangshan, and Cangzhou cities of Hebei province, respectively; four and six samples were from Jinzhou and Dalian cities of Liaoning province, respectively; four samples were collected from Jilin city, Jilin province; and two samples were from Yantai city of Shandong province (Figure 1). The samples were suspended in 2 mL phosphate-buffered saline (PBS) and stored at −80°C until further analysis.
2.2. CPV Detection and Amplification of VP2 Gene Sequences
The total DNA was extracted from PBS-diluted samples using an EasyPure Viral DNA/RNA Kit (Transgene Biotech, China). Specifical primers (Table S1) targeting the CPV VP2 gene were employed to screen the CPV nucleic acid using PrimerSTAR Max DNA Polymerase (Takara Bio, China) [27]. The complete VP2 gene sequence was amplified using primer pairs and sequenced using the Sanger method (Sangon, China).
2.3. CCoV Detection and Amplification of Partial S Gene Sequences
Total RNA was extracted from the PBS-eluted samples using an RNeasy Mini Kit (Qiagen, Germany). Total RNA was reverse transcribed to cDNA using a Transcriptor First Strand cDNA Synthesis kit (Roche, Germany). CCoV cDNA was detected by PCR based on specific primers [28] (Table S1). To distinguish between the genotypes CCoV-I and CCoV-II (containing subtypes CCoV-IIa and CCoV-IIb) in the CCoV-positive samples, four primers (Table S1) were used to amplify the targeting sequences of the S gene [20, 29], which were then sequenced using the Sanger method (Sangon, China).
2.4. Phylogeny
Complete CPV VP2 and partial CCoV S reference sequences were obtained from GenBank. The Muscle model aligned the sequences, and maximum likelihood (ML) trees were inferred by MEGA XI with 500 bootstrap replicates [30]. For the phylogeny of CPV VP2 sequences, the Tamura 3-parameter (T92) model with discrete gamma distribution (+G) and a certain fraction of sites that were evolutionarily invariable (+I) were selected as the fittest model [31]. In addition, TN93+G, T92+G, and T92+G were used to infer the phylogeny of the CCoV-IIa, CCoV-IIb partial S gene, and RBD sequences, respectively. The nucleotide and amino acid similarities of the sequences were analyzed using DNAMAN software in the MegAlign mode. Figtree was used to summarize and annotate these trees.
2.5. Histopathological and Electron Microscopy (EM) Observation
The small intestine tissues of naturally dead raccoon dogs (PP780836) were collected and fixed in 10% formalin. By following embedding in paraffin, tissues were cut into ~4 μm thickness sections to process the hematoxylin and eosin or immunohistochemistry (IHC) staining. The mouse anti-N protein of CCoV IgG1a (Zoonogen, China) and HRP-labeled horse anti-mouse IgG antibody (VectorLab, USA) were used as the primary and secondary antibodies. For EM observations, PBS-diluted CCoV-positive samples were fixed with glutaraldehyde and observed by EM (HITACHI, Japan).
2.6. MD Simulation
The MDs simulation portion of this study utilized the Alphafold2 molecular simulation tool to construct complex systems involving APN binding with RDCoV. These systems included CCoV-HuPn-2018+DogAPN, RDCoV+RD APN, and RDCoV+Human APN. APN sequences from various organisms were retrieved from UniProt [32]. The X-ray structure of CCoV-HuPn-2018+DogAPN (PDBID: 7U0L) served as a template for the automatic hetero-oligomer generation of the complexes. Subsequently, Amber MD simulations were conducted on these five complexes to elucidate the molecular interactions between RDCoV and different APNs by referencing their X-ray structure [33, 34]. The detailed method for the MD simulations can be found in Supporting Information on MD methods.
The root mean square deviation (RMSD) and root mean square fluctuation (RMSF) were computed using MD trajectories to assess the system dynamics. Furthermore, the Gibbs free energy between RDCoV and APN was determined using the molecular mechanics Poisson–Boltzmann surface area (MM-PB/GBSA) method [35].
3. Results and Discussion
3.1. PCR Detection of CPV and CCoV
CPVs and CCoVs are the major enteropathogens in wild and farmed carnivores, even in pangolins [6, 22, 29, 36, 37]. The PCR results demonstrated that 107 of the 133 (80.5%) samples were positive for CPV, which was highly positive in all investigated cities (Figure 1A). Meanwhile, 30 out of 133 samples (22.6%) tested positive for CCoV. Among these positive samples, 11 were identified as belonging to the CCoV-IIa genotype, with four of these 11 samples exhibiting coinfection with the CCoV-IIb genotype. Simultaneous CPV and CCoV infections among dogs are commonplace [38, 39] but are unexplored in raccoon dogs. In this study, we clearly demonstrated that 25 of 30 (83.3%) samples were coinfected with CPV and CCoV among farmed raccoon dogs (Figure 1B).
3.2. CPV VP2 Gene Amplification and Sequencing
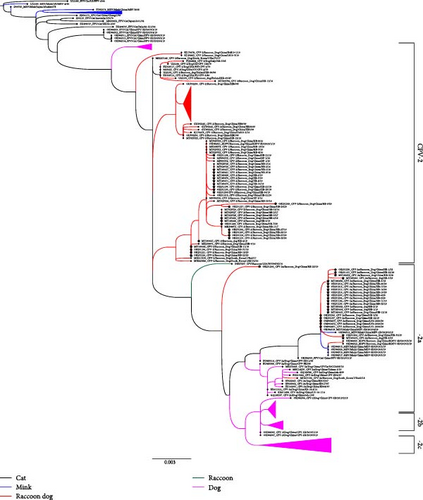
Since CPV emergence as a canine enteric pathogen in the 1970s, dog-origin CPV has evolved through various VP2 mutations, leading to multiple subtypes (CPV-2, CPV-2a, CPV-2b, CPV-2c). However, CPV-2 no longer infects dogs [40]. From the CPV nucleic acid-positive samples, we obtained 48 complete VP2 sequences, including 26 CPV-2 and 22 CPV-2a subtype amplicons, demonstrating that CPV-2 and CPV-2a are coprevalent in farmed raccoon dogs in China. The 26 VP2 sequences of CPV-2 clustered with those of other RD-ori CPV-2 strains isolated in China and South Korea between 2016 and 2017 (Figure 2, Figure S1). The mutations S297 and S297A were initially coprevalent in raccoon dogs during 2016–2017[6]; by 2018–2023, however, the S297A mutation had become fixed in all samples, suggesting that S297A has gradually replaced earlier strains, similar to the CPV-2a evolution in the 1990s [3].
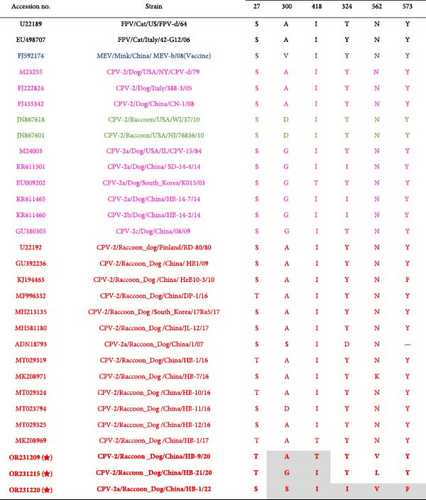
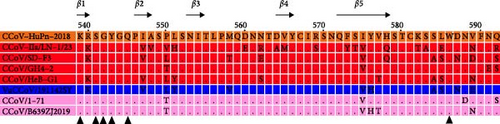
Recently, the substitution I418T was detected in RD-ori CPV-2, dog-ori CPV-2a, and CPV-2b [41, 42], and our results suggest that mutants with I418T are the predominant strain among raccoon dogs in China. Moreover, a rare 300G mutation, the key residue determining the host range of CPVs [5], was detected in CPV-2/Raccoon Dog/China/HB-21/20 (Figure 3). In addition, the L562V mutation was identified in a single amplicon (CPV-2/Raccoon Dog/China/HB-9/20) located near the 300-loop region (Figure 3) [6, 43]. Prior research demonstrated that the G300V/V562L mutation combination reduces CPV’s binding affinity to canine cells [44].
The new CPV-2a subtype, characterized by the S297A mutation, has likely spread further in the 1990s and has since become the predominant CPV subtype in global dog populations. In this study, 22 RD-ori CPV-2a strains were grouped into a distinct subclade along with other CPV and carnivorous parvovirus isolates from diverse hosts (Figure 2, Figure S1). The subclade fell between the CPV-2 and CPV-2a subclades (Figure 2, Figure S1). Furthermore, these amplicons retain 80R, 87L, 93N, 101T, 103A, 305Y, 323N, 564S, and 568G in VP2 and should be recognized as a CPV-2a subtype (Table S2) and reveal that new CPV-2a are emerging among farmed raccoon dog that are close to carnivore parvovirus sequences in Shandong province in China [45]. Consequently, these RD-origin CPV-2a strains clustered into an independent subclade, suggesting distinct molecular characteristics and evolution trends from those of dog-origin CPV-2a.
Additionally, all RD-ori CPV-2a subtype amplicons possessed the G300S, N562V, and Y573F mutations compared to dog-ori CPV-2a (Figure 3). N562V was detected in the RD-ori CPV-2a strain, which has not been previously described. Residue 562 was subjected to mutagenesis in an in vitro evolution experiment [40] (Figure 3).
Cross-species transmission of CPV is common among carnivores and other related animals [6, 37, 40]. The critical residues of CPV VP2 that interact with the host TfR were proposed to determine the host range of CPV, particularly residue 300, located in the loop region of the three-fold spike of the VP2 capsid [5]. Interestingly, the CPV sequences obtained from raccoon dogs possessed variable residue 300, including 300A, 300D, and 300G in the CPV-2 subtype and 300S in the CPV-2a subtype (Figure 2, Figure S1, Figure 3, Table S2). 300A mutants were found in most dog-ori CPV-2 subtype strains. However, it has been continuously replaced by the CPV-2a/CPV-2b/CPV-2c subtype strains with 300G since the 1985s [46–48]. In addition, 300S was found in masked civets (Paguma larvata) belonging to the family Viverridae, which is evolutionarily distant from raccoon dogs. The variable residue at position 300 of VP2 indicated that raccoon dogs were susceptible to different CPV subtypes (Figure 3, Table S2).
Recently, the chromosome-scale genome of raccoon dogs was first reported [49]. Based on this, we compared the TfR amino acid residues with those of other closely related carnivores. The TfR from raccoon dog (RDTfR) is evolutionarily similar to canine TfR (97% amino acid identity) but lacks the glycosylation site at residue 384, which plays a key role in blocking the binding of FPV-like viruses to canine TfR [50].
Prof. Colin R. Parrish’s group resolved the structure of the CPV-TfR complex by cryo-EM, revealing that the loop region consisting of β-strand II-1 and II-1 containing residue 217–222 of canine TfR were interacting with CPV VP2 capsid during the CPV-TfR binding process [51]. We compared the RDTfR amino acid residues of these regions with those of black-backed jackals (BBJ), dogs, vulpes, and raccoon TfR and found that RDTfR maintains a unique 225N mutation, which is different from all the species compared (Figure S2). Residue 225 of TfR can form hydrogen bonds with Lys-93 in FPV VP2; however, this can be blocked by Asn-93 in CPV [52].
3.3. CCoV Subtype Identification
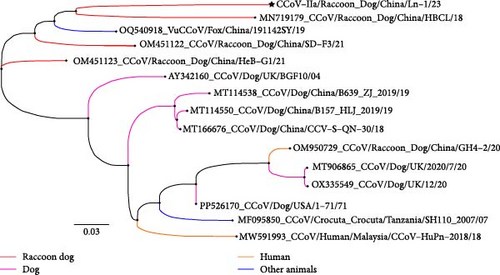
For the CCoV-IIa-positive samples, we analyzed 621 nucleotides (207 amino acids) of amplicons generated using primers S5 and S6 targeting S gene, as described by Pratelli et al. [20], with reference to CCoV-IIa strains from dog origin, RD-ori, and fox origin (vulpes-ori), as well as FIPV and TGEV strains. The nucleotide and amino acid identities among the sequences of the partial S gene from the 11 CCoV-IIa-positive samples were below 84% and 90%, respectively. These sequences shared ~87%–89% nucleotide identity and 92%–95% amino acid identity with the reference CCoV-IIa strain INSAVC-1 (#D13096). Phylogenetic analysis based on partial S gene sequences indicated that the sequences of these 11 CCoV-IIa samples could be divided into three distinct subclades: the amplicons of HB-10/18, HB-4/19, LN-1/20, LN-2/20, LN-3/20, LN-4/20, HB-38/20, and LN-1/23 clustered with other RD-ori CCoVs (#MN719179) and fox-origin CCoVs (#OQ540918); the amplicons of HB-6/18 and HB-17/18 clustered with other dog-origin CCoVs; and the amplicon of HB-19/18 clustered with raccoon dog samples (#EF192155) collected in 2003 (Figures S3 and S4). We compared the amino acid substitutions of residue 1181–1387 of the S protein (counted according to amino acid sequences of the INSAVC-1S protein). Nine of 11 amplicons contain the mutation of A1212S, T1222S, R1324K, and V1331T/A, V1363I/L, I1367M, D1370Q, and N1378D (Table S3). These residues are in the S2 region, with residues 1307–1378 of heptad repeat 2 (HR2), which plays a vital role in membrane fusion and viral entry [53]. Prof. Yongzhen Zhang reported that the genome of RD-ori CCoVs likely underwent a complex recombination process, particularly in the divergent S2 gene segment [26]. More importantly, raccoon dog CCoVs probably form a distinct monophyletic lineage based on the S gene segment [25].
In the four CCoV-IIb-positive samples, four amplicons were amplified based on the primers CEpol-1 and TGSP-2 [29]. These share 97%–100% and 94%–100% identities in nucleotide and amino acid levels, and 95%–98% and 90%–94% identities in nucleotide and amino acid levels with the RD-ori CCoV strain GH4-2 (#OM950729) collected from the central part of China [26]. Furthermore, we compared the first 50 residues of the NTD in the S protein with those of other CCoV-IIb and TGEV strains (strain Purdue as the reference sequence). No insertion was found in the RD-ori CCoV-IIb strain, including residue five, and the amino acid substitutions are shown in Table S4. The phylogeny of the CCoV-IIb strains revealed that the amplicons of the four samples obtained in this study clustered into a subclade close to the sequences of the raccoon dog samples GH4-2 and GH8-2 (Figures S5 and S6). Some CCoV-IIb strains (such as #MT906865) cause severe vomiting in dogs [54], and the RD-ori CCoV-IIb strain GH8-2 is close to #MT906865, but not the four RD-ori CCoV-IIb amplicons obtained in this study. Therefore, the clinical symptoms and pathological changes in raccoon dogs infected with GH4-2 should be further investigated in more extensive clinical samples.
Prof. Nicola Decaro and Canio Buonavoglia’s lab demonstrated that CCoV-II replicated more readily than CCoV-I in cell lines [20]. Viral isolation was performed using canine-derived A72 and MDCK cells. Unfortunately, no CPE or RNA replication was detected after treatment with the filtered CCoV-IIa- and CCoV-IIb-positive samples. Further exploration should be performed to isolate CCoVs after improving experimental conditions.
3.4. Pathological and Virus Particle Observation of CCoV-IIa Infection
Pathological observations were performed on a naturally dead 5-month-old raccoon dog infected with CCoV-IIa: CCoV-IIa/LN-1/23. After necrosis, mild congestion was observed in the small intestine and the mesenteric lymph nodes. No systemic pathological changes were observed (Figure 4A). Furthermore, Coronavirus particles with diameters of 100–120 nm were visualized and exhibited typical spikes found in purified small intestine samples (Figure 4B).
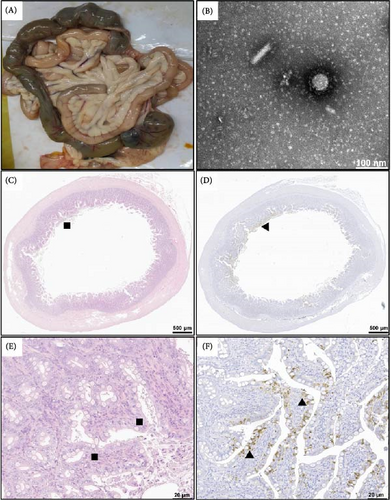
Similar to CCoV infections in dogs [55], the histopathological changes included inflammatory cell proliferation and lamina propria infiltration in the small intestine (Figure 4C,E). Furthermore, IHC staining revealed that CCoV antigens in the cytoplasm of the small intestinal villus epithelial cells occasionally extended to the junction of the crypt and epithelium in two CCoV-infected raccoon dogs (Figure 4D,F). Not surprisingly, histopathological changes and CCoV antigens were not observed or detected in the appearance of normal raccoon dogs.
3.5. RBD Analysis
The RBD sequences were amplified and analyzed for CCoV-IIa/LN-1/23 (PP780836). The phylogenetic results revealed that CCoV-IIa/LN-1/23 was evolutionarily close to another CCoV strain, HBCL, and vulpes coronavirus (Figure 5A). CCoV-IIa/LN-1/23 maintained the same residue at the vital position and interacted with APN such as K539, S541, G542, Y543, Q545, and W586 (Figure 5B, Table S5). APN is the primary cell-surface receptor for several alphacoronaviruses [15, 56]. Recently, Prof. Shuyi Zhang demonstrated that APN is a functional receptor of vulpes in vuples-ori CCoVs [14]. This study compared the amino acid residues of a major region that binds to the CCoV RBD. The results reveal that the α-sheet 18–20 region of APN is well-conserved among dogs, vulpes, and raccoon dogs, except for a rare mutation at residue 746 that did not directly interact with the CCoV RBD region (Figure 5C).

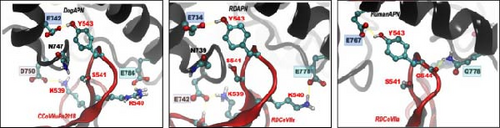
In addition to the rigid structural modeling of the CoV-APN systems (CoVHuPn2018+DogAPN, RDCoVIIa+RDAPN, and RDCoVIIa+HumanAPN) using Swiss-Model [57] and Alphafold3, we conducted all-atomic MDs simulations of the three systems to study binding details with consideration of protein dynamics (Figure 5D). The stability and residual mobility were analyzed using stable MD trajectories (Figure S7). Notably, RD-ori CCoV-IIa+RDAPN exhibited the lowest binding free energy, closely followed by CoVHuPn2018+DogAPN, indicating that RD-ori CCoV-IIa probably utilized APN as a functional receptor (Tables S6 and S7). Importantly, RD-ori CCoV-IIa exhibited no considerable binding to human APN, suggesting that RD-ori CCoV-IIa is less likely to infect humans (Figure 5D).
4. Conclusions
CPV and CCoV infections are well-documented in dogs, but limited studies have focused on raccoon dogs. Here, we demonstrated that CPV infections are prevalent in raccoon dogs, with a notable incidence of mixed CCoV infections. Both CPV-2 and CPV-2a subtypes coexist in these populations, showing variability in VP2 at residue 300, indicating an ongoing evolutionary process in RD-ori CPV. Pathological changes in CCoV-infected raccoon dogs were examined through IHC and EM, where viral particles were observed. Additionally, RD-ori CCoV likely utilizes the host APN receptor for cell entry. These findings suggest that raccoon dogs serve as reservoirs for CPV and CCoV, playing a crucial role in the viruses’ evolution.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Rongguang Lu: conceptualization, methodology, writing–original draft. Xue Bai: conceptualization, writing–review and editing, supervision, project administration. Haixu Cao, Wei Yang, Jianhai Fu, Tingli Qian, Chengqi Zhang, Na Feng, Bo Hu, Liwen Xu, Shuangshuang Li, Guanyu Zhao, Qiumei Shi, Deyue Zang, and Guobao Li: methodology, investigation. Rongguang Lu, Haixu Cao, Wei Yang, and Jianhai Fu contributed equally to this work. Rongguang Lu is the first author and Haixu Cao, Wei Yang, and Jianhai Fu are the co-first authors of this manuscript.
Funding
We sincerely thank the following funding sources: National Key Research and Development Program of China (grant nos. 2023YFD1800700 and 2021YFD1801101), National Natural Science Foundation of China (32100408), Jilin Provincial Science and Technology Development Plan Project (20210202050NC), Postdoctoral Science Foundation funded project (2023M742416), State Key Laboratory of Veterinary Etiological Biology, and Lanzhou Veterinary Research Institute, Chinese Academy of Agricultural Sciences, Grant/Award Number: SKLVEB2021KFKT015.
Acknowledgments
We thank Liliang Xia Heibei Shuobo Veterinary Company, Zhixin Fu, and Lifeng Chen from Hebei Normal University of Science & Technology for supporting the samples collection in Hebei province; Xinze Qu and Tao Zhang from Huaweite (Jiangsu) Biopharmaceutical company for the samples collection in Shandong province; Chen Wang for the samples collection in Liaoning province, we also thank Xueqing Liu for the samples collection in Jinlin province. We sincerely thank the following funding sources: National Key Research and Development Program of China (grant nos. 2023YFD1800700 and 2021YFD1801101), National Natural Science Foundation of China (32100408), Jilin Provincial Science and Technology Development Plan Project (20210202050NC), Postdoctoral Science Foundation funded project (2023M742416), State Key Laboratory of Veterinary Etiological Biology, and Lanzhou Veterinary Research Institute, Chinese Academy of Agricultural Sciences, Grant/Award Number: SKLVEB2021KFKT015.
Supporting Information
Additional supporting information can be found online in the Supporting Information section.
Open Research
Data Availability Statement
The complete sequences of CPV VP2 obtained in this study were submitted to GenBank under the following accession numbers: OR231190 to OR231220 and MT183657 to MT183673. The partial S genes amplified using primers S5/S6 [20] and CEpol-1/TGSP-2 [29] are OR248013 to OR248026 and OR724654. The accession number of RBD sequences is PP780836.




