Progress Toward Antigenic Epitopes of African Swine Fever Virus and Their Identification
Abstract
African swine fever virus (ASFV) is a highly contagious pathogen that causes pigs to develop high fever, vomiting, diarrhea, and respiratory distress, with an exceptionally high case fatality rate. Unfortunately, vaccine development is hindered by a limited understanding of the structure and function of the protein encoded by ASFV, as well as the mechanisms underlying infection and immunity. Among these factors, the lack of effective cellular epitopes represents a major obstacle to vaccine development. Epitopes serve as the smallest structural units capable of inducing immune responses and are critical targets for both vaccine design and diagnostic reagent development. Therefore, it is imperative to investigate antigens with high immunogenicity and elucidate their correlation with protective immune responses to provide scientific insights and a theoretical foundation for developing safe and effective ASFV vaccines. Currently, thorough reviews on the identification and functional characterization of ASFV antigenic epitopes remain scarce. To address this gap, this paper provides a comprehensive review of ASFV epitopes and their identification strategies. It initiates with a systematic classification of ASFV antigenic epitopes, followed by an extensive discussion of various methods for identifying ASFV epitopes, along with their advantages and limitations. Furthermore, the paper summarizes existing databases of characterized ASFV antigenic epitopes and highlights the most biologically significant ones. In addition, the paper explores emerging applications of ASFV epitopes while addressing the technical challenges in epitope-based research to provide valuable insights for ASFV vaccine development and production.
1. Introduction
African swine fever virus (ASFV) is a highly infectious DNA virus [1] and the only known insect-borne DNA virus [2], characterized by a complex genome and an ortho icosahedral structure, with lengths between 170 and 194 kb [1]. ASFV possesses a multilayered architecture organized into five layers from the interior to exterior: (i) inner core containing genomic DNA, (ii) nucleocapsid layer, (iii) inner envelope, (iv) protein coat, and (v) outer envelope [3–5]. The proteins encoded by the ASFV genome are categorized into structural and nonstructural proteins, depending on their involvement in the composition of the viral particle. Structural proteins play a crucial role in virus assembly and mediate interactions between the virus and the host cell, while nonstructural proteins participate in viral replication, gene expression regulation, and immune evasion. Among the above five layers, the outer membrane proteins, capsid proteins, and inner membrane proteins belong to structural proteins, and the remaining part of the genome encodes nonstructural proteins and virion-associated antigenic proteins are summarized in Figure 1.
ASFV primarily affects domestic and wild boars and produces a wide range of symptoms [6], from chronic and low-level disease to acute hemorrhagic fever and hyperacute death, with extremely high lethality, posing a serious threat to the global pig industry. Since it was first detected in the 1920s, the epidemic has spread widely across Central and Eastern Europe and Asia, resulting in large numbers of pig deaths. ASFV spreads through multiple transmission routes, including direct contact between infected and susceptible pigs via bodily fluids, indirect transmission through contaminated materials such as feed, bedding, and water, and vector-borne transmission via soft ticks (Ornithodoros moubata). Additionally, food-borne transmission occurs through the consumption of contaminated pork products, while long-distance dissemination is often facilitated by human-mediated transport of infected meat. Wild boar populations serve as reservoirs for ASFV, further complicating control efforts. The virus can also be mechanically transmitted by blood-feeding arthropods and persists on environmental surfaces, contributing to its resilience and potential for further spread. A limited understanding of ASFV infection and host immune responses constrains the prevention and control of ASFV. Therefore, a comprehensive investigation into the properties and functions of ASFV antigenic proteins is necessary.
The structure of ASFV from the outside to the inside is sequentially composed of five layers: envelope, coat, inner envelope, nuclear shell, and nucleoid. The subviral localization of 53 viral proteins among the five structural domains of the ASFV particle is shown. The bolded portions are the antigens highlighted in this article.
Antigenic epitopes, also known as antigenic determinants or antigenic determinants, are specific regions on the surface of an antigen that are recognized and bound by an antibody [7]. Virtually any surface region of an antigen that is identifiable by an antibody can serve as an antigenic epitope an antigenic epitope [8, 9]. As the smallest structural units capable of inducing an immune response, antigenic epitopes play a crucial role in understanding infection mechanisms and immune responses. They also contribute to elucidating the structure and function of viral proteins. Moreover, antigenic epitopes serve as essential targets for the development of epitope-based vaccines, monoclonal antibody (mAb) production, and diagnostic reagent development, making them fundamental to solving numerous epidemiological problems.
ASFV epitopes are classified into two types: B-cell epitopes (BCEs) and T-cell epitopes (TCEs), based on their interaction with antigen receptors on immune cells. BCEs are antigenic fragments that bind to antibodies and are recognized by B cells [10]. Similarly, T cells express a surface receptor known as the T-cell receptor (TCR) [11], and epitopes that specifically bind to TCRs are referred to as TCEs [9, 12, 13]. These two types of epitopes play distinct roles in immune responses, with BCEs inducing humoral immunity and TCEs triggering specific cellular immunity [14]. The structures of BCEs and TCEs are shown in Figure 2.
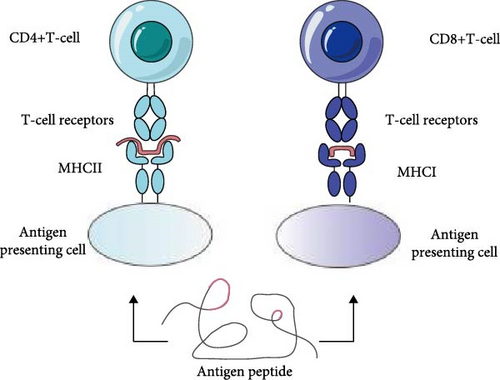
Based on the continuity of their amino acid sequences, ASFV epitopes are further divided into linear and conformational epitopes [15]. Linear epitopes, also known as continuous antigenic epitopes, consist of a contiguous sequence of amino acids within the peptide chain [16]. They are typically 9–12 amino acids in length, often adopting an amphipathic helical structure [17]. Linear epitopes are primarily found in TCEs and some BCEs. On the other hand, conformational epitopes are structurally more complex [18]; they are formed by spatially adjacent but sequential-discontinuous amino acids and are exclusive to BCEs [19]. Approximately 10% of BCEs are linear, and the rest are discontinuous in sequence and conformation [20].
The study of ASFV epitopes has multiple implications. In terms of vaccine development, although attempts to develop an ASFV vaccine have been made since the mid-1960s, a broad-spectrum commercial vaccine remains unavailable due to the complexity of the virus and immune evasion mechanisms [21–23]. Epitope-based vaccines contain appropriate amounts of epitopes and can be made into nucleic acid vaccines as well as peptide vaccines. BCEs within these vaccines induce the production of neutralizing antibodies, while TCEs activate protective T cells and cytotoxic T lymphocytes, generating a pathogen-specific and long-lasting immune response. Identifying key ASFV epitopes enables the rational design of vaccine candidates that elicit robust immunity [24]. Epitope-based vaccine strategies have been widely studied and successfully applied in developing and evaluating vaccines against various infectious agents, including influenza virus [25], human immunodeficiency virus (HIV) [26], and hepatitis B virus (HBV) [27]. The first proof of concept demonstration of such structure-based vaccine design in Phase I clinical trials was published for an immunogen mimicking a key conformational epitope of a respiratory syncytial virus [28, 29]. Since the efficacy of epitope-based vaccines largely depends on well-defined neutralizing epitopes, the sequence and structural characterization of antigenic epitopes are essential for guiding both therapeutic and prophylactic strategies. Therefore, obtaining well-validated epitope information is a critical prerequisite for the development of effective epitope-based vaccines.
In drug design, epitope information facilitates the development of small-molecule drugs that mimic or block the interaction of specific epitopes with immune cells. In diagnostics, antigenic epitope-based assays facilitate faster and more accurate detection. For example, using specific antigenic epitopes as targets, highly specific assays such as enzyme-linked immunosorbent assay (ELISA) can be developed [30, 31], which is crucial for outbreak surveillance and control. In immunological research, epitope studies provide insights into virus-host interactions and immune evasion mechanisms. They elucidate how viruses stimulate B-cell-mediated antibody production and T-cell-mediated immune responses, contributing to a deeper understanding of viral pathogenicity and host defense mechanisms. Additionally, in biosafety and disease surveillance, epitope identification contributes to the development of rapid and sensitive surveillance systems for detecting and monitoring the spread of pathogens.
Studying the conservation and variability of ASFV epitopes is critical for the long-term effectiveness of vaccines. Due to the high mutation rate of viruses, existing vaccines and treatments may lose efficacy over time [32]. However, conserved epitopes, which undergo minimal changes during viral evolution, represent promising targets for vaccine design. By identifying epitopes that remain conserved across different viral strains, researchers can develop vaccines that provide broad-spectrum protection against multiple viral variants. At the same time, understanding mutation-prone epitopes enables scientists to predict and counteract potential antigenic escape mechanisms.
In addition, certain antigenic epitopes may have similarities between different viruses. This provides an opportunity to develop vaccines that can provide cross-species protection. For instance, if we can identify antigenic epitopes that are common to ASFV and other swine-derived viruses like PRRSV and CSFV, vaccines targeting these epitopes may be effective in protecting against multiple swine-derived viruses.
In short, the development of effective epitope-based vaccines for ASFV prevention and control requires a comprehensive understanding of ASFV-associated epitopes and their identification methods.
2. Epitope Identification Methods
Epitope identification is the process of using bioinformatics tools and experimental methods to identify and determine specific regions (epitopes) on antigenic molecules that can be recognized by the immune system. These epitopes are typically protein segments capable of binding to antibodies or TCR, thereby stimulating an immune response [33]. Epitope identification plays a crucial role in vaccine design, disease diagnosis, immunotherapy, and basic immunology research.
Since epitope identification has profound impacts on public health, disease prevention, and treatment [34], the mastery of epitope identification methods is crucial for advancing epitope research. Methods of epitope identification are mainly categorized into experimental and computational (bioinformatics) methods. Table 1 summarizes commonly used epitope identification methods, along with their respective advantages and limitations. Furthermore, this paper categorizes these methods based on their application in BCE identification (Figure 3) and TCE identification (Figure 4).
| Methods | Advantages | Disadvantages | Reference |
|---|---|---|---|
| Experimental methods | |||
| ELISA | Easy-use, high-throughput, linear, and continuous epitopes can be identified | Inability to accurately identify conformational epitopes | [35] |
| Synthetic peptide method | High specificity, high throughput, wide applicability, lower cost | Inability to identify discontinuous epitopes, instability of synthetic peptides | [36] |
| Mutational analysis | Highly accurate, can perform functional analysis, widely applicable | May produce additional mutations, more technically demanding, time-consuming | [37] |
| MS | High precision, wide applicability, and information Richness | Expensive equipment, high sample requirements, and complex data analysis | [15] |
| X-ray crystallography | High-resolution structural information, precise atom positioning | Need for high-quality crystal samples | [38] |
| NMR | High specificity, nondestructive analysis | High requirements for sample purity and concentration | [39] |
| Protein microarrays | High flexibility, high throughput | High demands on sample preparation and fixation | [40] |
| Phage display | Easy to operate, low cost, and high throughput | Unable to identify larger epitopes | [41] |
| Bioinformatics methods | |||
| Bioinformatics tools | Quickly predict large numbers of potential table positions, saving time and cost | Accuracy is limited by algorithms and available data, require experimental validation | [20] |
| Molecular docking | Simulate the binding of epitopes to antibodies or T-cell receptors | Need for accurate protein 3D structure and experimental validation | [42] |
| Machine learning | High throughput | Requires large amounts of training data, require further experimental verification | [43] |
| Databases | All known epitope information can be queried | Completeness of databases and frequency of updates may affect the accuracy of information | [18] |
2.1. ELISA
The ELISA is commonly used to detect and quantify antigens or antibodies [46]. Additionally, indirect ELISA (iELISA) can identify precise epitopes by analyzing truncated peptides through absorbance measurements [47]. Epitope identification involves recognizing a specific part of the antigen recognized by a particular antibody. mAbs are highly specific antibodies that target specific epitopes. By utilizing mAbs, researchers can determine the antigenic epitopes they recognize. For instance, Li et al. [48] expressed ASFV DP96R protein in a prokaryotic expression system, immunized mice with it, and generated mAbs. These mAbs were subsequently characterized via western blotting, indirect immunofluorescence assay (IFA), and co-immunoprecipitation (Co-IP) experiments to identify the epitopes. To date, the majority of ASFV epitopes have been characterized using this approach [49–55].
2.2. Synthetic Peptide Method
The synthetic peptide method involves designing and synthesizing a series of short peptides that span the entire sequence of the target antigenic protein [56]. The exact epitope recognized by the antibody can be determined by combining these peptides with a solid-phase carrier (typically magnetic beads or ELISA plates) and detecting the binding of these peptides to a specific antibody. This approach is widely used for the identification of ASFV linear epitopes through synthetic peptide arrays [57].
2.3. Mutational Analysis
This process involves the targeted substitution of individual residues that make up functional epitopes, resulting in the complete loss of antibody binding [58]. Mutation analysis changes the amino acid sequence of antigenic proteins through genetic engineering techniques and obtains mutant proteins by introducing point mutations or deletion mutations [59]. After expression and purification of the mutant proteins, researchers can use specific antibodies in binding assays to determine epitope locations by comparing the binding affinities before and after the mutation.
2.4. Mass Spectrometry (MS)
MS is widely utilized to characterize the amino acid sequences of proteins, especially after enzymatic digestion (e.g., trypsin digestion) [60]. MS enables the identification of specific residues that constitute binding epitopes and paratopes, especially in the case of discontinuous epitopes [60]. By analyzing the mass and sequence of peptides, researchers can determine the precise epitope to which an antibody binds. In ASFV research, MS is frequently combined with protein interaction studies to elucidate the mechanisms of ASFV protein–host interactions. Recently, hydrogen–deuterium exchange (HDX) methods have been applied to epitope identification by some research teams [15].
2.5. X-Ray Crystallography
X-ray crystallography is a powerful technique that utilizes X-rays to analyze the arrangement of atoms in crystals. It serves as a fundamental method for determining the three-dimensional structure of proteins and other biological macromolecules. By analyzing the crystal structure of protein–antibody complexes, researchers can directly visualize the location and structural characteristics of epitopes [61]. X-ray crystallography is one of the gold standards for epitope identification. At the beginning of the ASFV outbreak, a large number of studies have applied this method for structural scanning and functional studies of ASFV proteins [61–69]. While this method has been less frequently applied to the study of ASFV structural protein epitopes, it is noteworthy that it has identified potential drug targets in several less-characterized antigenic proteins of ASFV [63, 64]. Recently, Liu et al. determined the structure of pP1192R across different enzymatic states using X-ray crystallography and single-particle cryo-electron microscopy (cryo-EM). Their findings elucidated the structural basis of pP1192R-mediated DNA topological changes and drug-protein interactions, providing crucial insights for developing anti-ASFV strategies targeting pP1192R.
2.6. Nuclear Magnetic Resonance (NMR)
NMR spectroscopy is an analytical technique that leverages the principles of NMR to provide detailed information about the structure and dynamics of molecules. It is widely used to investigate the three-dimensional structure of proteins and antibodies. Unlike X-ray crystallography, which requires crystallization, NMR allows for direct observation of epitope structures in solution. However, NMR has inherent limitations, such as its applicability is limited to small continuous proteins and peptides (below 30 kDa), and the purity and concentration of the complex should be high. In addition, due to the high level of expertise required and significant technical challenges, NMR is not commonly applied in ASFV research [70].
2.7. Protein Microarrays
Protein microarray technology, also known as protein microarray technology [71], is a high-throughput analytical method that allows for the simultaneous detection of interactions between multiple proteins and a range of different molecules (antibodies, small molecule drugs, other proteins, etc.). In protein microarrays, biomolecular probes (e.g., antigens and antibodies) are spatially immobilized on a surface. During analysis, the array is exposed to a sample containing potential binding partners (e.g., serum antibodies), enabling the detection of specific interactions between the immobilized probes and target analytes. Visualization of such interactions can be achieved by fluorescence labeling and imaging [40]. In ASFV epitope identification, protein microarrays are used to identify and analyze the interactions between specific antibodies and their target antigens to determine the location and characterization of epitopes. Desmet et al. [40] used this method to verify the conservation of the p54 protein and identified a prominent peptide sequence on this protein: IVLIYLFSSRKKKKAA.
2.8. Phage Display
Phage display library technology is a commonly used technique for identifying epitopes and screening antigen-specific peptides [72]. This technology enables high-throughput and cost-effective epitope screening, and the main methodological processes include library construction, phage packaging, biopanning, and identification of panning-positive clones. The principle is to fuse exogenous proteins or peptides with phage coat proteins, display them on the surface of phages, maintain a specific spatial conformation [73], and utilize the specific affinity interaction to screen for specific proteins or peptides. Currently, it is widely used in the development of new vaccines, screening of enzyme inhibitors, medical diagnosis and treatment [74], development of peptide drugs, antigen epitope analysis, screening of mAbs, and study of protein interactions. At present, phage display technology has been applied in the diagnostic research of ASFV. Gaiping Zhang’s team constructed a phage-displayed single-chain variable fragment (scFv) library using peripheral blood mononuclear cells (PBMCs) isolated from ASFV-recovered pigs. Through three rounds of biopanning, eight scFv clones that specifically targeted the ASFV p72 were isolated. Subsequently, they screened a conserved epitope peptide (221MTGYKH226) from a randomized peptide library using scFv-83. This peptide was further verified by flow cytometry and cellular uptake experiments, demonstrating its ability to promote the maturation of bone marrow-derived dendritic cells (BMDCs) and be efficiently uptaken by dendritic cells, suggesting its potential application in vaccine and diagnostic reagent development [75]. There is also a team combined with viral peptide libraries to express recombinant proteins of ASFV and prepared virus-like nanoparticle vaccines to immunize mice to achieve better immune effects [76]. However, to the best of our knowledge, there are few published reports on the use of phage display to determine ASFV antigenic epitopes.
2.9. Bioinformatics Tools
In addition to experimental methods, epitope prediction can also be performed by some epitope prediction software for bioinformatics, which can significantly enhance the efficiency of epitope identification when verified through experiments. These tools typically employ direct prediction methods based on the physicochemical characterization of individual amino acid residues that are common in known epitopes. These characteristics include properties such as β-turn propensity, charge, flexibility, antigenicity, amino acid frequency, surface accessibility, and secondary structure [44, 77–79]. Recently, an increasing number of teams have begun to utilize bioinformatic tools for ASFV epitope prediction.
2.10. Molecular Docking
Molecular docking is an approach to drug design by characterizing the receptor and the mode of interaction between the receptor and the drug molecule [80]. It is a theoretical simulation method that examines intermolecular (such as ligand and receptor) interactions and predicts their binding modes and affinities. While molecular docking has not been directly applied to ASFV epitope identification, Simbulan et al. [80] predicted 14 conserved ASFV epitopes and designed a subunit vaccine. Their study demonstrated that the vaccine effectively induced immune memory and mitigated subsequent pathogen infection by simulating ASFV interactions [80]. This approach offers a shortened vaccine development process, which can effectively address the rapid emergence or re-emergence of highly pathogenic infectious diseases.
2.11. Machine Learning (ML)
ML, a subfield of artificial intelligence (AI), utilizes statistical methods to improve the performance of computers [81]. ML techniques are generally categorized into three fundamental components: models, learning criteria, and optimization algorithms [20]. Two commonly used ML models include support vector machines (SVM) [82] and random forests (RFs) [83]. SVMs are generalized linear classifiers that use a maximal bounded hyperplane to separate different data. RFs combine multiple weak classifiers to produce voting or averaging predictions. The application of AI and ML to epitope prediction has made significant progress in recent years, especially in improving prediction accuracy, handling complex biomolecular interactions, and accelerating drug development. Consequently, an increasing number of research teams are developing and utilizing AI- and ML-based tools. Gajendra P. S. Raghava’s team presented HAIRpred, an ML-based tool for predicting residues in antigens that interact with human antibodies [84]. By combining relative surface accessibility (RSA) and evolutionary information (PSSM), HAIRpred achieves an AUC value of 0.72 on an independent test set, which significantly outperforms existing methods. In addition, HAIRpred utilized the Shapley Additive exPlanations (SHAPs) method to interpret the model predictions, revealing key features that influence the predictions. MAbTope is an AI-based epitope prediction tool that predicts antibody-binding epitopes by analyzing antibody-antigen interactions. Despite initial prediction limitations, the team led by Acar et al. [85] and Linos demonstrated that its accuracy can be enhanced through experimental validation and model refinement. Zhang et al. [86] proposed an in-context learning (ICL)-based approach for generating TCRs targeting novel epitopes. By introducing a small number of known TCRs as contextual information during the training, the model can better generate high-quality TCRs. In addition, self-contemplation prompting (SCP) augments the model’s generalization ability by employing automatically generated TCRs as contextual prompts, thereby establishing self-reinforcing feedback through these dynamically optimized cues. Majila et al. [87] developed a deep learning-based method, Disobind, for predicting interactions between intrinsically disordered protein regions (IDRs) and binding partners. Disobind utilizes the embedding information of the protein language model (pLM) in conjunction with evolutionary information and structural features to dramatically improve prediction accuracy. Compared to AlphaFold2 and AlphaFold3, Disobind performs better at multiple confidence thresholds, especially in predicting interactions of disordered residues. The rapid advancements in AI and ML have greatly contributed to epitope prediction, particularly by enhancing prediction accuracy, elucidating complex biomolecular interactions, and accelerating drug discovery. As a result, an increasing number of research teams are now employing ML methodologies in ASFV-related studies [88, 89]. Despite significant advancements in antigenic epitope identification techniques driven by AI and ML, challenges remain, including data scarcity, model complexity, uncertainty in predictions, and the high cost of experimental validation. Future research should focus on enhancing data collection, optimizing models, improving experimental validation strategies, and integrating multimodal data to increase the accuracy and efficiency of epitope prediction.
2.12. Databases
Recent advancements in biotechnology have led to the continuous generation and public availability of extensive epitope-related data, which can be efficiently accessed through specialized biological databases.
The Immune Epitope Database (IEDB) is an NIH-NIAID-funded publicly available database of TCEs and BCEs curated from the published literature or by direct submission from NIH-NIAID-funded large-scale epitope discovery contracts [90]. On the IEDB homepage, ASFV epitopes can be searched using selected criteria, and subsequent results can be further filtered with additional criteria, including specific assays or receptors. In addition to storing epitope-related data, the IEDB Analytics Resource contains a large number of different prediction tools to perform epitope prediction.
2.13. Summary of Epitope Identification Methods
In general, binding activity-based epitope identification techniques, such as ELISA, offer a cost-effective and rapid means to assess an epitope’s binding affinity to an antibody. However, they are unable to pinpoint the exact binding site and have limited resolution for conformational epitopes, making them more suitable for diagnostic or functional validation studies. Library-based screening techniques, such as phage display, are suitable for large-scale screening of antibody epitopes because they allow for high-throughput screening and identification of key amino acids. Mutation-based techniques can be accurate down to individual amino acids and are suitable for high-resolution epitope localization studies. MS-based techniques can analyze multiple epitopes simultaneously and provide dynamic binding information, which is suitable for conformational epitope and structural dynamics analysis. Structural analysis-based techniques, including X-ray crystal diffraction, cryo-EM, and NMR, elucidate the three-dimensional structure of epitopes, making them particularly valuable for structural investigations of antigen–antibody complexes. Computational approaches integrating AI-driven ML algorithms (e.g., molecular docking simulations and computational epitope mapping tools) enable high-throughput epitope screening with significantly reduced experimental expenditures. These methodologies prove particularly valuable in early-stage vaccine development, providing cost-effective solutions for preliminary epitope characterization and rational experimental design.
Different epitope identification methods have their strengths and limitations, and it is usually necessary to choose the appropriate method according to the research purpose and experimental conditions. For example, a large number of protein sequences can be analyzed in a short time using bioinformatics tools to predict potential BCEs and TCEs, perform preliminary screening of potential epitope regions, then synthetically express the epitopes obtained from the preliminary screening in a truncated form, and finally combine with immunological assays for precise epitope localization. In practice, integrating multiple techniques can enhance the accuracy and efficiency of epitope identification.
Despite the availability of various methods, ASFV antigenic epitope identification remains challenging. The primary obstacle is the high biosafety risk, restricting research to laboratories with BSL-3 facilities. Additionally, the absence of standardized protocols hinders data comparability across studies. These factors collectively impede progress in ASFV epitope identification. Notably, future identification of ASFV antigenic epitopes will benefit from advances in high-throughput screening technologies, computational prediction models, and standardized experimental frameworks. The development of safer and more accessible research models will help overcome biosafety constraints. Integrating these approaches will enhance the accuracy of epitope identification and drive the development of effective ASFV vaccines and diagnostic tools.
3. ASFV Existing Epitopes
The antigenic epitopes of ASFV are distributed across multiple proteins of the virus, including, but not limited to, structural proteins such as p22, p30, p54, p72 [91], and pB602L, as well as nonstructural proteins. These epitopes play a key role in viral infection and immune evasion.
BCEs are the part of the antigen capable of eliciting antibody production by B cells. In ASFV studies, several BCEs have been identified, which may be located on surface proteins of the virus, such as p12, p13, and p14 [92]. TCEs are antigenic parts that can activate T cells. TCEs have also been reported in ASFV studies, and these epitopes are essential for cellular immune responses. Some of the antigenic epitopes of ASFV may be protective, inducing the production of neutralizing antibodies, which are capable of preventing the binding of the virus to host cells and thus preventing viral infection. There may be variants in the antigenic epitopes of ASFV, which may affect the ability of the virus to escape immunity and the effectiveness of the vaccine.
As of February 6, 2024 (the epitope information of the proteins described below was all obtained from the database at this time), 747 ASFV epitopes have been identified and mapped in IEDB, spanning 118 antigenic proteins. The epitopes are mainly distributed on CD2v, p72, p54, p30, pp220, pEP135R, and MGF protein families, and this article mainly focuses on the review of the existing epitopes of five proteins, including CD2v, p72, p54, p30, and pB602L. 108 CD2v protein epitopes, 42 p72 protein epitopes, 35 p54 protein epitopes, 23 p30 protein epitopes, and 11 pB602L protein epitopes have been identified and stored in the IEDB database. Due to the large number of epitopes, it is impractical to describe each one individually. In this paper, we only showcase epitopes with significant research value. Additionally, we map the distribution of the above five protein epitopes obtained from the current studies (Figure 5).
Regarding epitope conservation analysis across different ASFV strains, while limited research has been conducted in this area, AI-driven tools offer a potential solution for sequence comparison and conservation analysis. By examining sequence similarities and variations, researchers can assess epitope conservation across ASFV strains. For example, Gaiping Zhang’s team [93] employed ClustalW to perform a comparative analysis of 20 sequences of the p54 protein in order to evaluate the conservation of the identified epitopes within the epidemic strains. Based on this analysis, they successfully designed highly conserved peptides that mimic the N-terminal domains (aa 1–29) of the ASFV p54 protein.
3.1. CD2v
CD2 is a T-cell protein involved in the synergistic regulation of cellular activation, and it has been shown that CD2v is the only known viral homolog of cellular CD2 [94]. The CD2v protein is encoded by the ASFV EP402R gene and shares similarities with the host CD2 protein. As a transmembrane protein, CD2v can effectively inhibit bystander lymphocyte proliferation in response to mitogens and mediate the absorption of red blood cells to ASFV-infected cells [95]. Malogolovkin et al. [96] demonstrated that the ASFV loci encoding CD2v and C-type lectin proteins mediate the serologic specificity of hemadsorption inhibition (HAI) and that CD2v/C-type lectin genotyping provides a simple method for grouping ASFV strains by serotypes. Shi et al. [49] further suggested that p22, p30, and CD2v elicit immune responses to a lesser extent and are potential candidates for future vaccine development. Given its role in immune modulation and serologic specificity, the study of CD2v epitopes is crucial for vaccine development.
Of the 108 CD2v epitopes stored in the IEDB database, 10 are experimentally validated BCEs and 99 TCEs, of which Galina Burmakina’s team identified six discrete TCE regions present on CD2v and C-type lectins by using an IFN-γ ELISpot assay and PBMCs from animals immunized against African swine fever [97]. Lu et al. [98] used an mAb to identify a novel BCE (264EPSPREP270) located in the CD2v intracellular region. This epitope is highly conserved and can elicit both humoral and cellular immune responses in a mouse model, and immunization of mice with this epitope induces a significant increase in IgG responses, as well as enhanced expression of CD8+ T cells and cytokines.
3.2. p30
The ASFV-encoded structural proteins p72, p54, and p30 are major targets for serologic diagnosis because of their high immunogenicity and conserved nature [94]. Among them, p30, which is encoded by the gene CP204L, exhibits the highest level of expression during the early stages of infection and possesses the greatest immunogenic properties [34].
As a structural protein encoded by the CP204L gene, p30 plays a critical role in viral internalization and entry into host cells [99]. It is one of the most immunogenic proteins expressed early during ASFV infection. This makes p30 an ideal serologic target for African swine fever detection and surveillance. Previous studies have demonstrated that p30 is detectable in macrophages as early as 2 h postinfection and throughout the infection cycle [100]. In addition, an antibody with a neutralizing effect on the p30 was detected as early as 8 days postinfection [101, 102], which was associated with inhibition of viral internalization. Given its immunogenicity and role in viral entry, determining the precise epitope of the p30 protein is crucial for vaccine development. Haixue Zheng’s team utilized p30 as a marker of ASFV replication to investigate metabolic alterations in host cells during infection and found that ASFV infection promotes tricarboxylic acid (TCA) cycling [103], providing insights for the development of novel preventive or therapeutic strategies targeting metabolic pathway alterations.
Among the 23 p30 epitopes documented in the IEDB, 22 are experimentally validated BCEs, and one is a TCE. Current strategies for BCE identification typically involve (i) generating mAbs using recombinant p30 protein, (ii) screening these mAbs against overlapping peptides spanning the antigen, and (iii) analyzing epitope conservation and variability through comparative bioinformatics to refine epitope characterization. Recently, Yu et al. [101] also employed a similar method to identify two novel BCEs (118SFETL122) and (76EHQAQEEWNMILHV89) of p30 protein. A comparative analysis with the IEDB database revealed that nearly all identified epitope sequences contain (118SFETL122), suggesting that the epitope is highly conserved. Due to its strong conservation (118SFETL122) holds potential as a diagnostic tool and a candidate epitope for vaccine development.
3.3. p54
p54, encoded by the E183L gene, is a surface-exposed viral protein that plays a crucial role in viral attachment to host cell receptors and subsequent viral replication. It facilitates viral transport by interacting with host dynamin proteins, allowing viral particles to reach the perinuclear region of the host cell [104]. All 35 p54 epitopes stored in the IEDB database are experimentally validated BCEs. Some researchers have mapped these epitopes, demonstrating several p54 epitopes can be recognized by mAbs and ASFV-positive pig sera [105]. Still, other research teams have begun to screen and characterize epitopes using bioinformatics primary screening of immunogens for synthetic peptides [104]. Recently, Zhao’s team made a significant breakthrough in identifying p54 antigenic epitopes. Their team developed a nanobody-based approach that successfully identified the smallest B-cell linear epitope of p54 (76QQWVEV81) [106]. This marks the first report on utilizing virus-specific nanobodies to detect epitopes that traditional mAbs fail to recognize, introducing nanobody technology as a novel tool for epitope identification. This finding not only expands the applications of nanobodies in epitope research but also provides new theoretical insights into p54-induced neutralizing antibody responses.
3.4. p72
p72 is encoded by the B646L gene, mainly involved in viral capsid assembly, and plays an important role in viral attachment and entry [107]. As the major capsid protein of ASFV, p72 is considered an important immunodominant antigen for serological diagnosis [108]. Notably, antibodies targeting p72 have been shown to block the infection of highly virulent ASFV isolates in Vero and macrophage cultures [109]. Despite the critical role of p72, few studies have focused on its epitopes, making it highly valuable to identify and characterize its neutralizing epitopes through gene cloning and epitope mapping.
Among the 42 p72 epitopes recorded in the IEDB database, 33 are experimentally validated BCEs and 9 are TCEs. In a study by Borca et al. [110], mAb 135D4, which recognizes a conformational epitope on p72, was identified as having partial neutralizing activity. Miao et al. [111] further advanced p72 epitope research by expressing and purifying full-length p72 protein using prokaryotic expression systems. The recombinant protein was used as the antigen to immunize mice, leading to the generation of 17 specific mAbs [111]. Subsequently, seven linear BCEs were identified using iELISA, three of which were consistent with the study by Heimerman et al. [57]. However, validation experiments revealed that antibodies generated against these epitopes did not exhibit neutralizing activity. Despite this, these epitopes were highly conserved among nine ASFV genotypes. Among them, the mAb 2B8D7 recognizing the third epitope (281PENSHNIQTA290) exhibited the highest negative/positive ratio (N/P ratio) in a competitive ELISA (cELISA), indicating that it was able to specifically distinguish between ASFV-positive and -negative sera. After this the team tested six known positive and one negative sera with 2B8D7. The result showed that the percent inhibition (PI) of the positive sera was 93.56%, 95.13%, 92.61%, 91.25%, 90.15%, and 88.63%, while the PI of the negative sera was 9.34%, indicating that 2B8D7 exhibits excellent sensitivity and specificity in ASFV diagnosis, implying its great potential as a diagnostic tool for cELISA. For diagnostics, an mAb targeting the p72 protein has been incorporated into the INGENASA sandwich ELISA kit developed in Spain [57]. It has been recognized by the World Organization for Animal Health as a reference for African swine fever diagnostic kits, demonstrating high specificity and sensitivity [112]. Given the importance of p72 in ASFV infection and immune recognition, advancing the study of the p72 epitope is extremely valuable for diagnosis and vaccine development strategies.
3.5. pB602L
pB602L is encoded by the B602L gene and acts as a molecular chaperone protein for p72 [113], which is involved in the folding of the p72 protein [114]. The absence of pB602L disrupts the formation of the virus’s characteristic icosahedral particle shape, rendering the virus structurally defective [115]. A previous study reported that pB602L caused a strong and specific response to recovering ASFV serum [116]. Therefore, increasing research efforts have been directed toward identifying its antigenic epitopes. Among the 11 pB602L epitopes recorded in the IEDB database, 4 have been experimentally validated as BCEs and 7 are confirmed as TCEs.
Wang et al. [117] prepared prokaryotic expression of recombinant pB602L protein, which was purified and used as an antigen to immunize mice to obtain a total of 8 mAbs. and a set of overlapping peptides of pB602L was used to screen its binding epitopes by Western blot. Finally identified three B-cell linear epitopes (366ANRERYNY373), (415GPDAPGLSI423), and (498EMLNVPDD505) by western blot using an overlapping set of polypeptides from pB602L. In another study, Song et al. [118] generated an mAb 7E7 against pB602L using the hybridoma technique. Western blot and immunofluorescence analyses demonstrated that mAb 7E7 specifically recognizes ASFV Pig/HLJ/2018/strain and eukaryotic recombinant ASFV pB602L proteins in vitro. Further epitope mapping identified the smallest BCE of pB602L as (474SKENLTPDE482) [118], marking a significant advancement in epitope characterization for ASFV diagnostics and vaccine development.
3.6. Others
In addition to studying well-characterized proteins whose functions are well understood or are critical in the process of viral invasion and infection, researchers are increasingly focusing on other conserved protein epitopes, such as the conserved epitope of pC129R identified by Wang et al. [52] using monoclonal technology (18KHYVLIPK25); Hagoss et al. [54] identified two conserved epitopes of pCP312R using the same approach (78DEEVIRMNAE87) and (122KNEQGEEIYP131); Gao et al. [55] identified two novel BCEs of pI215L (67LTFTSEMWHPNIYS80) and (167IEYFKNAASN176) by monoclonal technique, etc., further advancing the development of serological diagnosis and vaccine research for ASFV.
Ouyang’s team also performed high-throughput screening of ASFV epitopes using phage display technology and obtained a large amount of epitope information for the ASFV pig/China/HLJ/18 strain. Notably, the IEDB lists epitope data for only 17 proteins of this strain, predominantly including p72, pB602L, E2, pCP312R, pC129R, and dUTPase (deoxyuridine 5′-triphosphate nucleotidohydrolase). Their screening identified epitopes across 29 ASFV antigenic proteins. Beyond well-characterized antigens such as p30, p54, p72, and B602L, a novel DP238L protein was found to exhibit strong reactivity with convalescent swine sera despite minimal prior documentation. The corresponding epitope data will be published in the future, providing a new foundation for ASFV detection, diagnosis, and vaccine development.
4. Prospects for the Application of ASFV Epitopes
Currently, ASFV epitopes can be applied mainly in three areas (Figure 6). First, they are used in the study of the mechanism of interaction between the virus and the host immune system. Second, neutralizing epitopes can be utilized to develop epitope vaccines against ASFV, which can be prepared as epitope peptide vaccines by combining multiple epitopes and adjuvants or as epitope nucleic acid vaccines by combining multiple epitopes and specific delivery vectors, which can elicit an immune response. The third area of their application is that conserved nonneutralizing epitopes can be used to prepare diagnostic tools. In summary, through the identification and screening of epitope information, the neutralizing epitopes with viral neutralization effect can be used for the preparation of vaccines, the epitopes without neutralization effect but with high antibody-binding affinity can be used for the preparation of diagnostic reagents, and the highly conserved epitopes can be used for the study of host immune mechanism-related aspects.
According to the different binding activities of ASFV epitopes, they can be used for vaccine preparation, diagnostic reagent preparation, and host immunization mechanism studies.
4.1. Applications of Antigenic Epitopes in Mechanistic Studies
Investigating the interactions between epitopes and the immune system provides critical insights into the mechanisms of immune protection against ASFV. Through the analysis of the information on BCEs and TCEs, we can gain a deeper understanding of the viral invasion process, including its molecular targets and associated pathways within host cells. For example, the interaction of SARS-CoV-2′s spike glycoprotein (S protein) with angiotensin-converting enzyme 2 (ACE2) on the surface of the host cell is a key step in viral entry. By recognizing corresponding epitopes, researchers are able to understand how the virus interacts with the host cell, and similarly, by identifying the key epitopes of major antigenic proteins like p30, p54, p72, etc., we may be able to further understand the mechanism of ASFV invasion and immune evasion.
4.2. Applications of Antigenic Epitopes in Diagnosis
Early pathogen detection is critical for disease control, particularly in scenarios where vaccination is unavailable as a preventive measure. Epitope-based diagnostic strategies, characterized by the systematic integration of BCEs and TCEs combined with bioinformatic-driven selection of immunodominant epitopes, have emerged as powerful tools for serological detection of viral antibodies. There are several ASF serologic assays available, mainly based on most of the immunologic and structural proteins of ASFV, such as p72, p30, p54, pp62, and p22 [119]. A large number of studies have now prepared epitope-based diagnostic kits. For example, Jin et al. [31] designed a recombinant protein incorporating antigenic epitopes from p30, p54, and p72 after thorough screening and validation. The protein exhibited specific reactivity with mAbs targeting all three proteins, leading to the establishment of an iELISA assay. Clinical sample testing confirmed its high specificity and sensitivity, providing a novel approach for ASFV serodiagnosis and demonstrating the practical applications of epitope-based research. In addition to this, Dongming Zhao’s team also established a sensitive and specific IFA for ASFV antibody detection. The newly established IFA was highly specific and did not cross-react with sera-positive for six other important porcine pathogens [120]. Recently, Haixue Zhang’s team successfully generated four mAbs targeting the p72 protein by expressing recombinant protein and immunizing mice [121]. They further identified the epitopes recognized by these mAbs through truncation of overlapping peptides. Following a conservation analysis of these epitopes, the team developed mAbs based on the most highly conserved epitope, 20IILAQDLLNSRISNIKNVNKS39. A blocking ELISA method was developed for the detection of ASFV antibodies. This method has an AUC value of 0.9997, a diagnostic sensitivity of 100%, a specificity of 98.51%, and can detect positive standard sera up to a dilution of 1:512 with excellent reproducibility and accuracy.
4.3. Applications of Antigenic Epitopes in Vaccines
Antigenic epitopes can stimulate humoral immunity or specific cellular immunity, which is essential for inducing an antiviral immune response. The concept of epitope vaccines relies heavily on the prediction of immunodominant TCEs and BCEs that can trigger specific and protective immune responses. Traditional approaches to the development of ASFV vaccines have included inactivated viruses, recombinant proteins/peptides, viral vectors for antigen delivery, and live attenuated vaccines (LAVs); attempts at inactivated vaccines have failed, and both subunit vaccines and viral vectors provide only partial protection. As a result, LAVs remain the most promising strategy for inducing protective immunity. Nevertheless, LAVs pose significant risks. Despite attenuation, the virus retains some level of activity, raising concerns about potential reversion to virulence. Additionally, LAVs may not be suitable for immunocompromised individuals or individuals with weakened immune systems. In contrast, epitope-based vaccines offer a safer and potentially more effective alternative. These vaccines are derived from specific pathogen epitopes, eliminating the risk of virulence reversion. Moreover, through the selection and optimization of conserved epitopes across multiple ASFV strains, epitope vaccines hold promise for cross-protection. While no ASFV epitope vaccine has progressed to clinical-stage development, the foundational validity of this approach is evidenced by its successful implementation in human antiviral vaccines. For instance, Gregory A.’s team developed the FLU-v influenza vaccine candidate by chemically synthesizing six TCEs targeting conserved protein sequences of human influenza viruses [122], which successfully protected mice against a variety of unrelated strains of influenza viruses. This vaccine successfully protected mice against multiple unrelated influenza strains, offering a novel approach to overcoming the annual challenge of updating influenza vaccines in response to viral mutations.
5. Discussion and Outlook
ASF is characterized by rapid onset, high infectiousness, and high mortality, causing huge economic losses to the global pig industry. The main reason is the lack of adequate and effective prevention and control strategies. ASFV is structurally complex and the virus interferes with the host immune response through multiple cell signaling pathways. In addition to this, the diversity of virulent strains further complicates vaccine development. Nonetheless, through tireless efforts, Vietnam has developed an effective attenuated vaccine against ASF [123]. Early experiments have shown that the vaccine provides significant protection against homozygous genotype II strains (e.g., ASFV-G). For example, in an attack experiment, 100% of immunized pigs survived 28 days after vaccination, while all unimmunized pigs died. However, the vaccine did not provide sufficient protection against recombinant strains (e.g., recombinant type I/II strain VNUA/rASFV/TN1/23, which appeared in Vietnam in 2023). Experiments showed that immunized pigs still showed typical symptoms such as high fever and death after the attack, and surviving pigs showed sequelae such as lameness. In addition to this, the vaccine’s protective efficacy against nongenotype II strains remains unverified, raising concerns about insufficient cross-protection. Nevertheless, it is not difficult to believe that safer and more effective vaccines can be developed shortly.
Although the development of vaccines against ASF has never stopped, vaccine research still needs to be improved. Compared with conventional vaccines, epitope vaccines are safer, more stable and directly trigger immunity against the pathogenic microorganism. The core principle of epitope vaccine development is to induce protective immunity by identifying and utilizing key antigenic epitopes of ASFV. The development strategy for ASFV epitope vaccines consists of three key steps. The first step of the strategy of epitope vaccine development is the identification of protective epitopes, and the focus of this step is to pay attention to the type of antigen that can induce strong neutralizing antibodies. Secondly, based on the design concept of structural vaccinology, multiple protective epitopes can be integrated into a single carrier protein or nanoparticle to form a multivalent vaccine, which enhances the immunogenicity of the epitopes with the possible generation of cross-protective antibodies, and finally, selecting appropriate adjuvants and delivery systems to improve the presentation efficiency of the epitopes and enhance the immune response. The primary challenge in current epitope vaccine development is the potential complexity and variability of antigenic epitopes of ASFV, which increases the difficulty of identifying and screening epitopes. In addition, in the design of multiepitope vaccines, whether immune competition occurs between different epitopes and how to optimize the combination of epitopes so that each epitope can effectively induce immune responses is also a key issue that needs to be addressed. But it is worth mentioning that recent advances in technologies such as bioinformatics analysis and the availability of large amounts of genomic and proteomic data have accelerated the development of subunit vaccines, and in particular, the application of the latest immunoinformatics will improve our understanding of the nature of the epitopes and enable researchers to develop better strategies for immune intervention.
Finally, epitope research work still faces many challenges. Epitope prediction and validation remain key hurdles, particularly in distinguishing between linear and conformational epitopes. Linear epitopes are composed of consecutive amino acid sequences, which do not require complex folding to bind to antibodies. However, existing algorithms are based on sequence conservation or physicochemical properties of amino acids, ignoring conformational factors, resulting in oversimplified algorithms, and predicting short peptides that may lack the key spatial features of the natural conformation, which makes it difficult to trigger an effective immune response. Conformational epitopes are composed of amino acid residues that are spatially proximate but discontinuous in sequence, requiring precise three-dimensional structural data for accurate prediction. Experimentally determining these structures is both costly and time-consuming, and similar conformations in different proteins may lead to errors in antibody recognition.
In summary, linear epitope prediction requires balancing the effects of sequence features and potential conformations, while conformational epitope prediction requires high-precision structural modeling and molecular interaction simulation. Recent advancements in deep learning and structural prediction tools offer promising solutions: (i) utilize AlphaFold2 and RoseTTAFold for structural modeling, (ii) integrate MHC-binding prediction tools with local structural feature analysis to enhance linear epitope prediction, and (iii) apply molecular dynamics simulations to evaluate epitope stability and immunogenicity before experimental validation. Although various epitope prediction tools and methods are available, their application in vaccine design remains limited. The accuracy of epitope prediction still requires further validation through experimental studies. Additionally, while some antigenic epitopes capable of inducing neutralizing antibodies have been identified, there is still a lack of applied research on these epitopes. Diagnostic tools for conserved epitopes have been prepared, but most vaccine application studies are still conducted in vitro or in nonhost animals like mice. Additionally, since the epitopes are located on the viral surface, they may mutate during contact with the external environment. Therefore, future research should focus on advancing epitope prediction techniques to identify conserved antigenic epitopes. Optimizing algorithms and validating predictions through experimental verification will be essential to ensuring that the identified epitopes can be effectively utilized in vaccine development.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Writing–original draft preparation: Xingjun Ke and Zhi Cao. Illustrations: Mengjie Lian, Zhen Weng, Yin Xie, Tongyu Liu, Fengyu Wu, Shiying Zhou, Xinzhu Liu, Ruize Sun, Lerong Ma, Jiaqi Wang, Aishi Xu, and Hongsheng Ouyang. Writing–review and editing: Dongmei Lv, Linzhu Ren, and Daxin Pang. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the Jilin Province Science and Technology Development Plan Project (20230508117RC) and the Scientific Research Project of the Education Department of Jilin Province (JJKH20220971KJ).
Acknowledgments
The authors acknowledge the Jilin University Library for providing the full text of several inaccessible articles.
Open Research
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




