Fomites Could Determine Severity of SARS-CoV-2 Outbreaks in Low-Density White-Tailed Deer (Odocoileus virginianus) Populations
Abstract
The establishment of a reservoir species for zoonotic diseases is concerning for both animal and human health. Severe acute respiratory syndrome coronavirus (SARS-CoV)-2, the coronavirus responsible for the COVID-19 pandemic, has been detected in white-tailed deer (Odocoileus virginianus) in the United States. Since its initial detection, various studies have documented circulation and evolution of SARS-CoV-2 in deer, with human cases suspected of spill-back from infectious deer. A priority for mitigating SARS-CoV-2 outbreaks in deer populations is determining the contribution of direct (via aerosols and physical contact) and indirect (via contaminated objects and media) transmission pathways. We expanded existing epidemiological models founded on direct transmission pathways to include three indirect transmission pathways of infection for simulated deer populations, including contaminated water, food waste, and feed piles. Despite lower infection probabilities and transmission hazards (measured by force-of-infection (FOI)) posed solely by these indirect pathways compared to direct transmission pathways, the addition of indirect transmission pathways increased FOI, which had ramifications for the severity of SARS-CoV-2 outbreaks in simulated deer populations, particularly in populations with low degrees of spread between deer (measured by basic reproductive number; R0). We used contact rate models to estimate SARS-CoV-2 spread across deer range in the United States and identified widespread potential for indirect transmission to increase the severity of outbreaks in low-density deer populations. These results indicate that indirect transmission pathways need to be considered in the management of white-tailed deer as a reservoir species for SARS-CoV-2.
1. Introduction
Zoonotic diseases, or those that are transmitted between human and nonhuman hosts, account for over 60% of emerging infectious diseases in humans [1]. The maintenance of zoonotic pathogens in animal hosts is a threat to animal and human health, as these reservoirs increase the potential for pathogens to evolve or recombine into new strains or variants that have different epidemiological and pathological characteristics [2]. The persistence of pathogens in reservoir hosts can have broader population and ecosystem effects, in addition to risks to human public health [3]. Reservoir hosts can transmit pathogens to other susceptible species, leading to disease that can negatively impact host populations [4] and disrupt trophic interactions in the broader ecosystem [5]. Preventing the establishment of zoonotic disease reservoirs in nonhuman hosts is a priority for managing threats to public health [2, 6]. A key to prevention is a better understanding of transmission pathways that are critical to the introduction and persistence of zoonotic disease in potential reservoir species.
Coronaviruses are viral pathogens with broad host ranges and are responsible for numerous epizootics [7]. Coronaviruses have rapid mutation rates, which can lead to increased zoonotic potential during prolonged circulation in nonhuman hosts [8]. While zoonotic coronaviruses have emerged from bat species, multiple other animal species have served as intermediate or reservoir hosts, including masked palm civets (Paguma larvata) for severe acute respiratory syndrome coronavirus (SARS-CoV)-1 and dromedary camels (Camelus dromedarius) for Middle East respiratory syndrome coronavirus (MERS-CoV) [9–11]. The global pandemic caused by SARS-CoV-2 may have originated in horseshoe bats (Rinolophus spp.) and subsequently spread to other wildlife species, posing risks of nonhuman reservoirs producing emergent variants that could reinfect humans [8, 12].
One SARS-CoV-2 host of concern in the United States is white-tailed deer (Odocoileus virginianus, hereafter deer) because of its susceptibility, sociality with conspecifics, broadscale distribution, high abundance in some habitats, and potential for frequent interactions with humans. The captive deer industry, with 200,000 deer in captivity in 2012, adds an additional component of disease spillover potential to wild deer via fenceline transmission from captive to wild deer [13]. This concern is underscored by repeated detections of SARS-CoV-2 in captive and wild deer populations across broad geographical ranges [14]. Moreover, some SARS-CoV-2 variants circulating in deer populations descend from earlier human variants of concern that, once introduced into deer, have continued to adapt at a faster rate than observed in humans [15, 16]. These divergent variants have the potential to spillback to humans and affect the severity of disease and efficacy of treatments [15, 17].
Decision makers need accurate predictions of SARS-CoV-2 outbreak dynamics to better inform disease mitigation across wildlife, agricultural, and human health sectors (collectively termed One Health). There is a lack of information regarding the contributions of direct and indirect transmission pathways that lead to the persistence of SARS-CoV-2 in deer (Figure 1). The tissue tropism of SARS-CoV-2 favors upper respiratory tract tissues in humans, deer, and other wildlife species [18]. However, broader tissue tropism of SARS-CoV-2 in the oral cavity and nasopharynx suggests that indirect transmission through ingestion and contact with contaminated fomites and media poses another potential, but understudied, contribution to SARS-CoV-2 infection in deer populations, as observed in other wildlife diseases [19–21]. As deer continue to be infected with SARS-CoV-2 across the United States [22], ignoring the contributions of indirect transmission pathways may lead to incorrect assessments of population vulnerability to continued circulation of SARS-CoV-2 and the role of deer as a reservoir species. In total, this incomplete picture may limit management and regulatory opportunities across One Health sectors to reduce spread and evolution of novel variants in deer.
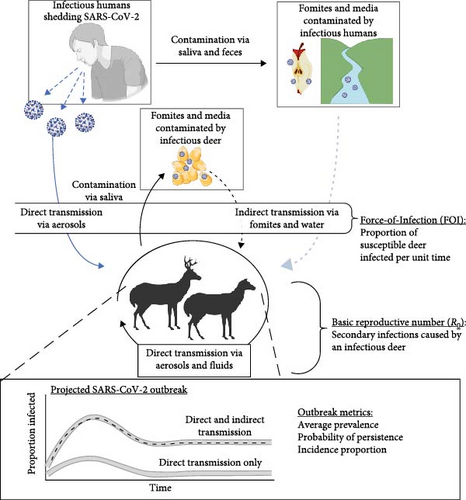
We evaluated direct and indirect pathways for their ability to sustain disease transmission in simulated wild deer populations (Figure 1). We modified existing epidemiological models of direct SARS-CoV-2 transmission in deer and humans to incorporate indirect transmission from three pathways, including ingestion of contaminated water sources, human food waste, and supplemental feed. We identify the epidemiological conditions under which outbreak severity is most sensitive to the cumulative risks posed by direct and indirect transmission and describe the spatial distribution of hazards according to varying conditions across deer range. Our simulation results highlight the importance of considering a broader range of transmission pathways when evaluating the potential for pathogen reservoirs to maintain infections in animal species and identify potential pathways that drive long-term spread and the evolution of novel variants which may be targeted for management.
2. Materials and Methods
We use three analyses to evaluate the contribution of indirect transmission to SARS-CoV-2 outbreaks in deer. First, we estimated the probability of infection and force-of-infection (FOI) for susceptible deer from three indirect transmission pathways: deer drinking from contaminated water sources, deer consuming contaminated food waste, and deer eating at communal and supplemental feed piles. We compared probability of infection and FOI for direct transmission from infectious humans via aerosols using estimates from Rosenblatt et al. [23]. We characterized the increase of cumulative FOI when considering both direct and indirect transmission pathways. Second, we extended the susceptible-infectious-recovered-susceptible (SIRS) approach used in [23] to project SARS-CoV-2 outbreaks in wild deer given both direct and indirect transmission pathways. We used these results to understand the sensitivity of various outbreak metrics to changes in FOI and spread of SARS-CoV-2 between deer (basic reproductive number; R0). Finally, with these insights into outbreak sensitivity to FOI and R0, we identified portions of the deer range in the contiguous United States where the additional hazards of indirect transmission pathways could alter SARS-CoV-2 outbreaks.
We focused on quantifying the contribution of indirect transmission in the introduction and spread of SARS-CoV-2 in wild deer inhabiting suburban areas (100 humans/km2) [24], because the indirect transmission pathways outlined here are clearly contextualized in a suburban setting. Calculations were conducted in R [25] and parameter values used in these calculations are listed in Table S1. The R code and associated data are publicly available from Rosenblatt et al. [26].
2.1. Probability of Infection
Contaminated water sources: We calculated probability of SARS-CoV-2 infection from human wastewater to a wild deer drinking from a contaminated water source. To simplify our calculations and to be conservative in our estimates, we assumed that contaminated wastewater was instantly well-mixed into natural waterways and that the virus did not settle into the sediment before ingestion by the deer.
Contaminated food waste: We calculated the probability of SARS-CoV-2 infection for a susceptible deer eating a single discarded apple core that was contaminated by an infectious human.
We converted published human salivary viral load () to PFU concentration using an estimate of the genomic copies to PFU, gcSARS−CoV−2/PFUSARS−CoV−2. We calculated the volume of human saliva deposited on the apple core as the product of the surface area of the apple core, approximating the apple core as a cylinder with radius rapple core and height happle core, the depth of saliva in the human’s mouth (dsaliva), and the proportion of that saliva transferred to the apple core (psaliva transfer). We estimated a range of apple core sizes by measuring a small and large apple. The core radii were 1.0 and 1.6 cm and heights were 5.4 and 7.6 cm, respectively.
We assumed that the apple core was completely consumed by a susceptible deer.
2.2. FOI
We calculated the probability of infection (σpathway) for contaminated water sources and fomites above (Equation 1). We simulated 1000 iterations of exposure rates to pair with the 1000 iterations of infection probability calculations detailed above. We compared resulting FOI estimates across pathways and with FOI estimates for direct transmission via aerosols from humans [23].
We calculated privers and pcontaminated for each county across the deer range in the contiguous United States (Table S1). The county-level iwater estimates were used to generate 1000 random draws to pair with our 1000 random draws of , to calculate FOIwater.
Contaminated food waste: We assumed that a susceptible deer ingests 0–10 contaminated apple cores per day, drawn from a uniform distribution (cfood waste). We have no empirical estimates available for this consumption rate, so we set this upper bound to represent exposure from backyard compost piles. Prevalence in humans (iH) was fixed at 5% to match conditions described in Rosenblatt et al.’s [23] epidemiological models.
We did not include feedpile transmission in this calculation as we were interested in the additional spillover hazard posed by human-contaminated fomites and water. We converted our continuous metric of direct transmission FOI to four categories using the getJenksBreaks() function from the BAMMtools package [36] to visualize sensitivity patterns. We also assigned cumulative FOI values with both direct and indirect transmission to these categories. We summarized the proportion of simulations that increased in FOI category with the inclusion of indirect transmission pathways.
2.3. Epidemiological Modeling
We used SIRS ordinary differential equations (ODEs) to project proportions of the wild population that are susceptible (sW), infected (iW), and recovered (rW), starting with a naïve population (sW,t=0 = 1; iW,t=0 = rW,t=0 = 0). We projected continuous SARS-CoV-2 introduction and spread over a 120-day fall season, considering both direct and indirect transmission pathways (Table S2). We ran 1000 iterations of a simulated outbreak for a wild deer population, with and without the presence of intensive captive facilities that would allow fenceline transmission between captive and wild deer (referred to as “wild-captive complex” and “wild,” respectively). Each iteration had a randomly drawn parameter set. We projected the proportion of deer that were susceptible, infectious, or recovered for 120 days for each iteration, using the ODE solver ode() from the deSolve package in R [25, 37]. We excluded feedpile transmission from the SIRS approach because our focus was to examine the role of indirect transmission from human-contaminated water sources and fomites.
We binned outbreak metrics from each simulation by R0 and FOI categories to test the sensitivity of outbreaks to the addition of indirect transmission. We defined R0 categories of R0 < 0.5, 0.5 ≤ R0 ≤ 1, 1 < R0 ≤ 2, 2 < R0 ≤ 3, and 3 < R0, spanning conditions where spread is unlikely, spread is characterized by stuttering chains, spread is likely sustained, and disease is widespread, respectively [41]. We summarized median outbreak metrics and 95% confidence for each combination of R0 and FOI category. We report this summary for wild deer simulations in the main text and report results for the wild–captive complex simulations in the supplemental materials.
2.4. Areas of Wild Deer Range Sensitive to Increases in FOI
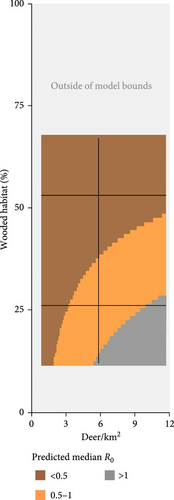
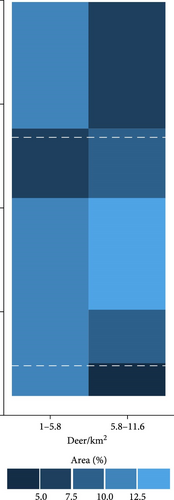
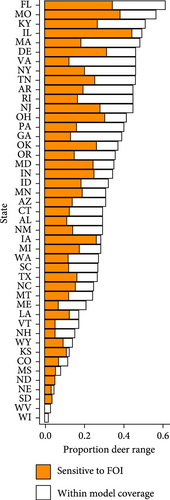
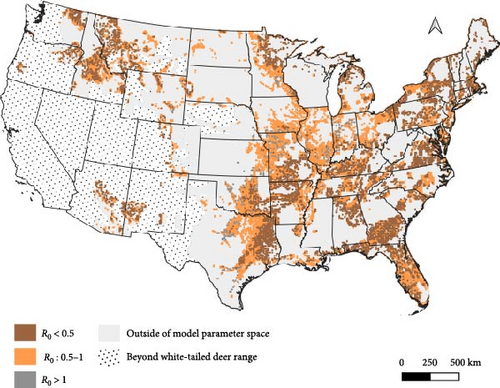
We used the proximity rate model developed by Habib et al. [42] to estimate deer–deer contact rates and median R0 across gradients of wooded habitat availability and deer density. This modeling approach described wooded habitat availability [43] and deer density [44] for 57% of the deer range containing lower density populations in the contiguous United States. Based on our sensitivity results, we identified combinations of wooded habitat availability and deer density that would predict R0 values sensitive to the additional hazard posed by indirect transmission pathways. We estimated contact rates and median R0 values across the deer range in the contiguous United States using land cover [43] and deer density [44] geospatial datasets rescaled to 100 km2 pixel resolution in QGIS (version 3.32.3-Lima; qgis.org). We note that the range-wide deer density dataset used for this analysis [44] presents data from 2003. To our knowledge, there are no equivalent datasets available from a more recent time period. We reported the proportion of pixels in each state that had any probability of median R0 falling within ranges sensitive to additional FOI from indirect transmission.
3. Results
Indirect transmission pathways were an important driver of SARS-CoV-2 dynamics in simulated wild deer populations because they increased the overall hazard of SARS-CoV-2 transmission (FOI) from humans to wild deer and the maintenance of simulated outbreaks. Relative to direct transmission from humans to deer via aerosols, indirect transmission pathways had lower and more variable FOI and were less likely to lead to human-to-deer infection (Figure 2A). Median FOI for pathways associated with contaminated water sources, human food waste, and deer feed piles were 10.5%, 0.5%, and <0.1% of the median FOI for aerosolized transmission from humans, respectively. The median probability of SARS-CoV-2 infection for these indirect pathways were all <0.1% of the median probability of infection for aerosolized transmission from humans. Despite small relative values, spillover hazards from indirect pathways, when added to hazards from direct transmission, resulted in an overall increase in FOI (Figure 2B).
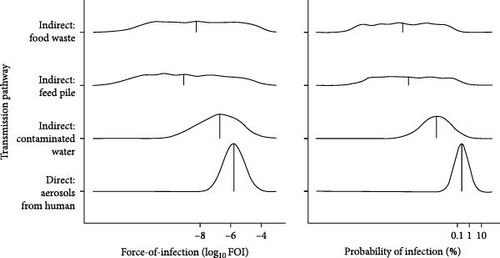
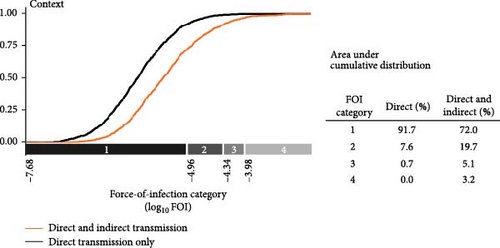
3.1. Indirect Transmission Pathways Pose Additional, Highly Variable Hazards of Spillover of SARS-CoV-2 to Wild Deer Populations
When we categorized FOI values by their natural breaks, we estimated that the addition of indirect transmission pathways to aerosol transmission from humans increased the number of simulations with higher FOI categories (categories 2–4) from 8.3% to 28% (Figure 2B). FOI rates increased by a median of 38.5% across all simulations with the addition of indirect transmission pathways, with high variation among simulations (2.5% quantile = 0.03%, 97.5% quantile = 14,633%). Most of these increases in cumulative FOI resulted in shifts from the lowest FOI category to the second lowest FOI category (70.4%), yet a substantial proportion (29.6%) of these cumulative FOI increases resulted in shifts to higher FOI categories (categories 3 and 4; Figure 2B).
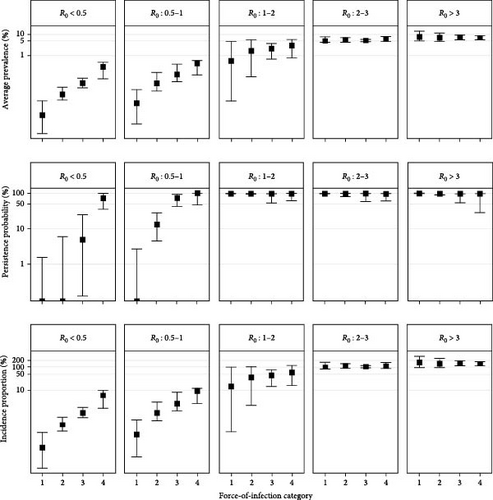
3.2. When R0 is Low, Indirect Transmission Pathways Could Increase the Severity of a SARS-CoV-2 Outbreak in Wild Deer
With their cumulative FOI hazard, direct and indirect transmission pathways alter disease outbreaks in wild deer compared to outbreaks projected with direct transmission pathways alone. However, the magnitude of this change depended on a simulation’s basic reproductive number (R0) or the number of secondary infections caused by a single infectious deer in a naïve population via direct transmission (e.g., direct contact and aerosol transmission). When we simulated disease outbreaks over a 120-day fall season and partitioned simulations by R0, we found that average prevalence, persistence probability, and incidence proportion were sensitive to FOI (Figure 3). These sensitivities were especially pronounced in simulations with a resulting R0 between 0.5 and 1. This R0 range can lead to stuttering chains of infection that will not be sustained without repeated introduction events, such as those occurring by indirect transmission pathways [41]. We found that in areas with low R0 values, that the added FOI hazard from indirect transmission pathways could increase the severity of a SARS-CoV-2 outbreak. We did not detect any changes to this sensitivity when wild deer faced additional hazards from fenceline interactions with captive deer also experiencing SARS-CoV-2 outbreaks (Figure S1).
3.3. The Potential for Deer to Serve as Reservoir Species for SARS-CoV-2 Is Likely Underestimated
There is widespread potential that low-density deer populations (≤11.6 deer/km2) could be sensitive to increases in spillover hazard from indirect transmission pathways. We mapped combinations of wooded habitat availability and deer density and highlight areas in 45 U.S. states, where we find the combination of deer density and habitat result in expected increases in spillover hazard from indirect transmission (Figures 4 and 5). The proportion of area within the sensitive range of R0 varied greatly between states, though this was partially influenced by the varying proportion of each state’s area that fell within the range of conditions that were supported by our model (Figure 4). Areas with sensitive epidemiological conditions (0.5 ≤ R0 ≤ 1) were widespread across deer range in the contiguous United States (Figure 5). Our results suggest that the maintenance of SARS-CoV-2 reservoirs in deer is likely to be underestimated if epidemiological models do not consider the contribution of indirect transmission pathways to the spillover hazard.
4. Discussion
We found that the severity and persistence of SARS-CoV-2 outbreaks in deer depended, in part, on indirect transmission pathways between humans and wild deer. We estimated lower and more variable probabilities of SARS-CoV-2 infection and FOI for indirect transmission pathways compared to direct transmission from humans through aerosols. However, when we integrated both direct and indirect transmission pathways into our epidemiological models, we found that increases in cumulative FOI was consequential for an outbreak’s severity. These differences were especially apparent when deer densities and forested cover favored lower rates of deer contact and deer-to-deer transmission. Recent work suggests that a single SARS-CoV-2 spillover event infecting even a small proportion of a deer populations can result in outbreak dynamics like those resulting from continual direct spillover from humans [23]. Here, we demonstrate that the marginal increase of spillover risk when considering indirect transmission pathways can change the outcome of spillover events, leading to higher probabilities of persistence that can set the stage for viral evolution to occur.
One Health decision-making is complex and in the case of SARS-CoV-2 in deer, our results suggest that indirect pathways warrant consideration when attempting to mitigate spillover and spread of SARS-CoV-2. Based on differences in spillover risk between direct and indirect transmission pathways, decision makers may consider a portfolio of management actions that mitigate multiple transmission pathways. In populations where wooded habitat conditions and deer density result in high rates of spread (R0 > 1), any form of spillover from any infectious host to deer, be it through direct or indirect transmission pathways, could result in a SARS-CoV-2 outbreak. In this case, decision makers may choose to focus on reducing both spillover and postintroduction spread, including reducing high deer densities, minimizing long duration of deer–deer proximity, and regulating use of attractants and supplemental feed [23, 45]. If a population’s spread of SARS-CoV-2 is low (R0 < 1), decision makers may instead prioritize actions to reduce spillover risks, either from direct transmission from humans or from indirect transmission pathways. This prioritization is critical when a population’s spread of SARS-CoV-2 could result in stuttering chains (0.5 ≤ R0 ≤ 1). Based on our work, direct transmission from humans poses the greatest spillover risk, and therefore, any reduction could alter rates of SARS-CoV-2 introduction into wild deer populations. However, management alternatives could be prioritized with focus on reducing spillover risk through indirect transmission pathways, particularly in areas with high human densities, and thus, higher risk of contaminated fomites.
This study demonstrates the potential risks posed by indirect transmission pathways and how they contribute to the maintenance of SARS-CoV-2 in wild deer populations. We report conditions where indirect transmission may be most important to consider across low-density deer ranges in the United States. While not sufficient as a standalone method to pinpoint specific locations and actions for risk mitigation, our findings suggest that decision makers could consider the roles of all viable transmission pathways, including those involving contaminated fomites when deciding whether and how to reduce the risk of SARS-CoV-2 spread in wild deer. These efforts could be aided by formal decision analysis methods, including structured decision making [46, 47], that may help in structuring decisions around localized contexts and that consider actions by multiple One Health sectors. Decision analysis methods can also inform where more precise parameter estimates would be helpful, including those involving indirect transmission pathways.
Our study may have broader implications for pathogen maintenance in a diversity of hosts and disease systems that include indirect transmission pathways. Wildlife species interact with their environment in complex ways that can facilitate indirect pathogen transmission, particularly through ingestion of concentrated food [48, 49]. Contamination of food surfaces and media from an individual host shedding pathogens can occur from settling respiratory droplets or biological fluid on contact [50]. Once contaminated, viral loads can persist on fomites for hours to days for some pathogens, including SARS-CoV-2; this prolonged viability external to hosts may lead to many opportunities for indirect transmission to occur [51]. Importantly, fomite transmission does not require both infectious and susceptible individuals to overlap in space and time [52]. Fomites present additional zoonotic transmission opportunities between humans and animals that may otherwise rarely overlap in proximity required for direct transmission [52].
Our SIRS approach and parameter set inherently assumed several characteristics of both simulated deer populations and the SARS-CoV-2 virus. Our inference that deer populations with low R0 are most sensitive to increases in FOI from direct and indirect transmission are based on naïve, randomly mixing populations exposed to a single variant of SARS-CoV-2. These conditions were appropriate for the purpose of this study as an initial exploration of disease dynamics; however, risk assessments for specific populations require careful consideration of these assumptions. Seroconversion has been detected in many wild deer populations across their range in the United States [14], potentially reducing the proportion of susceptible individuals in populations. Further, deer populations are likely not randomly mixed; social behavior and resource availability determine contact networks for a given deer population [53, 54]. Viral decay rate, infectivity, and the duration of infection and temporary immunity in deer likely vary across different SARS-CoV-2 variants, as documented in humans [55]. Modeling inferences and risk assessments could benefit from incorporating multiple variants and spatially explicit contact processes between individuals in simulations of populations previously exposed to SARS-CoV-2.
The indirect pathways altered outbreak dynamics in our simulations, yet more work remains in detecting and monitoring infectious SARS-CoV-2 virions on contaminated surfaces and in media. First, the efficacy for SARS-CoV-2 to infect a host when ingested during contact with fomites remains unknown. Our chosen dose–response function used parameters based on aerosolized transmission, which may overestimate the probability of infection from ingested virions [56]. While we reduced the dose received to account for the potential of reduced transmission efficiency (Equation 1 and Table S1), this knowledge gap warrants further study and may affect the risk posed by indirect transmission. Second, there have been limited studies demonstrating transmission risk via contaminated water sources, largely due to the challenges in isolating enveloped virions at low concentrations [57]. Secondary and tertiary wastewater treatments systems inactivate most SARS-CoV-2 virions, but the efficacy likely varies by treatment methodology [57, 58]. Further, untreated wastewater effluent is sometimes released into otherwise natural waterways during extreme flow events and mechanical failure at treatment facilities [29]. At the time of this study, there was no evidence of infectious virions in wastewater effluent. However, even a low risk of infection may cause spillover to wildlife populations drinking from these water sources, in addition to exposure to other contaminated fomites. Given our findings, further study of SARS-CoV-2 viability and dosages in and on contaminated fomites would be beneficial.
5. Conclusions
With the continued detection of SARS-CoV-2 in white-tailed deer and other wildlife species, the reservoir potential for SARS-CoV-2 in wildlife remains a concern. Despite a partial understanding of the modes of SARS-CoV-2 transmission, this study demonstrates that the rate of direct and indirect disease transmission may be important to pathogen circulation and maintenance. While the hazard posed by indirect transmission pathways are lower than direct transmission from humans, the cumulative hazard from both direct and indirect transmission pathways alter disease outbreaks beyond conditions predicted by epidemiological models that only consider direct transmission from humans. The apparent ease by which deer populations spread and sustain SARS-CoV-2 suggests that there may be multiple transmission pathways that contribute to the potential establishment of white-tailed deer as a reservoir species for a rapidly evolving zoonotic pathogen with profound human health implications.
Disclosure
Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government. This is publication #09 of the Disease Decision Analysis and Research Group (DDAR) of the U.S. Geological Survey.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This work was supported by the Coronavirus Aid, Relief, and Economic Security Act (P.L. 116-136).
Acknowledgments
We thank Dan Walsh and two anonymous journal reviewers for their comments that improved this manuscript.
Supporting Information
Additional supporting information can be found online in the Supporting Information section.
Open Research
Data Availability Statement
The R code underpinning this analysis is available through [57] and GitHub at https://github.com/erosenbl/Indirect_SARS.git. Data are publicly available in USGS ScienceBase https://doi.org/10.5066/P19KKRVV.




