Comparative Analysis of Salivary and Serum Inflammatory Mediator Profiles in Patients With Rheumatoid Arthritis and Periodontitis
Abstract
Background: Periodontitis (PD) and rheumatoid arthritis (RA) are chronic inflammatory conditions, characterized by dysregulated immune response and excessive production of inflammatory mediators. The oral disease PD is triggered by periodontal pathogens, leading to the destruction of tissues surrounding the teeth, whereas RA is a systemic autoimmune disease primarily affecting the joints. The objective of this study was to investigate the prevalence of PD and map the profile of salivary and serum inflammatory mediators of patients with RA, with respect to periodontal severity (PD stage II and PD stage III/IV).
Methods: For this cross-sectional cohort study, 62 patients diagnosed with RA were recruited. All participants underwent a full-mouth dental examination. Levels of various inflammatory mediators, including tumor necrosis factor (TNF) superfamily proteins, interferon (IFN) family proteins, regulatory T cell (Treg) cytokines, and matrix metalloproteinases were determined in saliva and serum samples from each participant using a human inflammation multiplex immunoassay panel.
Results: In the current RA cohort, all participants were diagnosed with PD, of which 35.5% were classified as PD stage II and 64.5% as PD stages III/IV. Inflammatory mediator levels were significantly higher in both saliva and serum samples from patients with RA and PD stages III/IV, compared to those with RA and stage II within the same cohort. These included higher serum levels of sCD30, IL-10, IL-19, osteopontin and elevated salivary levels of BAFF/TNFSF13B and IFN-α2. Additionally, APRIL/TNFSF13 levels were increased in both saliva and serum.
Conclusions: Among the studied patients with RA, the majority exhibited severe PD (stage III/IV), underscoring the importance of periodontal prophylaxis and treatment for this group of patients. Higher levels of inflammatory mediators were observed in both saliva and serum in those with PD stages III/IV, suggesting a potential link between the severity of PD and systemic inflammation in RA. Further research is needed to explore the clinical implications of these findings.
1. Introduction
Periodontitis (PD) is a chronic inflammatory disease that affects the structures surrounding and anchoring the teeth to the mandibular and maxillary bone. With an estimated prevalence of 42%–72.4% in the adult population, PD is one of the most prevalent chronic inflammatory diseases [1, 2]. Pathogenesis of PD is associated with a profound shift in the composition of subgingival bacterial communities and the accumulation of periodontal pathogens in the tooth-supporting tissues [3, 4]. It is known that periodontal pathogens collectively initiate an inflammatory response leading to the production of numerous inflammatory mediators, cytokines, proteolytic enzymes, prostaglandins, and toll-like receptors which can cause the destruction of alveolar bone and connective tissue [5, 6]. Proinflammatory cytokines associated with PD include interleukin (IL)-1, IL-6, IL-12, IL-17, IL-18, IL-21, tumor necrosis factor (TNF)-α, and interferon (IFN)-γ [5, 7]. Regulatory cytokines such as IL-4, IL-1Ra, and IL-10 are also suggested to be involved in the pathogenesis of PD [8]. Moreover, PD has previously been recognized for its systemic implications, suggesting a relationship to several systemic diseases such as cardiovascular diseases, diabetes mellitus, and rheumatoid arthritis (RA) [9–11].
RA is a chronic inflammatory disease with a prevalence of 1% of the world population, predominantly affecting women [12]. This disease is characterized by synovial joint inflammation and irreversible destruction of cartilage and underlying bone in the joints [13]. Synovial joint inflammation is associated with the accumulation of inflammatory mediators, including TNF, IL-1, IL-6, IL-16, and IL-17, driving the systemic inflammation [14]. Autoantibodies known as anticitrullinated protein antibodies (ACPAs) are established biomarkers of RA, present in ~70% of patients, often years before the clinical onset of the disease [15]. These autoantibodies target the citrulline sidechain, a posttranslational modification where arginine is converted to citrulline, exposed on proteins [15]. The oral pathogen Porphyromonas gingivalis, associated with PD, is the only identified bacteria known to catalyze citrullination, potentially contributing to the generation of ACPAs [16, 17]. Specifically, P. gingivalis infection might contribute to the development or exacerbation of RA by inducing the production of citrullinated proteins, which can trigger the autoimmune response in genetically susceptible individuals [18].
Multiple studies have suggested an epidemiological association between PD and RA [19, 20]. Both diseases share a similar profile of cytokines and inflammatory mediators that are present in synovial joints of patients with RA as well as in periodontal tissue in individuals with PD [17]. In addition, studies have reported an increased risk of PD in patients diagnosed with RA and vice versa an increased incidence of RA in patients with PD [21–23]. Previous research has shown a high prevalence of severe PD in patients with RA ranging from 29% to 48% [24, 25], compared to an estimated 7.8%–17.6% in general population [1, 2]. However, these observations were based on previous periodontal classifications. In this study, we aimed to assess the prevalence of PD in patients with RA, according to the currently used periodontal classification scheme, which encompasses both the severity and extent of the disease through stages [26]. Additionally, we aimed to profile salivary and serum inflammatory mediators in patients with RA, in relation to periodontal disease, according to the current classification stages.
2. Patients and Methods
2.1. Study Population and Patients
For this cross-sectional cohort study, 78 patients with RA were recruited. The inclusion criteria were a confirmed RA diagnosis and an age of 18 years or older. Exclusion criteria included recent antibiotic use or periodontal treatment within the past 3 months, pregnancy, lactation, or unwillingness to participate. As a result, 16 participants were excluded from the study. All participants were recruited from the Rheumatology Department at Karolinska University Hospital, Huddinge. Data regarding ACPA, rheumatoid factor (RF), C-reactive protein (CRP), and disease activity score (DAS-28) were collected from the responsible rheumatologist. In addition, all participants answered questionnaires regarding general health, medications, tobacco-smoking habits, personal information, e.g., level of education, and a health assessment questionnaire (HAQ). A dentist (K.E), calibrated by a periodontist (L.J), conducted a full dental examination of all participants at the Department of Dental Medicine at Karolinska Institutet, Huddinge. Dental examination included clinical periodontal variables at six sites on each tooth measuring: probing pocket depth (PPD) ≥4 mm, bleeding on probing (BOP), and clinical attachment loss (CAL). Plaque index (PI) was recorded at four sites on each tooth, excluding third molars. In addition, missing teeth, furcation involvement, and mobility were recorded. Dental radiographic examination was performed which comprised of bitewing X-rays and one orthopantogram. Periodontal diagnosis was based on the current periodontal classification scheme according to AAP/EFP [26]. Sample size calculation was performed using a statistical power analysis tool, based on data from our previous study [27], including inflammatory mediator levels in saliva and serum from patients with RA and PD (severe/moderate PD and gingivitis/mild PD). The analysis estimated a minimum of 59 participants to achieve a significance level of 0.05 and a statistical power of 0.95 (a = 0.05, β = 0.95).
2.2. Collection of Saliva and Serum Samples
Stimulated saliva and serum samples were collected from each study participants. The participants were not allowed to drink or eat 1 h prior to the examination, and saliva was collected before initiation of the oral examination. Stimulated saliva was collected by chewing on paraffin wax (1 g, Ivoclar Vivadent Liechtenstein) for a duration of 2 minutes, as previously described [28, 29]. After measuring the volume and flow rate, it was placed into 15 ml falcon tubes and centrifuged at 5°C with 500 × g for 10 min. Thereafter, the supernatants were aliquoted to 1.5 ml tubes and stored at −80°C until analysis of inflammatory mediators. Salivary total protein concentrations were measured using the DC (detergent compatible) protein assay (Bio-Rad Laboratories AB) following the manufacturer’s instructions. Blood samples were collected in BD Vacutainer SST tubes (MediCarrier AB, Stockholm, Sweden), which were then left at room temperature for 30 min to allow clot formation. The serum samples were then centrifuged at 200× g for 10 min at 20°C and aliquoted in 1.5 ml tubes at −80°C until further processing and analysis.
2.3. Analysis of Inflammatory Mediators in Saliva and Serum Samples
Levels of inflammatory mediators in saliva and serum samples were determined using human inflammation multiplex Immunoassay panel (Bio-Rad Laboratories, Hercules, CA, USA), which includes TNF superfamily proteins, IFN family proteins, regulatory T cell (Treg) cytokines, and matrix metalloproteinases (MMPs). The samples falling below the sensitivity level of the assay were replaced with the limit of detection (LOD) specific to each respective analyte, as specified by the manufacturer (Bio-Rad Laboratories). The analyzed inflammatory mediators (detection limits in pg/ml) were APRIL/TNFSF13 (190), BAFF/TNFSF13B (34.7), sCD30/TNFRSF8 (1.0), sCD163 (16.8), Chitinase 3-like 1 (10.3), gp130/sIL-6Rβ (16.9), IFN-α2 (0.7), sIL-6Rα (1.5), IL-8 (2.7), IL-10 (0.6), IL-11 (0.05), IL-12/p70 (0.1), IL-19 (0.2), IL-20 (3.6), IL-22 (1.1), IL-26 (1.2), IL-27/p28 (0.1), IL-29/IFN-λ1 (1.6), IL-32 (12.3), IL-34 (51.9), IL-35 (3.7), LIGHT/TNFSF14 (10.2), MMP-1 (33.7), MMP-2 (39.7), MMP-3 (28.5), osteocalcin (23.4), osteopontin (91.3), Pentraxin-3 (0.8), sTNF-R1 (0.2), sTNF-R2 (3.2), TSLP (0.8), and TWEAK/TNFSF12 (0.5), as previously described by Eriksson et al. [27]. The following inflammatory mediators were excluded from further analysis due to the majority of samples being below the LOD: in serum (IFN-α2, IL-8, IL-11, IL-12, IL-20, IL-22, IL-27, IL-32, IL-34, LIGHT/TNFSF14, MMP-1, and TSLP) and in saliva (sCD30/TNFRSF8, IL-11, IL-12, IL-20, IL-27, IL-29/IFN-λ1, IL-32, LIGHT/TNFSF14, MMP-1, MMP-2, osteocalcin, osteopontin, and TSLP).
2.4. Statistical Analysis
Descriptive statistics and statistical analyses were performed using R.4.2.0. and SPSS (IBM SPSS Statistics 21.0; SPSS Inc). For comparison between the groups, Wilcoxon rank sum test was used for numerical variables, and Chi-square test was used for categorical variables. The Spearman correlation analysis was adopted to investigate the correlations between RA duration and the demographic/anamnestic variables. For the logistic regression model, backward procedures were used with the occurrence of bleeding pockets (sites with PPD ≥4 mm and BOP ≤3.0, >3.0), bleeding index (BI) (≤30, >30), and PD stage III/IV versus stage II as the dependent variables and inflammatory mediators in saliva/serum and potential confounders as independent variables, included in the model if p < 0.10. Potential confounders were defined as variables correlated (Spearman correlation p < 0.010) to at least one of the inflammatory mediators and the dependent variable. The results were adjusted for age, ACPA status, and smoking status (current/former smokers). Inflammatory mediator levels were log-transformed to achieve normality. The level of statistical significance was set at p < 0.05. Random Forest analysis was done using R package randomForest v4.7.1.1. The randomForest mtry parameter (i.e., number of variables randomly sampled as candidates at each split) was tune with the function tuneRF, and the MeanDecreaseGini values were used as indicative of the feature importance. The train/test data split for Random Forest model calibration was carried out using a stratified sampling with the function initial split of the R package tidymodels v.1.1.1. To evaluate the model prediction power, two approaches were used: test dataset validation and when using the training dataset, an out-of-bag (OOB) approach [30]. Receiver operating characteristic (ROC) and area under the curve (AUC) were calculated using the R package pROC [31]. Principal coordinate analysis (PCoA) was performed using the R package vegan v2.6-4. The analysis was conducted using all periodontal variables and inflammatory mediators in saliva and serum samples. Before PCoA, the data were scaled by dividing each column by its standard deviation while retaining the original mean. To visualize correlations between salivary and serum inflammatory mediators, sparse partial least squares discriminant analysis (sPLS-DA) method was conducted, using mixOmics R package. Prior to the sPLS-DA analysis, the salivary and serum inflammatory mediator data was normalized to zero mean and unit variance.
3. Results
3.1. Characteristics of the Study Population
A total of 62 participants diagnosed with RA were included in this study. Demographic data collected from all participants, incorporating both the clinical examination and the health questionnaire, are shown in Table 1. Enrolled participants were predominantly women (90%), with a median age of 64 years and a median RA duration of 8.5 years from diagnosis of RA. All study participants exhibited the presence of PD; 35.5% (n = 22) were diagnosed with stage II, 48.4% (n = 30) with stage III, and 16.1% (n = 10) with stage IV. Among the participants, 8.1% (n = 5) were diagnosed with PD grade A, 80.1% (n = 50) with PD grade B, and 11.3% (n = 7) were diagnosed with PD grade C. As for comorbidities, 40.3% were diagnosed with temporomandibular joint (TMJ) disorders, 21% with gastrointestinal disorders, 20.1% with asthma, 19.4% with high blood pressure, and 12.9% with cardiac disorders. The majority of the study participants were positive for ACPA (77.4%) and RF (64.5%).
| Characteristics | All study participants (n = 62) | Periodontitis Stage II (n = 22) | Periodontitis stage III/IV (n = 40) | p ∗ |
|---|---|---|---|---|
| Female gender, n (%) | 56 (90.3) | 22 (100) | 34 (85) | 0.14 |
| Age, median (Q25%; Q75%) | 64 (55; 70) | 59 (41; 69) | 66 (62; 71) | 0.021 |
| BMI, median (Q25%; Q75%) | 24.2 (20.8; 27.8) | 24.7 (20.7; 28.3) | 23.6 (20.7; 27.6) | 0.94 |
| RA duration (years) median (Q25%; Q75%) | 8.5 (2.6; 16.0) | 10.0 (3.5; 16.0) | 8.0 (2.0; 16.7) | 0.61 |
| Comorbidities, n (%) | ||||
| Diabetes | 4 (6.5) | 1 (4.5) | 3 (7.5) | 0.67 |
| Cardiac disorders | 8 (12.9) | 3 (13.5) | 5 (12.5) | 0.91 |
| Vascular disorders | 5 (8.1) | 1 (4.5) | 4 (10) | 0.79 |
| High blood pressure | 12 (19.4) | 4 (18.2) | 8 (20) | 0.87 |
| Gastrointestinal disorders | 13 (21.0) | 2 (9.1) | 11 (27.5) | 0.19 |
| Osteoporosis | 5 (8.1) | 2 (9.1) | 3 (7.5) | 0.84 |
| Asthma | 13 (20.1) | 3 (13.6) | 10 (25) | 0.52 |
| Sjögrens syndrome | 6 (10.0) | 3 (13.6) | 3 (7.5) | 0.74 |
| Liver disease | 3 (4.8) | 0 (0) | 3 (7.5) | 0.48 |
| TMD | 25 (40.3) | 13 (59.1) | 12 (30) | 0.049 |
| Medication, n (%) | ||||
| Analgesics/NSAID | 31 (50) | 11 (50) | 20 (50) | 0.88 |
| DMARDs | 42 (67.7) | 15 (68.2) | 27 (67.5) | 0.96 |
| bDMARDs | 24 (38.7) | 10 (45.5) | 14 (35) | 0.59 |
| Glucocorticoids | 28 (45.2) | 8 (36.3) | 20 (50) | 0.44 |
| Current/former smokers, n (%) | 41 (66.1) | 10 (45.5) | 40 (77.5) | 0.010 |
| Current snuff, n (%) | 6 (9.7) | 0 (0) | 6 (15) | 0.14 |
| Alcohol consumption, n (%) | ||||
| Monthly | 37 (59.7) | 13 (59.1) | 24 (60) | 0.80 |
| Weekly | 32 (51.6) | 10 (45.5) | 22 (55) | 0.52 |
| Daily | 7 (11.3) | 1 (4.5) | 6 (15) | 0.41 |
| University degree, n (%) | 27 (43.5) | 11 (50) | 16 (40) | 0.30 |
| ACPA positive, n (%) | 48 (77.4) | 18 (81.2) | 30 (75) | 0.55 |
| DAS-28, median (Q25%; Q75%) | 3.3 (2.4; 4.1) | 3.1 (2.1; 3.9) | 3.3 (2.5; 4.3) | 0.44 |
| RF (IU/mL) positive, n (%) | 40 (64.5) | 12 (54.5) | 28 (70) | 0.62 |
| HAQ score, median (Q25%; Q75%) | 0.88 (0.38; 1.4) | 0.69 (0.25; 1.2) | 1.0 (0.5; 1.5) | 0.20 |
| CRP (ng/mL), median (Q25%; Q75%) | 3 (1; 5) | 2 (1; 5) | 3 (1; 5) | 0.34 |
- Note: Data are presented as median (Q25%; Q75%) or as number (percentage).
- Abbreviations: ACPA, anticitrullinated peptide antibodies; bDMARDs, biological disease modifying antirheumatic drugs; BMI, body mass index; CRP, C-reactive protein; DAS-28, disease activity score; DMARDs, disease-modifying antirheumatic drugs; HAQ, health assessment questionnaire for rheumatoid arthritis; NSAID, nonsteroidal anti-inflammatory drugs; RA, rheumatoid arthritis; RF, rheumatoid factor; TMD, disorders involving the temporomandibular joint.
- ∗Comparison between groups with periodontitis stage II and stage III/IV.
When comparing PD stages II with III/IV, demographic differences were observed. The median age in the group with stage III/IV was higher as compared to the group with stage II (Table 1). Concerning smoking, more former/current smokers were present in the group with the severe forms of PD (stage III/IV:77.5% vs stage II:45.5%, p = 0.01). Furthermore, the prevalence of TMJ also differed between the groups (stage II:59.1% vs stage III/IV:30%, p = 0.049). There were no significant differences between the two groups regarding gender, body mass index (BMI), RA duration, alcohol consumption habits, or level of education. Additionally, RA duration was not correlated with these variables. Furthermore, no significant differences were observed between the two groups regarding the use of analgesics, nonsteroidal anti-inflammatory drugs (NSAIDs), disease-modifying antirheumatic drugs (DMARDs), biological disease-modifying antirheumatic drugs (bDMARDs), or glucocorticoids. Similarly, comorbidities such as diabetes, cardiac and vascular disorders, gastrointestinal disorders, osteoporosis, asthma, Sjögren’s syndrome, and liver diseases did not differ significantly between groups. Moreover, no significant differences were found in ACPA, RF, DAS-28, HAQ, or CRP when comparing RA patients with PD stage II to those with PD stage III/IV (Table 1).
3.2. Clinical Periodontal Variables in Relation to the Severity of PD
Next, we investigated the clinical periodontal characteristics stratified into two groups based on PD stages II and III/IV. Periodontal variables including PI, BOP, PPD, CAL, number of missing teeth, mobility, and furcation involved teeth as well as stimulated salivary rate flow, collected during the clinical dental examination, are demonstrated in Table 2. In the group with stage III/IV, an increase (p < 0.05) was found in sites with PPD ≥4 mm with BOP, PPD 4-5 mm and PPD > 5 mm, interproximal sites with PPD ≥5 mm, CAL 3-4 mm, CAL ≥5 mm, as well as interproximal sites with CAL ≥4 mm, and CAL ≥6 mm, number of missing teeth, and furcation involvements, as compared to group stage II. The presence of CAL 1-2 mm, however, was higher (p = 1.34 × 10−6) in PD stage II as compared to PD stage III/IV. No significant differences could be observed in PI, BOP, stimulated salivary flow rate, or total protein concentrations when comparing the two groups.
| Periodontal variables, median (Q25%; Q75%) | Periodontitis stage II (n = 22) | Periodontitis stage III/IV (n = 40) | p ∗ |
|---|---|---|---|
| Plaque Index | 52.2 (42.4; 68.2) | 47.3 (34.8; 62.5) | 0.12 |
| Bleeding Index | 27.7 (16.2; 60.9) | 32.9 (22.7; 49.2) | 0.98 |
| Sites with PPD ≥ 4 mm and BOP | 1.2 (0.60; 3.3) | 3.9 (1.3; 8.8) | 0.0031 |
| Sites with PPD 4–5 mm | 1.2 (0.60; 4.2) | 6.7 (2.3; 12.2) | 0.00047 |
| Sites with PPD >5 mm | 0.0 (0.0; 0.0) | 0.70 (0.0; 2.4) | 4.43e–05 |
| Interproximal sites with PPD ≥5 mm | 0.0 (0.0; 0.25) | 2.0 (0.0; 4.0) | 0.00065 |
| Sites with CAL | |||
| 1–2 mm | 71.5 (59.4; 84.1) | 35.2 (15.9; 58.9) | 1.34e–06 |
| 3–4 mm | 25.1 (10.0; 25.1) | 54.3 (31.5; 66.1) | 1.16e–05 |
| ≥5 mm | 0.0 (0.0; 0.85) | 5.1 (2.4; 14.8) | 1.75e–07 |
| Interproximal sites with CAL | |||
| ≥4 mm† | 1.0 (0.0; 2.2) | 6.0 (4.0; 14.7) | 7.92e–07 |
| ≥6 mm | 0.0 (0.0; 0.0) | 0.0 (0.0; 3.0) | 0.00017 |
| Missing teeth | 3.6 (0.0; 7.1) | 10.7 (3.6; 20.5) | 0.0028 |
| Mobile teeth | 0.0 (0.0; 0.0) | 0.0 (0.0; 13.9) | 0.14 |
| Furcation involvement | 6.4 (2.7; 21.4) | 17.0 (10.3; 23.5) | 0.016 |
| Stimulated salivary flow rate (ml/min) | 1.7 (1.2; 2.3) | 1.5 (0.72; 2.0) | 0.29 |
| Total salivary protein (mg/ml) | 1.8 (1.6; 2.5) | 2,0 (1.5; 2.7) | 0.42 |
- Note: Data are presented as median (Q25%; Q75%) or as number (percentage).
- Abbreviations: BOP, bleeding on probing; CAL, clinical attachment loss; PPD, probing pocket depth.
- ∗Comparison between groups with periodontitis stage II and stage III/IV.
- †For periodontitis stage II, interproximal sites with CAL did not exceed 4 mm.
3.3. Levels of Inflammatory Mediators in Saliva Samples
Levels of numerous inflammatory mediators, included in the human inflammation panel, were analyzed in stimulated saliva samples. Among the analyzed inflammatory mediators (Table 3), salivary levels of APRIL/TNFSF13, BAFF/TNFSF13B, and IFN-α2 were significantly higher (p < 0.05) in the group with a severe form of PD (stage III/IV) compared to the group with PD stage II (Table 3, Supporting Information 1: Figure S1A). The remaining inflammatory mediators were not significant (p > 0.05) and are listed in Table 3.
| Inflammatory mediators in saliva, median (Q25%; Q75%) | Periodontitis stage II (n = 22) |
Periodontitis stage III/IV (n = 40) |
p ∗ |
|---|---|---|---|
| APRIL/TNFSF13 | 33,572 (15215; 61750) | 60,643 (34359; 175252) | 0.018 |
| BAFF/TNFSF13B | 2072 (1561; 3088) | 2995 (2306; 4146) | 0.039 |
| sCD163 | 731 (17; 1676) | 927 (17; 2502) | 0.60 |
| Chitinase 3-like 1 | 6287 (3488; 13861) | 6576 (3261; 11011) | 0.53 |
| gp130/sIL-6Rβ | 7958 (1636; 10397) | 6767 (2773; 12948) | 0.63 |
| IFN-α2 | 28 (8.1; 73) | 59 (33; 112) | 0.019 |
| sIL-6Rα | 151 (54; 471) | 238 (96; 571) | 0.38 |
| IL-8 | 750 (453; 1418) | 893 (416; 1451) | 0.71 |
| IL-10 | 2.5 (0.82; 5.2) | 3.8 (2.1; 6.5) | 0.067 |
| IL-19 | 86 (53; 263) | 177 (45; 338) | 0.45 |
| IL-22 | 6.2 (1.1; 11) | 7.9 (2.5; 20) | 0.095 |
| IL-26 | 6.4 (2.6; 8.4) | 6.8 (2.7; 15) | 0.18 |
| IL-34 | 125 (52; 377) | 226 (58; 449) | 0.35 |
| IL-35 | 123 (63; 231) | 187 (91; 340) | 0.15 |
| MMP-3 | 83 (28; 594) | 297 (28; 936) | 0.33 |
| Pentraxin-3 | 180 (73; 416) | 265 (145; 597) | 0.31 |
| sTNF-R1 | 432 (215; 1033) | 672 (288; 1159) | 0.44 |
| sTNF-R2 | 22 (5.7; 884) | 62 (23; 310) | 0.68 |
| TWEAK/TNFSF12 | 25 (14; 64) | 45 (21; 106) | 0.12 |
- Note: Data are presented as median (Q25%; Q75%).
- Abbreviations: APRIL/TNFSF13, a proliferation-inducing ligand; BAFF/TNFSF13B, B-cell-activating factor; gp130/sIL-6Rβ, glycoprotein 130; IFN-α2, interferon alpha 2; IL, interleukin; MMP-3, matrix metalloproteinase-3; sCD163, soluble cluster of differentiation 163; sIL-6Rα, soluble interleukin-6 receptor alpha; sTNF-R1, soluble TNF receptor 1; sTNF-R2, soluble TNF receptor 2; TWEAK/TNFSF12, tumor necrosis factor-like weak inducer of apoptosis.
- ∗Comparison between groups with periodontitis stage II and stage III/IV.
3.4. Levels of Inflammatory Mediators in Serum Samples
Levels of corresponding inflammatory mediators determined in serum samples of participants, with different stages of PD, are presented in Table 4. When comparing PD stage II with PD stage III/IV, levels of APRIL/TNFSF13, sCD30/TNFRSF8, IL-10, IL-19, and osteopontin were higher (p < 0.05) in the PD group with stage III/IV (Table 4, Supporting Information 1: Figure S1B). The inflammatory mediators in serum that were not statistically significant are presented in Table 4.
| Inflammatory mediators in serum, median (Q25%; Q75%) | Periodontitis stage II (n = 22) |
Periodontitis stage III/IV (n = 40) |
p ∗ |
|---|---|---|---|
| APRIL/TNFSF13 | 62,921 (39482; 103618) | 128,109 (65667; 152030) | 0.0013 |
| BAFF/TNFSF13B | 7828 (5658; 9446) | 9675 (6851; 14375) | 0.083 |
| sCD30/TNFSF8 | 176 (133; 321) | 266 (197; 419) | 0.028 |
| sCD163 | 46,318 (35479; 65115) | 50,938 (36564; 80064) | 0.47 |
| Chitinase 3-like 1 | 12,500 (9148; 17420) | 14,823 (8743; 22568) | 0.48 |
| Gp130/sIL-6Rβ | 27,528 (24303; 32064) | 28,855 (23286; 31126) | 0.80 |
| sIL-6Rα | 12,302 (9467; 14765 | 12,729 (10312; 13386) | 0.81 |
| IL-10 | 8.3 (2.6; 18) | 17 (11; 19) | 0.024 |
| IL-19 | 9.6 (2.6; 26) | 27 (16; 33) | 0.0050 |
| IL-26 | 54 (38; 63) | 61 (51; 75) | 0.084 |
| IL-29/IFN-λ1 | 14 (7.7; 86) | 49 (2.3; 98) | 0.58 |
| IL-35 | 32 (19; 63) | 43 (31; 86) | 0.17 |
| MMP-2 | 3731 (3226; 7680) | 4716 (3703; 7171) | 0.43 |
| MMP-3 | 4611 (3468; 9286) | 6577 (3967; 10312) | 0.18 |
| Osteocalcin | 1557 (937; 1710) | 1588 (1106; 2294) | 0.14 |
| Osteopontin | 18,284 (14434; 23828) | 27,096 (20544; 34139) | 0.014 |
| Pentraxin-3 | 176 (87; 224) | 192 (156; 289) | 0.095 |
| sTNF-R1 | 1817 (1280; 2482) | 2243 (1902; 3069) | 0.079 |
| sTNF-R2 | 2,172 (1402; 3934) | 2361 (1497; 5049) | 0.65 |
| TWEAK/TNFSF12 | 161 (133; 184) | 167 (139; 198) | 0.64 |
- Note: Data are presented as median (Q25%; Q75%).
- Abbreviations: APRIL/TNFSF13, a proliferation-inducing ligand; BAFF/TNFSF13B, B-cell-activating factor; Gp130/sIL-6Rβ, glycoprotein 130; IL, interleukin; MMP, matrix metalloproteinase; sCD163, soluble cluster of differentiation 163; sCD30/TNFSF8, soluble CD30; sIL-6Rα, soluble interleukin-6 receptor alpha; sTNF-R1, soluble TNF receptor 1; sTNF-R2, soluble TNF receptor 2; TWEAK/TNFSF12, tumor necrosis factor-like weak inducer of apoptosis.
- ∗Comparison between groups with periodontitis stage II and stage III/IV.
3.5. Relation of PD Severity with Levels of Salivary and Serum Inflammatory Mediators
Next, we performed logistic regression analysis including salivary and serum inflammatory mediators as independent variables, adjusted for age, ACPA, and smoking (former/current smokers) (Tables 5 and 6). None of the potential confounders were included in the models (p > 0.10). Concerning salivary mediators, when using PPD ≥4 mm and BOP as the dependent variable, the cytokines APRIL/TNFSF13, IL-34, sTNF-R2, and TWEAK/TNFSF12 were positively correlated with PPD ≥4 mm and BOP (Table 5). Furthermore, when using BI as the dependent variable (Table 5), the highest OR among salivary inflammatory mediators was found for BAFF/TNFSF13B and sTNF-R2 both showing a positive correlation with BI. On the contrary, the inflammatory mediators sCD163, IL-19, and MMP-3 were negatively correlated with BI. Moreover, when using stage III/IV vs. stage II as the dependent variable, positive correlations were found for salivary IFN-α2 levels and age (Table 5). Overall, for the logistic regression analyses using the three periodontal parameters: PPD ≥4 mm and BOP, BI, and stage as the dependent variable; 83%, 81%, and 80%, respectively, of the cases were correctly classified by the model.
| Dependent variables | Independent variables | OR | 95% CI | p |
|---|---|---|---|---|
| PPD ≥4 mm and BOP | APRIL/TNFSF13 | 1.71 | 1.3–2.85 | 0.039 |
| IL-10 | 2.58 | 0.96–6.95 | 0.060 | |
| IL-34 | 0.12 | 0.03–0.49 | 0.003 | |
| sTNF-R2 | 1.58 | 1.01–2.48 | 0.047 | |
| TWEAK/TNFSF12 | 3.55 | 1.11–11.33 | 0.032 | |
| Bleeding index | BAFF/TNFSF13B | 10.71 | 1.33–85.95 | 0.026 |
| sCD163 | 0.59 | 0.35–0.98 | 0.041 | |
| IL-10 | 2.42 | 0.98–6.02 | 0.056 | |
| IL-19 | 0.39 | 0.18–0.85 | 0.017 | |
| MMP-3 | 0.51 | 0.27–0.97 | 0.039 | |
| sTNF-R2 | 1.97 | 1.14–3.38 | 0.015 | |
| Stage III/IV vs. stage II | Age | 1.07 | 1.01–1.14 | 0.033 |
| IFN-α2 | 1.75 | 1.01–3.03 | 0.046 |
- Note: The results were adjusted for age, ACPA status, and former/current smokers. Odds ratios (OR) with 95% confidence intervals (CI) are presented.
- Abbreviations: ACPA, anti-citrullinated protein antibody; APRIL/TNFSF13, a proliferation-inducing ligand; BAFF/TNFSF13B, B-cell-activating factor; BOP, bleeding on probing; IFN-α2, interferon alpha 2; IL, interleukin; MMP-3, matrix metalloproteinase 3; PD, periodontitis; PPD, probing pocket depth; sCD163, soluble cluster of differentiation 163; sTNF-R2, soluble TNF receptor 2; TWEAK/TNFSF12, tumor necrosis factor-like weak inducer of apoptosis.
| Dependent variables | Independent variables | OR | 95% CI | p |
|---|---|---|---|---|
| PPD ≥4 mm and BOP | Gp130/sIL-6Rβ | 34.09 | 2.11–549.63 | 0.013 |
| IL-26 | 4.93 | 0.75–32.26 | 0.096 | |
| Bleeding index | Former/current smokers | 0.21 | 0.05–0.92 | 0.038 |
| IL-19 | 1.96 | 0.96–4.03 | 0.065 | |
| sCD163 | 0.30 | 0.07–1.17 | 0.083 | |
| Stage III/IV vs. stage II | Age | 1.11 | 1.02–1.20 | 0.013 |
| ACPA positive | 0.07 | 0.01–0.74 | 0.027 | |
| IL-19 | 3.00 | 1.37–6.57 | 0.006 |
- Note: The results were adjusted for age, ACPA status, and former/current smokers. Odds ratios (OR) with 95% confidence intervals (CI) are presented.
- Abbreviations: ACPA, anti-citrullinated protein antibody; BOP, bleeding on probing; Gp130/sIL-6Rβ, glycoprotein 130; IL, interleukin; PD, periodontitis; PPD, probing pocket depth; sCD163, soluble cluster of differentiation 163.
With regard to serum samples, using PPD ≥4 mm and BOP as the dependent variable, a positive correlation was found with the levels of Gp130/sIL-6Rb (Table 6). Additionally, using BI as the dependent variable, former/current smoking status showed a negative correlation (Table 6). When employing stage III/IV vs. stage II as the dependent variable, a positive correlation was observed between the levels of IL-19 and age. ACPA positivity was shown to be negatively correlated with stage III/IV. In the logistic regression analyses using PPD ≥4 mm and BOP, BI, and PD stages as the dependent variables, the model correctly classified 69%, 66%, and 73% of the cases, respectively.
3.6. Characterization of Similarities and Differences between PD Stage II and Stage III/IV
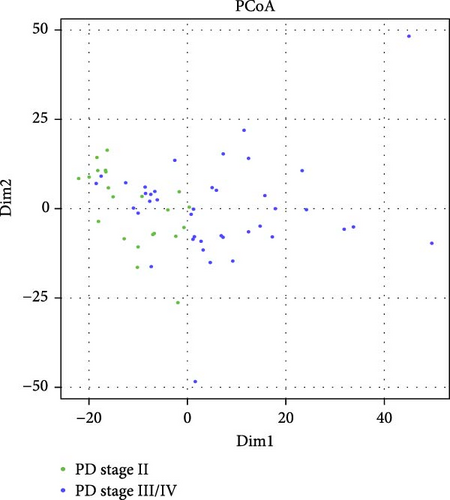
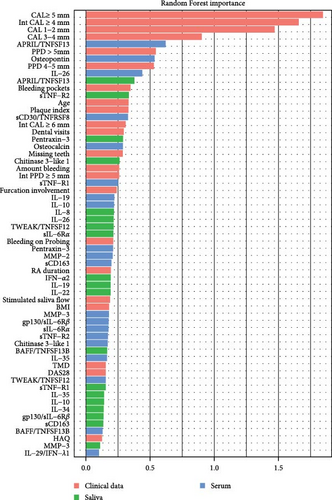
To visualize similarities and dissimilarities between the group with PD stage II and stage III/IV in patients with RA, PCoA was performed based on Manhattan distances (Figure 1A). Trends leaned toward the left side for the group with PD stage II, while the group with PD stages III/IV showed less uniformity. To further investigate whether the collected clinical data and salivary and/or serum inflammatory mediators could be used to distinguish between PD stage II and stage III/IV, we applied a Random Forest model (Figure 1B). The dataset was split (stratified sampling), where 46 samples were used to train the model and 16 samples to test the model. The analysis revealed the periodontal variables: CAL ≥5 mm, intCAL ≥4 mm, CAL 1-2 mm, CAL 3-4 mm, and PPD >5 mm and the serum inflammatory mediators APRIL/TNFSF13 and osteopontin as important variables (MeanDecreaseGini values >0.5) distinguishing between PD stage II and PD stage III/IV in patients with RA. The OOB approach on train dataset (46 samples) showed an F1 score of 0.852, and the model validation using the test dataset (16 samples), showed an F1 score of 0.737 and an AUC of 0.85.
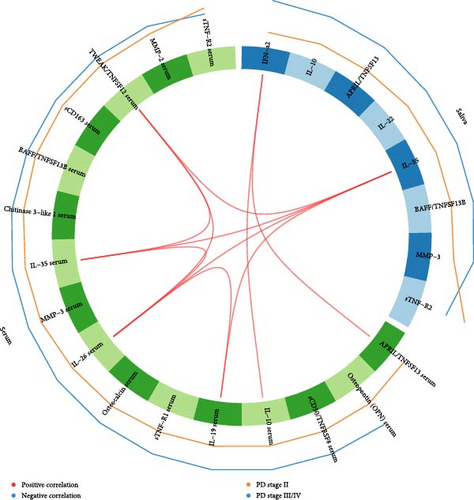
3.7. Correlations between Salivary and Serum Inflammatory Mediators
Figure 2 shows a Circos plot illustrating inter- and intracorrelations between salivary and serum inflammatory mediators. Positive correlations were observed between salivary IFN-α2 and serum levels of APRIL/TNFSF13 and IL-10, as well as between salivary IL-35 and serum TWEAK/TNFSF12, IL-35, IL-26, and IL-19. Additionally, positive intracorrelations were identified in serum between IL-26 and TWEAK/TNFSF12, IL-35 and IL-19, as well as between TWEAK/TNFSF12 and IL-35 (Figure 2).
4. Discussion
The objectives of this study were to investigate periodontal status based on the current periodontal classification and assess the levels of local and systemic inflammatory mediators in patients with RA. Our findings demonstrate a high prevalence of PD stage III/IV in patients with RA and elevated levels of the cytokine APRIL/TNFSF13 in both saliva and serum of RA subjects with severe PD (stage III/IV).
In our RA cohort, 100% of the participants exhibited clinical signs of periodontal disease (PD stage II–IV). Previous studies investigating the prevalence of PD in patients with RA have reported an increased risk and higher prevalence of severe PD when compared to non-RA controls [21, 24, 25]. Research on the prevalence of PD in patients with RA, in relation to the current AAP/EFP periodontal classification scheme, is scarce. In a previous study, our research group reported that 75% of the RA participants exhibited moderate to severe PD, whereas the remaining individuals exhibited no or mild PD, based on the former PD classification criteria from the year 2007 [27]. Regarding the general prevalence of PD using the current periodontal classifications scheme, a study investigated the prevalence of PD in a Norwegian population. Based on their findings, the prevalence of PD in adults was 72.4%, whereas 41% was diagnosed with PD stage II and 17.5% with stage III/IV [2]. Findings from a Swedish study that used similar diagnostic criteria for PD revealed a 40% prevalence of moderate and severe PD [32]. Furthermore, a recent global study reported that 12.5% of individuals had severe PD [33]. In this study, 64.5% had more severe PD (stage III/IV), which suggests that patients diagnosed with RA have a high prevalence of severe PD. The variation in PD prevalence may be influenced by the periodontal classification systems and employed case definitions used [34, 35] potentially explaining the observed discrepancies. In addition, the prevalence of PD can vary significantly based on factors such as age, geographic location, ethnicity, educational level, availability of health services, and systemic health [36, 37].
In chronic inflammatory diseases, such as PD and RA, inflammatory mediators play an important role in promoting and maintaining the state of chronic inflammation, eventually leading to the destruction of tissue and bone [5, 38]. The cytokine APRIL/TNFSF13, from the TNF superfamily, is a proliferation ligand known to be involved in autoimmune diseases including RA [39, 40]. In our study, both salivary and serum levels of APRIL/TNFSF13 were higher in RA patients with severe periodontal disease (PD stage III/IV) as compared to PD stage II. In addition, our results showed that salivary levels of APRIL/TNFSF13 were positively correlated with inflamed periodontal pockets. Salivary levels of BAFF/TNFSF13B, another member of the TNF superfamily, were also higher in RA patients with PD stage III/IV compared to stage II as well as positively correlated with BI. Interestingly, in non-RA patients with PD, the levels of APRIL/TNFSF13 and BAFF/TNFSF13B were found to be increased in periodontal lesions and suggested to contribute to periodontal bone destruction [41]. Moreover, in line with our results, increased serum levels of APRIL/TNFSF13 and BAFF/TNFSF13B have been observed in patients with RA and PD compared to non-RA individuals with PD [39]. Members of the TNF superfamily play a crucial role in mediating inflammation, partly by affecting B-cell survival and activation of signal pathways [42]. In RA, B-cells can give rise to the production of autoantibodies such as RF and ACPAs and contribute to RA pathogenesis by the production of different cytokines and chemokines [42]. Similarly in PD, BAFF/TNFSF13B contributes to B-cell activation and survival during periodontal inflammation [43]. Notably, levels of both APRIL/TNFSF13 and BAFF/TNFSF13B are reported to be higher in serum samples of patients with RA and PD compared to non-RA with PD [39]. Serum levels of APRIL/TNFSF13 correlated with salivary levels of IFN-α2, which was also significantly correlated with PD stage III/IV in the logistic regression analyses. Taken together, it is plausible that these cytokines, by activation of B-cells, may contribute to elevated levels of inflammatory mediators, sustaining periodontal inflammation and leading to severe PD in patients with RA.
Regarding other cytokines within the TNF superfamily, salivary levels of sTNF-R2 and TWEAK/TNFSF12 were positively correlated with periodontal inflammation in terms of inflamed periodontal pockets. Additionally, salivary sTNF-R2 levels were also correlated with BI and may be of importance in distinguishing between PD stage III/IV and stage II in patients with RA. In line with our findings, a study conducted by Kibune et al. [44] demonstrated an association between elevated levels of salivary sTNF-R2 and periodontal inflammation. Elevated levels of TWEAK/TNFSF12 have been suggested to be related to the exacerbation of periodontal diseases and contribute to RA pathogenesis by inducing the production of proinflammatory cytokines [45]. This cytokine was also observed to be significantly higher in patients with PD and RA in saliva samples, compared to patients with PD without RA [46]. Moreover, the soluble TNF-α receptors, sTNF-R1, and sTNF-R2 have been suggested to be involved in modulating and balancing TNF-α activity in inflammation [47].
In serum, patients with PD stage III/IV exhibited elevated levels of the cytokines: sCD30, IL-10, IL-19, and osteopontin, as compared to stage II. Moreover, serum levels of osteopontin may also be important for classifying between PD stage III/IV and stage II. Osteopontin, an inflammatory glycoprotein associated with bone resorption [48], has previously been reported to be increased in both plasma and gingival crevicular fluid (GCF) samples from sites with periodontal destruction [49], indicating its association with aggravated periodontal disease, further supporting the results from this study. Regarding the cytokine IL-19, our research group has previously reported higher levels of IL-19 in GCF of patients with RA and moderate/severe PD compared to RA subjects with no/mild PD [27]. These findings are consistent with current results showing increased levels of IL-19 in PD stage III/IV. Interestingly, IL-19 has also been suggested to possess anti-inflammatory properties in RA [50], which is in line with the negative correlation between salivary IL-19 levels and BI observed in this study. Thus, IL-19 may play a complex role in the immune system, and further studies are needed to clarify its role in patients with PD and RA. Like IL-19, the cytokine IL-10 also has anti-inflammatory properties and inhibits inflammation, autoreactivity, and production of pro-inflammatory cytokines [51]. Serum levels of IL-10 have previously been shown to be increased in patients with RA, and prior research suggests that IL-10 may play a contradictory role in RA by enhancing the humoral autoimmune response [52, 53]. This dual function may explain the elevated serum levels of IL-10 observed in this study. While IL-10 is primarily known for its anti-inflammatory properties, IL-6 is known to be pro-inflammatory and has a crucial role in modulating immune responses and inflammation. The soluble forms of the IL-6 receptor, sIL6Rα, and gp130/sIL-6Rβ are suggested to play an important role in the IL-6 signaling pathway, potentially contributing to the inflammatory cascade observed in chronic inflammatory diseases such as PD and RA [54]. The aforementioned is in line with the results from the current study, demonstrating that serum levels of gp130/sIL-6Rβ are correlated with inflamed periodontal pockets.
In this study, salivary levels of IL-34 were negatively associated with inflamed periodontal pockets (PPD ≥4 mm and BOP). In line with our results, salivary levels of IL-34 have previously been demonstrated to be associated with periodontal health and negatively correlated with parameters of periodontal inflammation such as PPD and BOP [55]. However, levels of IL-34 in serum and GCF samples have been shown to be increased in patients with chronic PD, potentially contributing to alveolar bone loss in periodontal lesions [56]. These findings suggest that IL-34 expression may vary between saliva, GCF, and serum samples.
As expected, former/current smoking habits were negatively correlated with BI, corresponding to previous studies suggesting a strong association with reduced BI in smokers [57]. Additionally, the prevalence of former/current smokers was higher in the group with PD stage III/IV compared to stage II, which is consistent with the well-established association between smoking and increased risk for both PD and RA [58]. Furthermore, a higher prevalence of PD has been demonstrated in current smokers with ACPA-positive RA [59]. In the current study, ACPA positivity negatively correlated with PD stage III/IV, and no medication differences were observed between ACPA-positive and ACPA-negative patients (Supporting Information 2: Table S1). Nevertheless, recent findings indicate that a subset of ACPA might have protective potential in RA [60, 61], highlighting its multifaceted role in disease management and progression.
One of the study’s strengths was the use of both saliva and serum samples from each participant. This dual-sample analyzing methodology enhanced our findings to compare potential biomarkers locally and systemically. This approach provided a better understanding of the interactions occurring in the mouth and throughout the body in patients with PD and RA. Additionally, one single dentist examined all participants excluding interexaminer variability. Another strength of this study was the confirmation of well-known risk factors (former/current smoking habits). This study also has some limitations. Since RA is more common in females compared to males [12], we were not able to match the groups for gender which resulted in a higher prevalence of females compared to males. Additionally, incorporating a comparison group with PD but without RA could have provided further insights. However, our group addressed this in a previous study, comparing salivary inflammatory mediators in individuals with chronic PD, with or without RA. In that study, we reported that TWEAK/TNFSF12, IFN-α2, and IL-19 were higher in saliva samples from patients with both PD and RA compared to those with PD alone [46]. Moreover, some participants had systemic diseases such as diabetes and cardiovascular conditions that are suggested to be associated with PD. However, no significant differences were found regarding systemic diseases between the groups. Another limitation of this study is the relatively small sample size of 62 participants. While our power analysis estimated a minimum of 59 samples, the cohort size may still limit the generalizability of the findings in our well-characterized cohort.
To our knowledge, this is the first study to investigate the prevalence of PD using the current periodontal classification scheme and compare salivary and serum inflammatory mediators in relation to periodontal stages in patients with RA. Our findings revealed several significantly higher inflammatory mediators in both saliva and serum when comparing PD severity within the RA cohort. These results suggest that PD may contribute to increased systemic inflammation in RA, as indicated by higher serum inflammatory mediator levels in patients with severe PD (stage III/IV) compared to those with moderate PD (stage II).
5. Conclusion
In this cohort of RA patients, the majority were diagnosed with severe PD (stage III/IV), highlighting the necessity of periodontal prophylaxis and treatment for this group of patients. Elevated levels of inflammatory mediators were detected in both saliva and serum in patients with severe PD, suggesting a potential relationship between the severity of periodontal disease and increased systemic inflammation in RA. Further research is required to evaluate the clinical significance of these findings and the potential benefits of early intervention.
Ethics Statement
Ethical permits have been obtained from the Regional Ethical Review Board in Stockholm (2009/792-31/4, 2014/1588–32/3, and 2015/766-32) and registered at ISRCTN.com (ISRCTN16761141). Written consent has been obtained from all participants. This study followed the principles for medical research according to the Helsinki Declaration and the Good Clinical Practice (GCP) guidelines.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Tülay Yucel-Lindberg and Kaja Eriksson contributed to the conception and design of the study. Kaja Eriksson performed all dental examinations and/or collected the saliva and serum samples in collaboration with Caroline Lindström, Martina Ericson, Shigufta Syed, Mehrad Mohammadi, and Leif Jansson. Kaja Eriksson, Carina Fei, and Tülay Yucel-Lindberg contributed with the preparation of the samples and performed the analysis of inflammatory mediators. Luis Fernando Delgado Zambrano, Leif Jansson and Carina Fei contributed to statistical analyses and data interpretation. Guozhong Fei, Amel Guenifi, and Georgios Tsilingaridis contributed to the recruitment and clinical management of the patients. Carina Fei, Kaja Eriksson, Tülay Yucel-Lindberg, and Rikard Holmdahl contributed to drafting and revising the manuscript. All authors have made substantial contributions to the design, methodology, data collection, and/or preparation of the manuscript, and all have given final approval for the version to be published.
Funding
This project was supported by the KI/SLL steering group for odontological research (SOF), the Stockholm County Council (ALF), Ulla and Gustaf af Uggla Foundation, the Swedish Rheumatism Association, the Swedish Research Council (2019-01209), and the Swedish Patent Revenue Fund for Research in Preventive Odontology.
Acknowledgments
We would like to acknowledge all the study participants for their contribution and participation of this study and the Rheumatology Unit at Karolinska University Hospital in Huddinge. We would also like to thank TePe for providing toothbrushes to the study participants.
Supporting Information
Additional supporting information can be found online in the Supporting Information section.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




