Modified Gegen Qinlian Decoction Ameliorates DSS-Induced Colitis in Mice via the Modulation of NF-κB and Nrf2/HO-1 Pathways
Abstract
Background: This study aims to reveal the potential molecular mechanisms of modified Gegen Qinlian decoction (MGQD) in relieving ulcerative colitis (UC).
Methods: C57BL/6J mice were used to establish experimental colitis via dextran sodium sulfate (DSS). Body weight, disease activity index (DAI), spleen weight, colon length, and histopathologic features were measured to evaluate the therapeutic effects of MGQD on mice with UC. The ELISA kits were employed to assess the concentrations of interleukin (IL)-6, IL-1β, tumor necrosis factor-α (TNF-α), glutathione (GSH), reactive oxygen species (ROS), and malondialdehyde (MDA). Western blot analyses were used to assess the levels of IκBα, p65, p-IκBα, p-p65, HO-1, and Nrf2. Moreover, the protein levels of Nrf2 and p-p65 were assessed by immunofluorescence.
Results: Colitis-related symptoms in mice were significantly alleviated by MGQD. Moreover, MGQD inhibited the levels of TNF-α, IL-1β, IL-6, MDA, and ROS and increased the level of GSH in mice with UC. Mechanistically, MGQD prevented the activation of the NF-κB pathway and concomitantly promoted the activation of the Nrf2/HO-1 pathway.
Conclusion: MGQD alleviated UC by suppressing inflammation and oxidative stress via the modulation of NF-κB and Nrf2/HO-1 pathways, suggesting that MGQD may be a candidate therapy for UC.
1. Introduction
Ulcerative colitis (UC) is characterized by the recurrent and widespread mucosal inflammation [1]. The prevalence and incidence of UC have been increasing globally [2]. Typical symptoms of UC are bloody diarrhea, abdominal pain, and rectal bleeding, which greatly reduce the patients’ quality of life and increase their financial burden [3]. The disruption of epithelial barrier and abnormal inflammatory responses has long been considered to be responsible for the development and progression of UC [4]. Treatments for UC include 5-aminosalicylic acid (5-ASA), corticosteroids, biologics (e.g., anti-integrins and anticytokines), thiopurines, and small molecules (sphingosine-1-phosphate receptor modulators and Janus kinase inhibitors) [5]. Although the therapeutic options are expanding, patients with UC are not always satisfied with the outcome of their treatment, and 10%–20% of patients still require proctocolectomy for medically refractory disease [1]. Therefore, research into complementary and alternative therapies for the treatment of UC is of great interest [6].
Gegen Qinlian decoction, which is composed of Glycyrrhiza uralensis Fisch, Coptis chinensis French, Scutellaria baicalensis Georgi, and Pueraria lobata (Willd.) Ohwi [7], is beneficial in the treatment of UC [8]. Under the guidance of clinical practice and Chinese medicine theory, we have added Zingiber officinale Roscoe and Talcum to Gegen Qinlian decoction, thus formulating modified Gegen Qinlian decoction (MGQD) [9–11]. Our previous studies [9–11] revealed that MGQD can effectively alleviate UC, but the specific mechanisms remain poorly understood. Therefore, this study aimed to reveal the molecular mechanisms by which MGQD ameliorates UC through a mouse model of colitis.
2. Materials and Methods
2.1. Preparation of MGQD
MGQD (G. uralensis Fisch [6 g], C. chinensis French [9 g], Scutellaria baicalensis Georgi [9 g], P. lobata (Willd.) Ohwi [24 g], Z. officinale Roscoe [9 g], and Talcum [9 g]) was provided by the pharmacy of Xi Yuan Hospital. Talcum was decocted for 30 min, and then the other herbs were added and decocted for 1.5 h and finally filtered. The filtered residue was added to six times the volume of distilled water and boiled again for 1 h and then filtered. The filtrate obtained on both occasions was mixed and concentrated to 0.5, 1, and 2 g/mL of drug, respectively.
2.2. Experimental Protocol for Mice
Male C57BL/6J mice (6–8 weeks old) were purchased from SPF Biotechnology Co., Ltd. (license number: 2019-0010, Beijing, China). Mice were housed in the experimental animal center of Xiyuan Hospital (12/12 h light/dark cycle, room temperature 24 ± 1°C) with free access to food and water. Mice were randomly assigned to the groups of control, dextran sulfate sodium (DSS), 5-ASA, high-dose MGQD (GH), medium-dose MGQD (GM), and low-dose MGQD (GL), with n = 8 mice per group. Acute colitis was induced by adding 3% DSS to the drinking water of all mice except the control group. Meanwhile, mice in the GL, GM, and GH groups were given 5, 10, and 20 g/kg MGQD by gavage once daily for 7 days according to the previous protocol [9–11]. For mice in the 5-ASA group, 0.1 g/kg dose of 5-ASA was intragastrically administered once a day for 7 days. In addition, the equal volume of saline gavage was administered to the DSS and control groups. The experimental procedure is presented in Figure 1A.
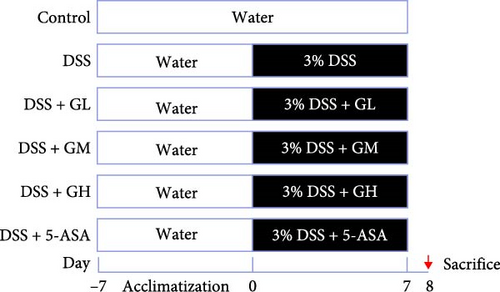



2.3. Disease Activity Index (DAI)
Body weight, fecal consistency, and blood stools were measured daily in mice, and DAI scores were calculated according to previously reported methods (Table 1) [12].
| Score | Weight loss | Stool consistency | Occult/gross rectal bleeding |
|---|---|---|---|
| 0 | None | Normal | Normal |
| 1 | 1%–5% | — | — |
| 2 | 5%–10% | Loose stools | Hemoccult+ |
| 3 | 10%–20% | — | — |
| 4 | >20% | Diarrhea | Gross bleeding |
- Note: The clinical criteria used to evaluate the grade of extent of intestinal inflammation. Scores were tallied for each category and then divided by three to obtain the DAI.
- Abbreviation: DAI, disease activity index.
2.4. Histological Analysis
Following 4% paraformaldehyde fixation, the acquired colon tissue samples were paraffin embedded. Before hematoxylin and eosin (H&E) staining, the paraffin-embedded tissue blocks were sliced to 5-µm-thick sections. Sections were scored for histopathology according to previously published criteria (Table 2) [12].
| Score | Inflammation severity | Inflammation extent | Crypt damage | Percent involvement |
|---|---|---|---|---|
| 0 | None | None | None | 0% |
| 1 | Mild | Mucosa | Basal 1/3 damage | 1%–25% |
| 2 | Moderate | Submucosa | Basal 2/3 damage | 26%–50% |
| 3 | Severe | Transmural | Crypt lost; surface epithelium present | 51%–75% |
| 4 | — | — | Crypt and surface epithelium lost | 76%–100% |
- Note: The score ranges from 0 to 14 (total score), which represents the sum of scores from 0 to 4 for severity and extent of inflammation, crypt damage, and percent of the colon involved. All evaluations were performed by observers unaware of the treatment groups.
2.5. ELISA Analysis
The ELISA kits were employed to assess the concentrations of interleukin (IL)-6 (Ruixin Biotechnology, RX203049M), IL-1β (Ruixin Biotechnology, RX302869R), tumor necrosis factor-α (TNF-α) (Ruixin Biotechnology, RX202412M), glutathione (GSH) (Ruixin Biotechnology, RX202918M), reactive oxygen species (ROS) (Ruixin Biotechnology, RXSH0666), and malondialdehyde (MDA) (Ruixin Biotechnology, RXWB0005) in the colonic tissue samples of mice in accordance with the instructions provided by the manufacturer.
2.6. Immunofluorescence
The colon tissue was fixed with 4% paraformaldehyde, paraffin-embedded, and sectioned at 5 μm. Tissue slides were cleaned with deionized water after being dewaxed with xylene and rehydrated with gradient ethanol. Antigenic repair techniques were carried out employing microwaves and antigenic repair solutions. The slides were sealed with 10% normal goat serum for 1 h at room temperature. They were then incubated with primary antibodies (Nrf2 [Proteintech, 80593-1-RR] and p-p65 [Proteintech, 82335-1-RR]) at 4°C overnight before being stained with fluorescent (CY3) or fluorescent (FITC)-labeled secondary antibodies. Following the second antibody incubation, the tablets were stained with DAPI solution and sealed with a sealing solution containing an antifluorescence quenching agent. All operations were performed in dark conditions. Caseviewer and Leica LAS image acquisition systems were applied to observe sections.
2.7. Western Blot Analysis
Proteins were isolated from colon tissue samples of mice and quantified. After being separated into proteins of various molecular weights using gel electrophoresis, the proteins were transferred to polyvinylidene difluoride membranes and blocked with skim milk. Then, membranes were mixed with IκBα (Proteintech, 10268-1-AP), p65 (Proteintech, 10745-1-AP), p-IκBα (Proteintech, 82349-1-RR), p-p65 (Proteintech, 82335-1-RR), HO-1 (Proteintech, 27282-1-AP), and Nrf2 (Proteintech, 80593-1-RR) primary antibodies, respectively, and incubated at 4°C overnight. The samples were then treated for 2 h at room temperature with HRP-labeled rabbit or mouse IgG secondary antibodies. ECL solution (P0018, Beyotime) was used to visualize the protein signals.
2.8. Statistical Analysis
The mean ± standard error of the mean was used to express all data. For comparisons between two or more groups, one-way analysis of variance was employed. All statistical analyses were carried out by SPSS 26.0 and GraphPpad Prism version 9 software. A statistically significant difference was defined as p < 0.05.
3. Results
3.1. MGQD Alleviated the Symptoms of Mice with UC
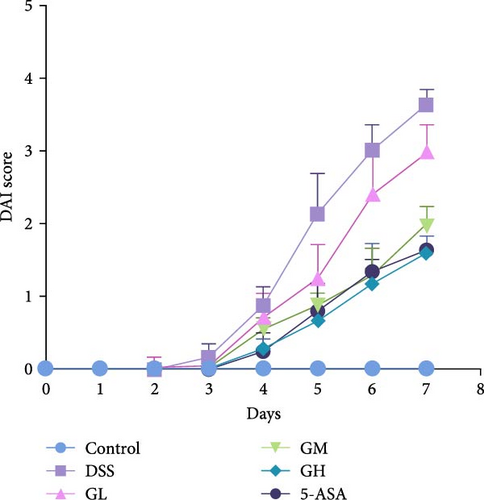
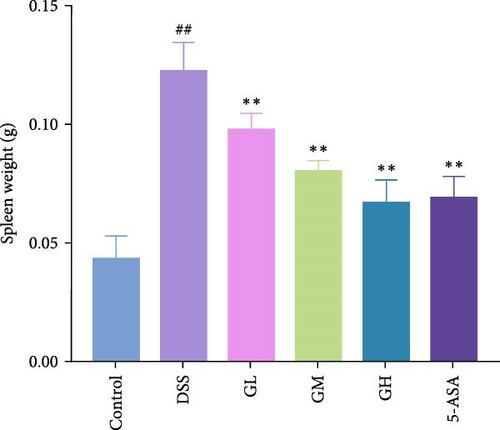
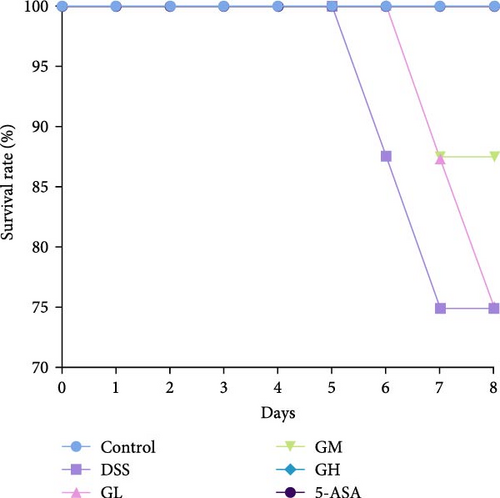
In comparison to the control group, mice elicited by DSS displayed evident indications of colitis, such as weight alleviation, bloody stools, loose stools, shortened colon length, and increased spleen weight (p < 0.05, Figure 1). Interestingly, mice treated with MGQD and 5-ASA recovered their body weight and colon length and had significantly lower DAI scores and spleen weight compared to the DSS group (p < 0.05, Figure 1B–F). In addition, the mortality rate of DSS-induced colitis mice was as high as 25% compared with that of mice in the control group (p < 0.05, Figure 1G). Compared with mice in the DSS group, the mortality rate of mice treated with MGQD was significantly reduced (p < 0.05), with the survival rate of mice in the GH group reaching 100% (Figure 1G). Notably, MGQD showed a dose-dependent improvement of symptoms associated with colitis, and the anticolitis effect of MGQD at 20 g/kg was similar to that of 5-ASA (p > 0.05).
3.2. MGQD Alleviated the Pathology of Mice with UC
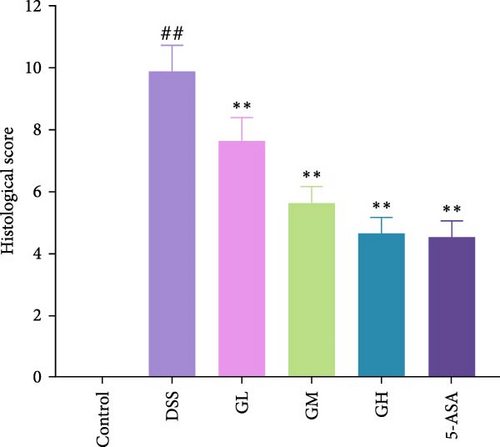
In comparison to the control group, mice with UC exhibited mucosal injury, infiltration of inflammatory cells, goblet cell loss, abnormal crypts, and higher histopathologic scores (p < 0.05, Figure 2). Interestingly, pathological changes and histopathological scores of colonic tissues were significantly improved in mice treated with MGQD and 5-ASA compared to the DSS group (p < 0.05, Figure 2).

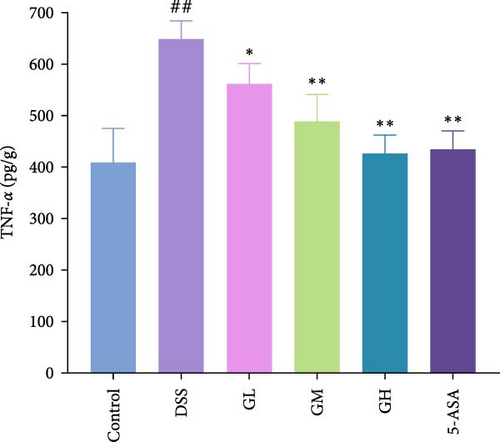
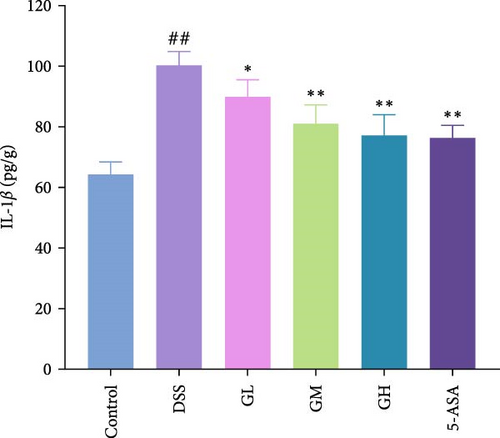
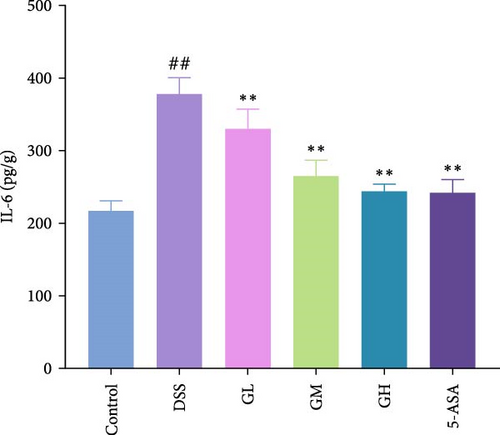
3.3. MGQD Inhibited Cytokine Production and Oxidative Stress
In comparison to the control group, the colonic tissues of mice with UC showed considerably higher expression levels of the proinflammatory cytokines, including IL-1β, IL-6 and TNF-α (p < 0.05, Figure 3). Interestingly, MGQD and 5-ASA treatment significantly reversed elevated proinflammatory cytokine levels in mice with UC (p < 0.05, Figure 3).
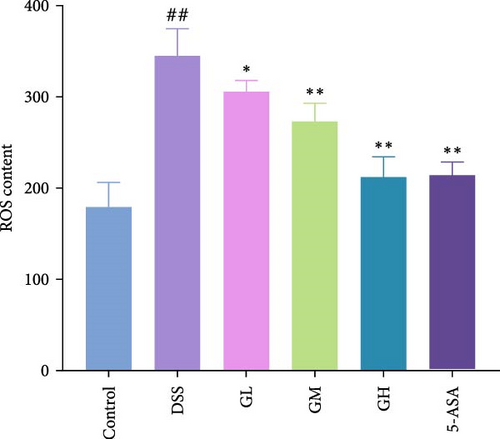
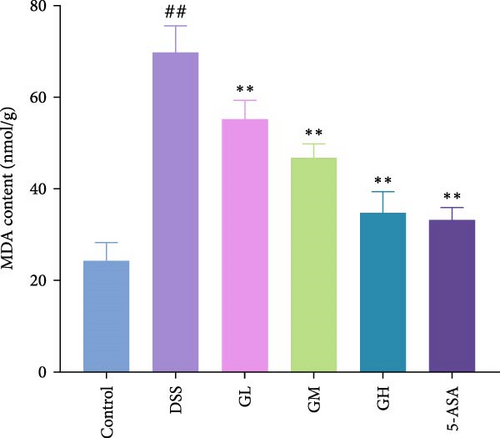
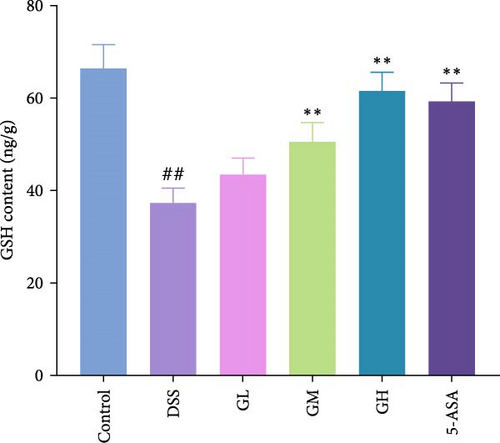
In comparison to the control group, the colonic tissues of mice with UC showed considerably higher levels of ROS and MDA while lower level of GSH (p < 0.05, Figure 4). Interestingly, MGQD and 5-ASA therapies significantly reversed the abnormal alterations in these metrics in mice with UC (p < 0.05, Figure 3).
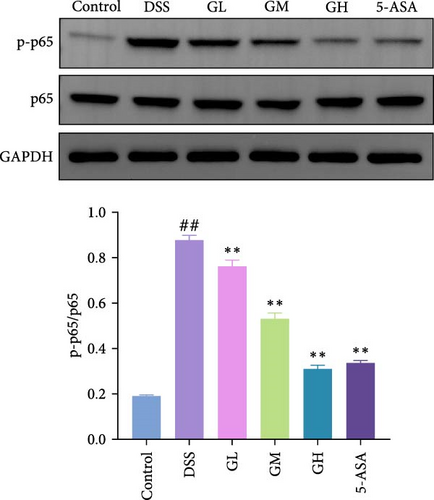
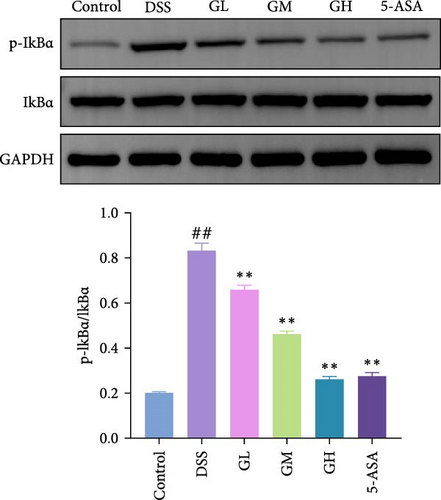
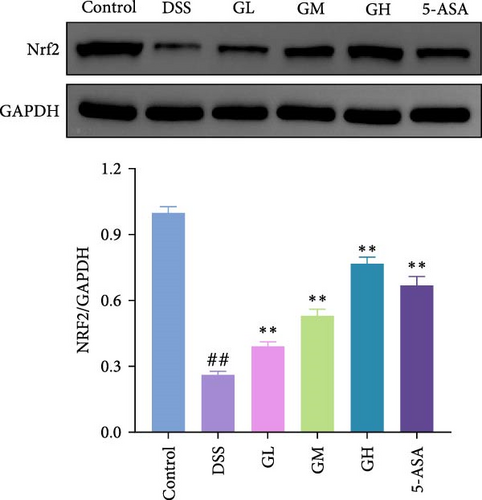
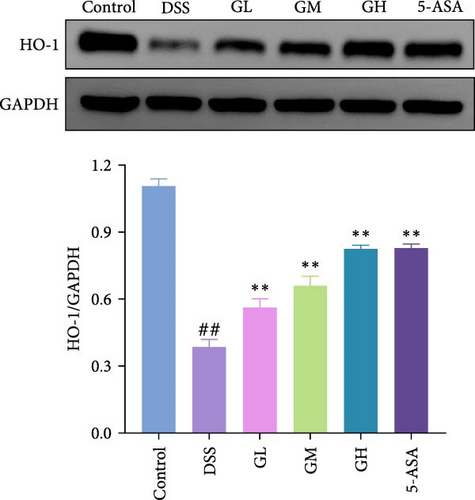
3.4. MGQD Inhibited the NF-κB Pathway and Activated the Nrf2/HO-1 Pathway in Mice With UC
The NF-κB pathway was activated in the colonic tissue of mice with UC, as evidenced by considerably increased phosphorylation protein expression of p65 and IκBα when compared to that of mice in the control group (p < 0.05, Figure 4A–B). Interestingly, the elevated levels of p-p65 and p-IκBα in the colonic tissues of mice with UC treated with MGQD and 5-ASA were significantly reversed (p < 0.05, Figure 4A–B). In line with this, the results of immunofluorescence assay showed that p-p65 was significantly inhibited by MGQD and 5-ASA treatment in mice with UC (Figure 5A).
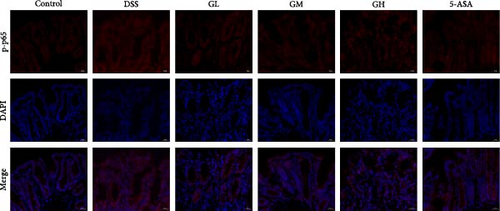

The Nrf2/HO-1 pathway was suppressed in the colonic tissue of mice with UC, as evidenced by considerably decreased protein expression of Nrf2 and HO-1 when compared to that of mice in the control group (p < 0.05, Figure 4C–D). Interestingly, the decreased levels of Nrf2 and HO-1 in the colonic tissues of mice with UC treated with MGQD and 5-ASA were significantly reversed (p < 0.05, Figure 4C–D). In line with this, immunofluorescence assay showed that MGQD and 5-ASA significantly upregulated the expression of Nrf2 in mice with UC (Figure 5B).
4. Discussion
UC has been characterized by inflammation of the mucosa [13]. TNF-α and ILs are important mediators of inflammatory reactions in UC [14]. Fundamental to the regulation of inflammation, NF-κB is a key transcriptional regulator of innate and adaptive immunity that regulates multiple signaling pathways and the generation of proinflammatory cytokines. UC and experimental intestinal inflammation models including DSS, trinitrobenzene sulfonic acid-induced colitis, and IL-10 knockout mice are characterized by NF-κB activation and increased expression of proinflammatory NF-κB target genes [15, 16]. When activated, NF-κB complex translocates to the nucleus, where it induces the expressions of proinflammatory genes including those of cytokines and chemokines [17]. Thus, the activation of NF-κB is known to be extremely important in the pathogenesis of UC and has been proposed as a major culprit and therapeutic target for UC [18]. Targeted inhibition of the NF-κB pathway has been shown to be a therapeutic strategy with ameliorative effects on UC. By targeting the NF-κB pathway, a variety of natural substances have been found to alleviate intestinal inflammation [19, 20]. Similarly, we also observed the suppression of NF-κB pathway and the decrease of proinflammatory cytokines levels in UC mice intervened by MGQD in the present study, suggesting a mechanism by which MGQD alleviates colonic inflammatory injury by inhibiting the NF-κB pathway.
Accumulating evidence suggests that oxidative stress plays an important role in triggering an inflammatory response and inducing UC pathogenesis, which impacts on the progression of the disease in multiple ways [21]. Nrf2 is an important transcriptional regulator involved in redox homeostasis and has an irreplaceable role in promoting antioxidant responses in organisms [22]. Among the genes upregulated by Nrf2, HO-1 has received significant attention because the products of HO-1-induced heme catabolism have well-recognized antioxidant and anti-inflammatory properties [23]. Evidence for the involvement of Nrf2/HO-1 pathway in the course of UC was first published in 2006, when it was reported that DSS-induced colitis was associated with increased expression of Nrf2 regulatory enzymes and that the increased susceptibility of Nrf2-deficient mice to DSS-induced colitis may be due to decreased expression of HO-1 as well as increased expression of proinflammatory mediators [24]. In addition, the levels of proinflammatory cytokines and lipid peroxides were significantly increased in the colon of Nrf2 knockout mice, whereas the expression levels of antioxidant enzymes were decreased [25]. Therefore, the Nrf2/HO-1 pathway may represent a promising avenue to treat UC, and the mechanism of action of targeting this pathway involves regulating oxidative stress, reducing intestinal inflammation, repairing the intestinal mucosal barrier, and preventing ferroptosis in intestinal epithelial cells [26]. By targeting the Nrf2/HO-1 pathway, several natural substances with therapeutic potential for UC have been identified [26]. Similarly, we also observed the activation of Nrf2/HO-1 pathway and the decrease of oxidative stress index in UC mice intervened by MGQD in the present study, suggesting a mechanism by which MGQD suppresses oxidative stress by activating the Nrf2/HO-1 pathway.
Limitations should be acknowledged. First, our previous study [9] identified aicalin, puerarin, palmatine chloride, wogonin, and berberine chloride as the major components of MGQD by high-performance liquid chromatography, but we have not explored the effects of the individual components of MGQD on UC in isolation, and therefore, we were unable to identify the specific components that have a mitigating effect on UC. Second, the signaling pathways associated with UC are multiple, and MGQD may have multiple targets in the treatment of UC; further studies to explore other pathways involved in the treatment of UC with MGQD are still necessary.
5. Conclusion
MGQD alleviated UC by suppressing inflammation and oxidative stress via the modulation of NF-κB and Nrf2/HO-1 pathways, suggesting that MGQD may be a candidate therapy for UC.
Nomenclature
-
- UC:
-
- Ulcerative colitis
-
- MGQD:
-
- Modified Gegen Qinlian decoction
-
- DSS:
-
- Dextran sulfate sodium
-
- ROS:
-
- Reactive oxygen species
-
- GSH:
-
- Glutathione
-
- MDA:
-
- Malondialdehyde
-
- 5-ASA:
-
- 5-Aminosalicylic acid
-
- GM:
-
- Medium-dose MGQD
-
- GL:
-
- Low-dose MGQD
-
- GH:
-
- High-dose MGQD
-
- H&E:
-
- Hematoxylin and eosin
-
- DAI:
-
- Disease activity index.
Ethics Statement
This work was approved by the Animal Ethics Committee of Xi Yuan Hospital of China Academy of Chinese Medical Sciences (Approval No. 2019XLC003-2).
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Jinke Huang designed the research. Jinke Huang, Zhihong Liu, Jiaqi Zhang, Jing Ma, and Yifan Wang performed the experiments. Jinke Huang analyzed the data and wrote the paper. Fengyun Wang and Xudong Tang contributed to review and editing.
Funding
This work was funded by The Specific Research Fund for TCM Science and Technology of Guangdong Province Hospital of Chinese Medicine (Grant YN2024GZRPY030), the National Natural Science Foundation of China (Grant 82341233), and Spleen and Stomach Disease Inheritance and Innovation Team of Science and Technology Innovation Project of China Academy of Chinese Medical Sciences (Grant CI2021B005).
Acknowledgments
The authors have nothing to report.
Open Research
Data Availability Statement
The datasets generated for this study are available upon request to the corresponding author.




