Effects of High Lipid Diet and Bromelain Enzyme on Body Weight, Lipase Gene Expression, and Blood Parameters in Mice BALB/c
Abstract
The study examined the effects of high protein and lipid diets and bromelain enzyme supplementation on weight, liver enzymes, hematological, and lipid profiles of mice. A total of 25 male mice BALB/C with 7 weeks of age were used in the experiment. The treatment groups were as follows: (1) Control group (no addition in diet), (2) high lipid (HL, 4 g/mice), (3) high protein (HP, 4 g/mice), (4) HL + bromelain enzyme (HLB, 4 mg/mice), and (5) HP + bromelain enzyme (HPB, 4 mg/mice). Body weight gain rate of the control group was lower than the HL group, and the HLB group had a higher rate than the HPB group (p < 0.01). Liver enzyme levels were higher in the high-fat diet group, but bromelain mitigated this effect. Hematological results showed that the high-fat diet decreased platelet counts while increasing RBC, HGB, and WBC, whereas the protein diet improved platelet formation. Bromelain improved RBC and HGB levels in the high-fat diet, indicating a protective effect. Lipid analysis showed that the high-fat diet elevated harmful lipid levels, while the high-protein diet reduced some. Bromelain improved lipid profiles across both diets, suggesting cardiovascular benefits. The high-fat diet also raised creatinine and urea levels, indicative of kidney impairment, an effect exacerbated by a high-protein diet; however, bromelain supplementation reduced these levels, indicating kidney protection. The study found that high-fat diets increased oxidative stress markers, which were alleviated by bromelain. While PMN viability remained unchanged, phagocytosis rates increased with a high-protein diet and were further enhanced with bromelain in high-fat diets but not in high-protein diets. Moreover, bromelain significantly increased lipase gene expression in the high-fat diet, indicating enhanced fat metabolism, though it lowered lipase expression when combined with protein. Histological analyses illustrated severe liver and kidney damage in the high-fat diet group, marked by liver congestion and fatty degeneration, alongside renal damage, including atrophy and hemorrhage.
1. Introduction
Nutrition regulates fundamental processes of the body and directly affects the gene expression of several enzymes and proteins involved in metabolism. It also significantly affects the functions of various organs [1]. Lipase plays an important role in fat metabolism since it participates in the hydrolysis of triglycerides into fatty acids and glycerol and aids in the absorption of fatty acids used for energy and storage. People who have physically demanding jobs or live in cold climates find free fat to be a concentrated source of energy since it provides a high percentage of calories to power everyday activities [2]. Furthermore, free fat contains fat-soluble vitamins, such as vitamin A, which promotes eye health and the immune system and vitamin D, which increases calcium absorption and so strengthens bones and teeth. Free fat also includes vitamin E, an antioxidant that protects cells from free radical damage and reduces the risk of chronic illnesses such as heart, kidney, and liver disease [3]. Free fat contains short- and medium-chain fatty acids, such as butyric acid, which has anti-inflammatory effects that help to maintain gut and digestive health [4]. Butyric acid has been found to nourish intestinal cells and enhance digestion, therefore lowering the risk of some intestinal illnesses [5]. A moderate intake of free fat may improve heart health since it includes conjugated linoleic acid (CLA) which can help reduce visceral fat and increase blood lipid levels, potentially lowering the risk of obesity and associated heart diseases [6, 7]. Furthermore, free fat contains healthy cholesterol, which is required for hormone synthesis and the regulation of critical bodily activities in particular. Free fat fosters a healthy neurological system and helps in brain functions especially of youngsters and elderly [8]. Various nutritional factors, especially type of fat, carbohydrate, and protein content of the diet, influence lipase enzyme activity. Lipase gene expression has been established to be associated with immune system functions since fat metabolism influences the behavior of various immune cells. Such functions also contribute to the regulation of complex immune signals via cytokine production and macrophage [9]. Changes in functions of T cell and B cells in fat intake can alter immune responses by enhancing or suppressing immune activity with consequences for host resistance to infection and the development of inflammatory and autoimmune diseases [10]. There has been work on BALB/c strain of laboratory mice for their vigorous immune response and adaptability to environmental changes [11]. These mice offer a chance to study how diet-induced changes in lipase genes affect downstream immune responses [12]. Such changes can reveal potential links between metabolism and immunity and act as a basis for possible nutritional strategies to enhance overall health and immunity [13]. Fats are complex chemical substances that are an important element of the diet and they serve an important function in the body and offer several advantages since they are a concentrated source of energy, offering 9 calories per gram of fat as opposed to carbs and proteins, which include 4 calories per gram [14]. In addition to delivering fat-soluble vitamins (A, D, E, and K), fats act as an insulator and protective of essential organs, a sheath for nerve tissues and help to transmit signals between cells in the body. Fats under the epidermis also help to keep the body temperature stable [15]. The US Food and Drug Administration (FDA) recommends ingesting 20%–35% of total daily calories from fats, with a preference for monounsaturated and polyunsaturated fats over saturated and trans fats [16].
2. Material and Methods
2.1. Experimental Design
Animal care procedures for the experiment have been approved by the local Ethic Committee of Animal Experiments, Erciyes University, Kayseri, Türkiye (No: 22/204). Experiments were carried out in Saladin Governorate and Anbar Governorate of Iraq. Present experiments involved 25 male albino laboratory mice with 7 weeks of age and 208 g body weight. The standard diet was formulated based on internationally accepted NRC guidelines. Fat was sourced from cow’s milk in the form of ghee, while albumin served as the protein source. Bromelain enzyme was extracted from pineapple. Bromelain extraction was performed using fondness chromatography, achieving approximately 80% recovery, followed by dialysis to eliminate small molecules. Ghee was prepared by melting butter and simmering it while stirring to prevent the burning of milk solids. Experimental groups were designated as follows: T1 received a control diet and water; T2 received the standard diet with high fat (ghee) added; T3 received the standard diet with high protein (albumin); T4 received the standard diet with high fat and bromelain; T5 received the standard diet with high protein and bromelain.
2.2. Animals and Feeds
The animals were divided into five groups and were fed manually using a dosing tool. In the protein group (albumin extracted from eggs), 2 mL was given in the morning and 2 mL in the evening. In the lipid group (fat extracted from cow’s milk), 2 mL was given in the morning and 2 mL in the evening. In enzyme group (bromelain enzyme extracted from pineapple), 0.2 mL was given in the morning and 0.2 mL in the evening [17, 18].
2.3. Preparation of Bromelain Enzyme
Fondness chromatography offers a more fruitful method for bromelain extraction. The method is able to recover about 80% of bromelain from the pineapple fruit by ammonium sulfate in the same range of saturation. After the precipitation, the precipitate was dialyzed to remove the small molecule compounds [19].
2.4. Preparation of Albumin and Ghee
To make ghee, place the butter in a saucepan over the stove and warm it up. Once the butter melts completely, let it simmer. As the butter simmers, it will start foaming and splutter. Continue cooking the ghee on low heat for 20–25 min, stirring occasionally to make sure the milk solids do not burn at the bottom [20]. A simple method, based on ethanol fractionation, for the preparation of highly purified serum albumin with a higher yield than that of the conventional ethanol procedures is described. It consists of two purification steps, namely, precipitation of most of the other plasma proteins from a 3-fold diluted plasma with ethanol at 42% concentration, pH 5.75 and −5°C, leaving over 96% pure albumin in the supernatant, followed by isoelectric precipitation of albumin from the supernatant at pH 4.8 and −5°C. The resultant paste was processed into the final albumin solution according to the conventional methods. The yield of the final albumin with a purity of over 99% was equivalent to 29.5 g/L of plasma representing a recovery of over 93% [21, 22].
2.5. Preparation and Collection of Blood Samples
The tools that will be used to draw blood samples from the animals have been prepared after anesthetizing them with chloroform solution [23]. After the end of the experiment period, which lasted for 30 days, the animals were starved for 10 h to ensure the accuracy of the results, and then, the animals were anesthetized with ketamine, and after the animals became numb, the animal’s limbs were fixed with stabilizers, and then, the abdomen was incised longitudinally and blood was drawn through a cardiac puncture. In a size of (3-4 mL) using disposable medical syringes, then part of the blood was transferred to tubes containing EDTA to prevent clotting in order to conduct blood tests including (PLT–WBC–RBC–HGB). The other part of the blood was transferred to Gel tubes for separating the serum and performing the remaining tests manually.
2.6. The Experimental Investigation
Weekly body weight gains were calculated with a specific formula. Blood samples [24] were categorized for various tests: one for complete blood count (CBC) analysis and the other for enzyme assays like glutamic oxalate transaminase (GOT), glutamic pyruvic transaminase (GPT), and alkaline phosphatase (ALP) [25]. Blood analyses were carried out using commercial kits and spectrophotometer [26]. After dissection, organ samples were preserved for histological examination [27]. The study detailed methods for quantifying white blood cells (WBCs) and red blood cells (RBCs), estimating serum lipids, and assessing kidney function through serum urea and creatinine levels [28]. WBCs were counted with a Neubauer chamber after dilution and staining, while RBCs were counted using a hemocytometer following dilution [29]. Lipid levels were estimated enzymatically, and kidney functions were evaluated with specific test kits [30]. Liver enzyme activities were measured using Biosystems kits [31].
2.7. Cellular Immunity Tests
2.7.1. Isolation of Polymorphonuclear Macrophages/Neutrophils (PMNs) From Peripheral Blood
2.7.1.1. PMNs Cell Migration Under Agarose
2.8. Gene Expressions
- 1.
After treatment, bacterial growth in nutrient broth with different concentrations (sub mic for each isolate) was harvested and bacterial cells were placed into a microcentrifuge tube and centrifuged for 1 min at 13,000 rpm. This step was repeated until getting a sufficient amount of pellet. The pellet was resuspended by 0.5 mL of triazole reagent.
- 2.
Blood, any tissue suspends it with a triazole of 0.6 mL.
- 3.
Fifteen minutes of incubation to permit complete dissociation of the nucleoprotein complexes.
- 4.
The chloroform was added as 200 μL of TRIzol Reagent used for lysis.
- 5.
Incubation for 2-3 min at room temperature.
- 6.
The sample was centrifuged for 10 min at 10,000 rpm. The mixture was separated into a lower red phenol–chloroform, interphase, and a colorless upper aqueous phase.
- 7.
The aqueous phase containing the RNA was transferred to a new tube.
- 8.
The RNA was precipitated by adding 200 μL of isopropanol or absolute ethanol to the aqueous phase.
- 9.
The mixture was incubated for 2 min at room temperature.
- 10.
The mixture was centrifuged for 10 min at 10,000 rpm. Total RNA precipitated on filter of spin column tube.
- 11.
The supernatant was discarded.
- 12.
The column gets resuspended by 0.5 mL of washing buffer 1.
- 13.
The tube was centrifuged for 2 min at 10,000 rpm.
- 14.
The supernatant was discarded.
- 15.
The column gets resuspended by 0.5 mL of washing buffer 2.
- 16.
The tube was centrifuged for 2 min at 10,000 rpm.
- 17.
The supernatant was discarded.
- 18.
The column gets preheated elution by 75 µL and centrifuged for 1 min at 10,000 rpm.
- 17.
Total RNA samples were stored in deep freezer.
2.9. cDNA Synthesis
- 1.
Eighteen microliters of RNA extract was added to the microfuge tube.
- 2.
Two microliters of hexamer primer (for prokaryotic cells) or oligo dt (for eukaryotic cells) was added and then mixed well.
- 3.
The mixture was incubated in PCR with the following cycles, 37°C for 10 min followed by 42°C for 1 h and 95°C for 5–10 min in one cycle.
- 4.
Synthesized cDNA was immediately used as template for qRT-PCR or for long-term storage at −20°C.
2.10. Quantitative Reverse Transcription PCR (qRT-PCR)
- 1.
Two microliters of cDNA was added to PCR tube.
- 2.
One microliter of each primer was added.
- 3.
The volume was completed to 20 μL with DNase free distilled water.
- 4.
The mixture was mixed well and put into the qPCR machine. Table 1 reveals the PCR conditions.
| Step | Temperature (°C) | Duration | Cycles |
|---|---|---|---|
| Initial denaturation | 95 | 3 min | 1 |
| Denaturation | 95 | 15 s | 40 |
| Annealing | 55 | 45 s | |
| Extension | 72 | 60 s | |
2.11. Calculation of the Fold of Gene Expression
2.12. Preparation of Histological Sections
The preparation of histological sections according to [38] involves several key steps: First, organs are fixed in a 10% formalin solution for 24 h. After fixation, they are washed to remove the formalin. The tissues undergo dehydration through a series of increasing concentrations of ethyl alcohol (70%–100%) over approximately 2 h. Next, the samples are cleared using xylene to eliminate alcohol, followed by infiltration in a xylene–paraffin wax mixture at 60°C. The embedding process entails placing the samples in paraffin wax molds until they harden. Using a rotary microtome, sections of 5–7 μm are cut and placed in a warm water bath to prevent aggregation before being treated with Mayer’s albumin solution and left to dry. The slides undergo a series of washes to remove paraffin and are then subjected to staining with hematoxylin and eosin to color the nuclei and cytoplasm, respectively. Finally, a drop of Canada balsam is applied for mounting, preparing the sections for microscopic examination.
2.13. Statistical Analysis
The Statistical Package for the Social Sciences (SPSS) Version 16 was used to analyze the initial data of the study results. Experimental data were subjected to an analysis of variance (ANOVA) test, and significant means were compared with the use of the least significant difference (LSD) test at a statistical probability level of p < 0.01.
3. Results
3.1. Effect of Treatments on the Weight Gain Rate of BALB/c Mice
Table 2 shows the effect of treatments on the weight gain rate of BALB/c mice. The results showed differences in the weights of the experimental animals between the different treatments. The control group, which relied on a standard feed + drinking water diet during 30 days from the start of the experiment, gained 37 g as the weight increased from 208 to 245 g, at a weight gain rate of 1.23 g per day. The lipid group, whose diet relied on high levels of fat, experienced a weight gain of 75 g over 30 days, increasing from 210 to 285 g at a weight gain rate of 2.5 g per day. The protein group, which relied on high levels of protein, had a weight gain rate of 1.7 g per day. The fourth group, which relied on a high-fat diet with bromelain enzyme extracted from pineapple, had a weight gain of 23 g over 30 days and a weight gain rate of 0.7 g per day. However, a decrease in the weights of animals was observed in the fifth group, which was fed a high-protein diet enhanced with the enzyme bromelain (4 mg). The average weight decrease was −16 g, at an average weight decrease rate of −0.53 g per day as the weight decreased from 211 to 195 g.
| Treatment | Weight before (g) | Weight after (g) | Change in weight (g) | Weight gain rate (g per day) |
|---|---|---|---|---|
| Control | 208 | 245 | 37 | +1.23 |
| Lipid | 210 | 285 | 75 | +2.5 |
| Protein | 209 | 261 | 52 | +1.7 |
| Lipid + bromelain | 212 | 235 | 23 | +0.7 |
| Protein + bromelain | 211 | 195 | −16 | −0.53 |
3.2. Effect of Treatments on Liver Enzyme Levels of BALB/c Mice
Table 3 shows the effect of treatments on liver enzyme levels of BALB/c mice. The “Lipid” group had the highest level of ALP, followed by the “Lipid + Bromelain” and “Protein” groups. The ALP levels of the “Control” and “Protein + Bromelain” groups were significantly lower. This suggests that a high-fat diet may increase ALP levels, while bromelain enzymes may have an inhibitory effect. ALT levels were higher in the Lipid and Lipid + Bromelain groups, suggesting a potential negative effect of a high-fat diet on liver health. ALT levels were lower in the Control and Protein + Bromelain groups, possibly suggesting a protective role of bromelain when added to the protein. The highest AST levels were in the “Lipid” group, which may indicate that the high-fat diet caused an increase in liver enzyme activity. The “Control” and “Protein + Bromelain” groups had lower levels of AST, supporting the conclusion that bromelain reduced liver damage associated with a high-protein or high-fat diet.
| Treatments | (AST–GOT) U/L |
(ALT–GPT) U/L |
ALP U/L |
|---|---|---|---|
| Control | 135.00 ± 2.54d | 31.000 ± 1.22c | 41.600 ± 0.92c |
| Lipid | 225.400 ± 11.69a | 50.200 ± 1.88a | 61.800 ± 1.68a |
| Protein | 162.400 ± 1.63c | 39.400 ± 0.92b | 49.800 ± 0.86b |
| Lipid + bromelain | 191.600 ± 1.77b | 49.200 ± 0.66a | 52.600 ± 0.92b |
| Protein + bromelain | 139.00 ± 0.70d | 32.400 ± 0.74c | 40.600 ± 0.92c |
- Note: Different letters (a, b, c, d) indicate significant differences at p < 0.05. Values are presented in mean ± standard error.
3.3. Effect of Nutritional Quality and Enzymes on Some Blood Variables of BALB/c Mice
Table 4 shows the effect of treatments on some blood variables of BALB/c mice. The control group reflected the normal levels of blood variables in BALB/c mice without any nutritional or enzymatic intervention as compared to the fat group with a significant decrease in PLT and a significant increase in RBC, HGB, and WBC levels compared to the control group. This suggests that a high-fat diet significantly affected platelet count, decreasing platelet count while increasing RBC, hemoglobin, and WBC production. These changes may be associated with increased blood viscosity and the risk of thrombosis and inflammation. The protein group showed an increase in PLT compared to control, suggesting that a high-protein diet may enhance platelet formation. An increase was also observed in RBC and HGB levels, but their values were lower than in the fat group. When bromelain was added to the high-fat diet, a slight increase was observed in PLT compared to the fat-alone group, but it was still lower than the control and protein groups. RBC and HGB also decreased to levels closer to control, suggesting that bromelain may have a protective effect against the negative effects of fat on these hematological variables. WBC values remained elevated, possibly indicating an ongoing immune response. In the protein plus bromelain group, PLT levels were almost identical to the protein-alone group, indicating that bromelain does not significantly affect platelets in the presence of the protein. On the other hand, RBC and HGB decreased to levels closer to control, suggesting a balancing effect of bromelain. WBC also decreased to a level lower than the protein-alone group and closer to the control group compared to the rest of the groups, which may reflect an anti-inflammatory effect when bromelain was added to the group of animals fed on protein fortified with bromelain enzyme.
| Treatments | WBC | HGB | RBC | PLT |
|---|---|---|---|---|
| Control | 9.100 ± 0.360d | 13.22 ± 0.16c | 5.00 ± 0.11c | 208.80 ± 4.11b |
| Lipid | 15.36 ± 0.27a | 16.42 ± 0.24a | 6.94 ± 0.12a | 152.60 ± 1.83d |
| Protein | 11.96 ± 0.092b | 15.16 ± 0.12b | 6.06 ± 0.10b | 229.60 ± 4.15a |
| Lipid + bromelain | 12.10 ± 0.16b | 13.42 ± 0.08c | 5.06 ± 0.12c | 181.40 ± 9.37c |
| Protein + bromelain | 10.18 ± 0.12c | 13.18 ± 0.08c | 4.96 ± 0.08c | 228.60 ± 5.91a |
- Note: Different letters (a, b, c, d) indicate significant differences at p < 0.05. Values are presented in mean ± standard error.
3.4. Effect of Nutritional Quality and Enzymes on Lipid Levels of BALB/c Mice
Table 5 shows the effect of treatments on lipid levels of BALB/c mice. The control group showed moderate levels of lipid levels, which is the basis for comparison with the other groups. The values indicate a normal level of lipids in the blood without any dietary or enzymatic intervention. In the lipid group, a significant increase was observed in the levels of LDL, TG, VLDL, and TC, indicating that a diet rich in fats increases the levels of harmful fats in the blood: LDL (bad cholesterol), which may increase the risk of atherosclerosis, while HDL (good cholesterol), important in metabolic processes and hormones, decreased. In the protein-only group, there was a decrease in VLDL and TG and a slight increase in HDL compared to the fat group. This suggests that a high-protein diet may be less harmful to blood lipid levels than a high-fat diet, but it still has a significant effect on LDL levels. In the lipid + bromelain group, it was noted that adding bromelain to the high-fat diet led to a significant decrease in LDL and TC compared to the lipid-alone group, with an increase in HDL. This indicates that bromelain may contribute to reducing the harmful effects of fat on blood lipid levels and improving cholesterol levels. As for the protein group with bromelain, an improvement in all lipid indicators was seen with a decrease in LDL and an increase in HDL. This indicates that bromelain may enhance the protective effects of a high-protein diet on blood lipid levels.
| Treatments | T.C | T.G | HDL | LDL | VLDL |
|---|---|---|---|---|---|
| Control | 69.80 ± 2.90c | 84.80 ± 3.33b | 42.00 ± 1.09b | 10.84 ± 3.01c | 16.9 ± 0.66c |
| Lipid | 95.60 ± 1.36a | 106.80 ± 5.2a | 21.20 ± 1.65d | 53.04 ± 3.23a | 21.36 ± 1.04a |
| Protein | 83.20 ± 3.66b | 65.60 ± 4.51 | 29.40 ± 0.50c | 40.68 ± 2.97b | 13.12 ± 0.90b |
| Lipid + bromelain | 73.20 ± 0.86c | 84.40 ± 4.38 | 45.20 ± 1.59ba | 11.12 ± 2.42c | 16.88 ± 0.87c |
| Protein + bromelain | 73.80 ± 1.28c | 84.40 ± 2.54b | 48.20 ± 0.86a | 8.72 ± 0.70c | 16.88 ± 0.50c |
- Note: Different letters (a, b, c, d) indicate significant differences at p < 0.05. Values are presented in mean ± standard error.
3.5. Effect of Treatments on Creatinine and Urea Levels of BALB/c Mice
Table 6 shows the effect of treatments on creatinine and urea levels of BALB/c mice. The high-fat diet led to a significant increase in creatinine and urea levels compared to the control group. This suggests a possible negative effect on kidney function. In the protein group, a greater increase was seen in creatinine and urea levels compared to the fat and control groups, indicating that a high-protein diet may have a more harmful effect on kidney function, as excess protein may increase the burden on the kidneys, leading to the accumulation of nitrogenous substances such as creatinine and urea in the blood. Adding bromelain to a high-fat diet reduced creatinine and urea levels compared to the fat-only group, indicating that bromelain helped to reduce the negative impact of fat on kidney functions due to its effective role in breaking down peptide bonds and stimulating enzymes responsible for digesting protein and converting it into smaller molecules that are easier to excrete from the body. In the protein and bromelain group, an improvement in creatinine and urea levels was also observed when bromelain was added to the protein-rich diet. Although the values were still high compared to the control group, they were lower than those observed in the protein-alone group, indicating a balancing effect of bromelain when added.
| Treatments | Urea | Creatinine |
|---|---|---|
| Control | 41.20 ± 1.68d | 1.10 ± 0.07d |
| Lipid | 52.40 ± 1.07b | 1.80 ± 0.11b |
| Protein | 60.20 ± 0.73a | 2.20 ± 0.09a |
| Lipid + bromelain | 48.40 ± 0.50c | 1.38 ± 0.08c |
| Protein + bromelain | 49.60 ± 0.50bc | 1.52 ± 0.03c |
- Note: Different letters (a, b, c, d) indicate significant differences at p < 0.05. Values are presented in mean ± standard error.
3.6. Effect of Treatments on the Viability of Polymorphonuclear Leukocytes of BALB/c Mice
Table 7 shows the effect of the quality of nutrition and enzymes on viability of polymorphonuclear leukocytes of BALB/c mice. Experimental treatments had no significant effect on the viability of PMNs) of BALB/c mice, as the PMNs value was 93.20 ± 0.86 in the control group, 92.00 ± 0.31 in the high-fat diet (lipid) group, and 92.00 ± 0.54 in the high-protein diet (protein) group. The value was 95.20 ± 1.56 in the group that consumed fat with the enzyme (lipid + bromelain) and 92.80 ± 1.31 in the group that consumed protein with the enzyme (protein + bromelain). Statistical analyses showed no significant differences between the different groups (p > 0.05), indicating that the studied factors (nutrition quality and bromelain enzyme addition) did not significantly affect the viability of PMNs.
| Treatments | PMNs |
|---|---|
| Control | 93.20 ± 0.86a |
| Lipid | 92.00 ± 0.31a |
| Protein | 92.00 ± 0.54a |
| Lipid + bromelain | 95.20 ± 1.56a |
| Protein + bromelain | 92.80 ± 1.31a |
- Note: Different letters (a, b, c, d) indicate significant differences at p < 0.05. Values are presented in mean ± standard error.
3.7. Effect of Treatments on Phagocytosis of BALB/c Mice
Table 8 shows the effect of treatments on phagocytosis of BALB/c mice. The quality of nutrition and enzymes significantly affected the levels of phagocytosis in BALB/c mice. Phagocytosis rates were measured at three different time points: 90, 60, and 30 min after exposure to the studied agents. The control group showed the lowest phagocytosis rates with values of 71.20 ± 0.58 at 90 min, 71.60 ± 0.50 at 60 min, and 72.20 ± 1.06 at 30 min. These values were significantly lower than the other groups. In the high-fat diet (lipid) group, phagocytosis rates increased significantly compared to the control group, reaching 82.80 ± 1.01 at 90 min, 83.00 ± 0.94 at 60 min, and 84.00 ± 0.83 at 30 min. Significant differences indicate superiority over the control group. The protein-rich feeding group showed the highest phagocytosis rates, which were 90.40 ± 0.87 at 90 min, 92.80 ± 1.42 at 60 min, and 93.40 ± 1.50 at 30 min. These values were significantly higher than those recorded in the previous two groups (lipid and control). The group of fats with the enzyme bromelain (lipid + bromelain) showed similar results to the protein group, with values reaching 92.60 ± 0.50 at 90 min, 94.20 ± 1.15 at 60 min, and 95.20 ± 1.56 at 30 min. These values were significantly higher than the rest of the groups. Finally, the protein + bromelain group showed lower phagocytosis rates than the protein and lipid + bromelain groups, with values of 72.80 ± 1.06 at 90 min, 74.00 ± 1.70 at 60 min, and 74.00 ± 1.37 at 30 min. These values were not significantly different from the control group but lower than the other groups.
| Treatments | Phago 30 m | Phago 60 m | Phago 90 m |
|---|---|---|---|
| Control | 72.20 ± 1.06c | 71.60 ± 0.50c | 71.20 ± 0.58d |
| Lipid | 84.00 ± 0.83b | 83.00 ± 0.94b | 82.80 ± 1.01c |
| Protein | 93.40 ± 1.50a | 92.80 ± 1.42a | 90.40 ± 0.87b |
| Lipid + bromelain | 95.20 ± 1.56a | 94.20 ± 1.15a | 92.60 ± 0.50a |
| Protein + bromelain | 74.00 ± 1.37c | 74.00 ± 1.70c | 72.80 ± 1.06d |
- Note: Different letters (a, b, c, d) indicate significant differences at p < 0.05. Values are presented in mean ± standard error.
3.8. Effect of Treatments on the Gene Expression of Lipase Enzyme in BALB/c
Table 9 shows the effect of nutritional quality and bromelain supplementation on the gene expression of lipase enzyme in BALB/c mice. The results reflect changes in gene expression levels, which are measured by fold change. Lipid intake significantly increased the gene expression of lipase enzyme, which increased by 149.09 times compared to the control sample. This result indicates that lipid intake alone led to a very large increase in the gene expression of lipase enzyme and this increase may be due to lipid stimulation of the digestion and metabolism of fats, which requires increased production of lipase enzyme to effectively break down fats. In the treatment with protein, protein intake significantly increased the expression of the gene for the enzyme lipase by 22.01 times compared to the control sample. Protein intake also increased the expression of the gene for the enzyme lipase, but at a much lower level compared to the lipid group. Protein may stimulate the secretion of amino acids necessary for many metabolic processes, including the production of lipase, but its effect is less than that of lipids due to the different nature of metabolism. When bromelain enzyme extracted from pineapple was added to the diet containing fat, the gene expression of lipase enzyme was significantly enhanced, with a relative change of 427.57 times compared to the control sample. The results showed that the presence of the bromelain enzyme doubled the response of fat cells and led to a significant increase in the production of the lipase enzyme, indicating a cooperative interaction between fat and bromelain in enhancing fat-related metabolic activity. In the group fed with albumin protein to which bromelain enzyme was added, the results showed a significant decrease in the gene expression of lipase enzyme, with a relative change of only 0.69 times, indicating a decrease in the levels of gene expression of lipase enzyme compared to the control sample.
| Treatments | CT gene | CT HK | ΔCTE | ΔCTC | ΔΔCt | Fold change2−ΔΔCt |
|---|---|---|---|---|---|---|
| Control | 26.75 | 0 | 0 | 0 | 0 | 1 |
| Lipid | 26.78666667 | 31.04333 | −4.26 | 2.96 | −7.22 | 149.0859 |
| Protein | 24.62666667 | 26.21 | −1.5 | 2.96 | −4.46 | 22.00867 |
| Lipid + bromelain | 23.64 | 29.42 | −5.78 | 2.96 | −8.74 | 427.5650147 |
| Protein + bromelain | 25.27666667 | 21.77333333 | 3.5 | 2.96 | 0.54 |
3.8.1. Group 1: Control Diet and Water
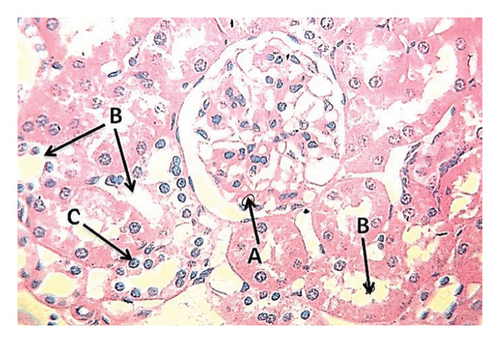
The results of the first group, which was fed a standard diet with drinking water, showed normal histological imaging of the liver, where the central vein, hepatocytes, and hepatic sinusoids were normal (Figure 1). The results of histological imaging of kidney sections showed a normal glomerulus consisting of the lumen of the capillaries, Bowman’s capsule and proximal tubules were normal, and the interstitial tissue was also normal (Figure 2).
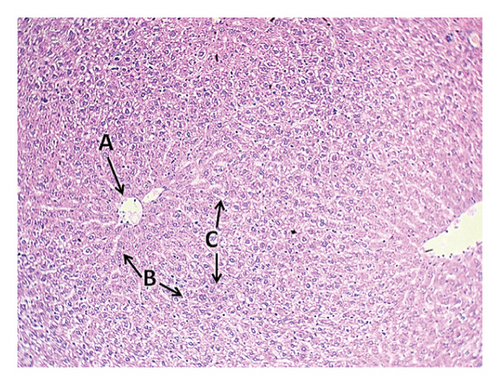

3.8.2. Group 2: High-Fat Diet (High-Fat Diet, 4 g of Free Fat Ghee Divided Into Two Doses, Morning and Evening)
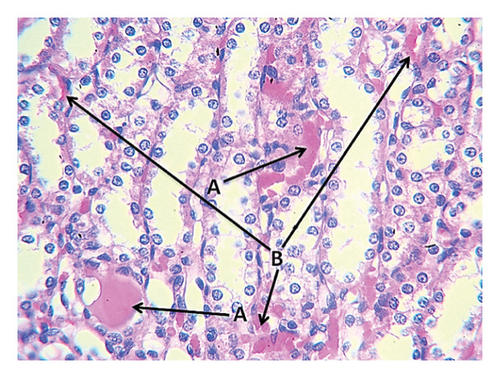
The second group (high-fat diet, 4 g of free fat divided into two doses, morning and evening) showed congestion in the central vein of the liver tissue with fatty degeneration and irregularity of fat cells (Figure 3). It also showed the presence of large clots in the central vein in the portal area with congestion in the central vein and aggregation of mononuclear inflammatory cells. Aggregation of lymphocytes in the hepatocytes and acute cellular degeneration and hyperplasia of the bile ducts in the portal area were also observed, in addition to a significant increase in the number of Koffer cells. Liver tissue of the second group (high-fat) also showed an increase in the number of bile ducts in the portal area with hyperplasia of its epithelium and atherosclerosis of the arteries in the portal area in addition to the explosion of hepatocytes. Kidney tissue of this group showed the presence of interstitial hemorrhage between many tissue slices of the kidneys, atrophy of the renal glomeruli, and severe star-shaped cellular degeneration (Figure 4). Also observed in the other kidney tissues of the individuals of this group were a thrombus in the renal vein, fat deposition around the renal vein surrounded by inflammatory cells, in addition to blood congestion in the renal vein, infiltration of lymphocytes in the interstitial tissue, and the presence of hyaline casts in the distal renal tubules.
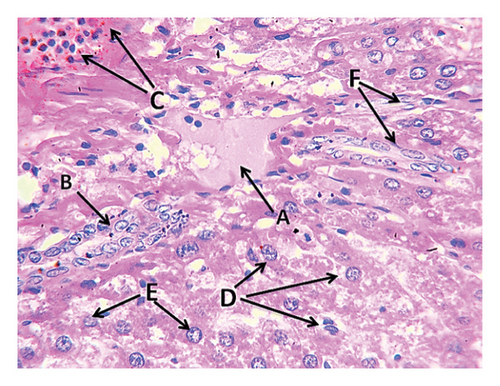
3.8.3. Group 3: Fat + Bromelain (4 mg of Bromelain Enzyme With Fat Divided Into Two Doses Daily)
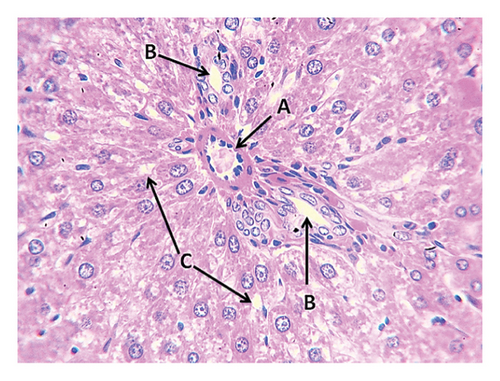
The fourth group (high-fat diet 4 g + bromelain 4 mg divided into two doses, morning and evening) showed a gradual return to normal tissue when compared with the liver tissue of the individuals in the control group, where the liver cells and bile duct appeared normal (Figure 5), while other histological examinations among individuals in this group showed slight dilatation and congestion of the central vein and slight rupture of hepatic cells less than what was found among the tissues in the protein and fat group without additives. However, in the tissues of the remaining individuals, the liver tissue was normal, the central vein appeared normal, and the interstitial tissue was normal. In the kidney tissues of this group, the glomeruli and renal tubules were normal, as was the case with the lymphocytes, which returned to normal, and there were no cellular infiltrations as was the case with the kidney tissue among the individuals of the control group (Figure 6).
4. Discussions
The study investigated the effects of dietary fat, protein (albumin), and the enzyme bromelain on weight gain, metabolic health, and immune functions of experimental animals. Results indicated that moderate increases in animal weight correlated with the nutritional content of their feed, but high-fat intake (4 g daily) led to significant weight gain due to the high caloric density of fat (9 calories per gram) and its impact on appetite regulation via leptin, a satiety hormone [39, 40]. The albumin (protein) group also experienced increased weight gain, attributed to its essential amino acids and effects on hunger-regulating hormones [41]. When bromelain was added to a high-fat diet, weight gain was reduced, likely due to its role in fat absorption and metabolism, as well as its anti-inflammatory properties [42]. Bromelain also enhanced protein digestion, leading to increased energy availability and reduced appetite, which contributed to weight loss [43, 44]. The addition of bromelain to both fat and protein diets improved liver health by reducing liver enzyme levels and oxidative stress, indicating a protective effect against fatty liver disease [45]. The study also found that high-fat diets resulted in lower platelet counts and increased WBC counts, indicating chronic inflammation and potential clotting issues [46]. In contrast, the protein group showed higher platelet levels due to the amino acids provided for platelet production [47]. The introduction of bromelain normalized platelet levels and reduced inflammation [48]. In terms of lipid profiles, the fat-only (lipid) group exhibited elevated levels of LDL, triglycerides, and total cholesterol, while the protein group showed lower liver fat levels, suggesting better metabolic health [49]. Bromelain supplementation improved lipid profiles by enhancing liver function and reducing fat accumulation [50]. Kidney function was also affected, with increased urea and creatinine levels observed in the fat and protein groups, indicating potential renal stress [51]. Bromelain helped alleviate this burden by improving protein digestion and enhancing renal blood flow [52]. Phagocytosis levels were highest in the fat and protein groups, with bromelain further enhancing phagocytic activity, likely due to its effects on immune cell function and cytokine production [53]. The study concluded that the dietary combinations used were safe and beneficial, enhancing metabolic activity and immune responses without compromising cell viability [45]. Gene expression analysis revealed that fat intake increased lipase enzyme expression, crucial for fat metabolism, while bromelain significantly boosted this effect when combined with fat [40, 54]. However, bromelain’s interaction with albumin reduced lipase expression, suggesting a complex interplay between dietary components and metabolic regulation [55].
5. Conclusion
The study discovered that weight gain, metabolism, and immune system functions were affected by dietary fat, protein, and bromelain. A high-fat diet resulted in considerable overweight, low-grade inflammation, and metabolic disturbances, while protein consumption improved metabolic health but placed greater stress on the kidneys. Supplementation with bromelain inhibited those adverse actions through stimulation of lipid metabolism, reduced inflammation, enhanced hepatic and renal functions, and boosted immune response. Overall, these are great findings, suggesting that bromelain supplementation may be a beneficial dietary addition to balance the effects of fat and protein-rich diets without adverse effects.
Ethics Statement
Animal care procedures for the experiment have been approved by the local Ethic Committee of Animal Experiments, Erciyes University, Kayseri, Türkiye (No: 22/204).
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Omar Salah Ahmed: methodology, investigation, data curation, formal analysis, writing – original draft, visualization; Yusuf Konca and Najeeb Mohammed Hussein: conceptualization, data curation, formal analysis, methodology, investigation, visualization, resources, project administration, funding acquisition, supervision, writing – original draft and review and editing.
Funding
This work was supported by Erciyes University, Scientific Research Projects Authority under Grant No. 22-204-January 2024 for PhD studies in the field of Therapeutic Nutrition and Enzymes.
Acknowledgments
The authors thank the central laboratories of the College of Agriculture and Veterinary Medicine at Kayseri University/Türkiye and the University of Anbar and the University of Tikrit/Iraq for the support of research works.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




